Abstract
Objectives: Midkine, a heparin‐binding growth factor, promotes population growth, survival and migration of several cell types, but its effect on articular chondrocytes remains unknown. The aim of this study was to investigate its role on proliferation of articular chondrocytes in vitro and in vivo.
Materials and methods: Bromodeoxyuridine incorporation and MTT assays were performed to examine the proliferative effect of recombinant human midkine (rhMK) on primary articular chondrocytes. Activation of extracellular signal‐regulated kinase (ERK) and phosphatidylinositol 3‐kinase (PI3K) was analysed using western blot analysis. Systemic and local delivery of rhMK into mice and rats was preformed to investigate the proliferative effect of rhMK in vivo, respectively. Histological evaluation, including measurement of articular cartilage thickness, cell density, matrix staining and immunostaining of proliferating cell nuclear antigen was carried out.
Results: rhMK promoted proliferation of articular chondrocytes cultured in a monolayer, which was mediated by activation of ERK and PI3K. The proliferative role of rhMK was not coupled to dedifferentiation of culture‐expanded cells. Consistent with its action in vitro, rhMK stimulated proliferation of articular chondrocytes in vivo when it was administered subcutaneously and intra‐articularly in mice and rats, respectively.
Conclusion: Our results demonstrate that rhMK stimulates proliferation of primary articular chondrocytes in vitro and in vivo. The results of this study warrant further examination of rhMK for treatment of animal models of articular cartilage defects.
Introduction
Articular cartilage is a hypocellular and avascular connective tissue with dense collagen and proteoglycan matrix that provides a low‐friction and highly durable wear‐resistant surface (1). It is structurally divided into three zones, namely superficial, intermediate, and a deep zone, each with unique cell morphology and arrangement of type II collagen fibres (1). Articular chondrocytes, comprising only 5–10% of cartilage, are the only cell type found within the tissue but produce the extensive extracellular matrix and maintain its physiological function (2). Chondrocytes in the superficial zone are flattened, with collagen fibrils arranged parallel to the surface. In the intermediate zone, they are located in the lattice and are round in shape, with collagen fibres being less organized. In the deep zone, chondrocytes and collagen fibres are oriented in vertical columns perpendicular to the surface (1). Under normal physiological conditions, in vivo metabolism of mature articular cartilage is regulated by growth factors, originating from both the cartilage and the surrounding synovial tissue and fluid (3).
Several growth factors have been investigated extensively for their functions in chondrocyte proliferation, differentiation and synthesis of extracellular matrix in vitro, such as fibroblast growth factor‐2 (FGF‐2), FGF‐18, insulin‐like growth factor‐1 (IGF‐1) and CCN family 2/connective tissue growth factor (CCN2/CTGF) (4, 5, 6, 7, 8, 9, 10, 11). In addition, FGF‐2 has been shown to stimulate increase of articular cartilage in young rats in vivo after intra‐articular injection (12). The major signalling pathway of FGF‐2 for cell proliferation and differentiation is known to activate mitogen‐activated protein kinases (MAPKs) (5). MAPKs including ERK, p38 and JNK are the central regulators that control cell proliferation and survival as well as matrix synthesis (13).
Midkine (MK) is a member of the pleiotrophin (PTN)/MK family and was originally described as a retinoic acid‐inducible, developmentally regulated, heparin‐binding, secreted neurotrophic factor (14, 15). MK is highly expressed in the brain during midgestation and is down‐regulated at birth. In the adult, MK has a very restricted pattern of expression with highest transcript levels in the intestine (16). MK is also expressed in hypertrophic chondrocytes of pre‐bone cartilage rudiments and in the epiphyseal growth plate during embryogenesis (17). Previous studies have mostly focused on MK effects in neurons and neurite outgrowth (18), bone fracture healing (17), neutrophil activation and osteoclast differentiation in rheumatoid arthritis (19), tumorigenesis and tumour progression (20), cerebral infarction (21), cardiac remodelling (22) and liver regeneration (23). However, the potential roles of MK in proliferation and differentiation of articular chondrocytes remain undetermined.
In the present study, we investigated effects of recombinant human midkine (rhMK) on proliferation of primary and passaged articular chondrocytes, dedifferentiation of articular chondrocytes, and its role in enhancement of articular cartilage, after systemic and local delivery.
Materials and methods
rhMK protein expression and purification
A DNA sequence (GenBank NM_001012334) encoding mature human MK (hMK) protein was expressed in Escherichia coli BL21 (DE3) using pET30a (+) vector (Novagen, Madison, WI, USA) as described previously (24). Briefly, expression of rhMK was induced by isopropylthio‐β‐d‐galactoside (IPTG) at final concentration of 1 mm. After induction of rhMK expression, bacteria were harvested and sonicated. The pellet of the inclusion body was washed, precipitated and solubilized in 6 m guanidine HCl. Solubilized proteins were refolded by drop‐wise dilution into defined protein folding buffer, followed by centrifugation at 18 000 g at 4 °C for 30 min. The supernatant was purified using S‐Sepharose columns (Amersham, Piscataway, NJ, USA). Column fractions were analysed by SDS–PAGE and western blotting. Purity of purified protein was >98% as assessed by reverse phase high performance liquid chromatography (RP‐HPLC), and proved to be biologically active by NIH3T3 cell proliferation assay. Purified protein was passed through a sterile 0.22 μm filter and stored at −80 °C. Endotoxin level of the final formulated rhMK protein solution was <0.03 EU/μg protein.
Isolation of primary articular chondrocytes
Rat articular chondrocytes were isolated from articular cartilage of 8‐week‐old male Sprague–Dawley rats as described previously (25). Briefly, rat articular cartilage was removed using a scalpel from the tibial plateau and femoral condyle, and diced into small pieces. Cartilage specimens were then treated with 0.25% trypsin solution containing 0.02% EDTA. Chondrocytes were released by digestion with 0.2% type II collagenase (Invitrogen, Shanghai, China) in Dulbecco’s modified Eagle’s medium (DMEM) (high glucose) (Invitrogen) for 16 h and filtered through a 100 μm nylon filter (Falcon, Franklin Lakes, NJ, USA). Cells were seeded in 6‐, 24‐, or 96‐well tissue culture plates in DMEM containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) and incubated in a humidified atmosphere of 5% CO2 at 37 °C.
BrdU incorporation assay of chondrocyte proliferation
Rat primary articular chondrocytes were plated at cell density of 1 × 104 cells/well in 96‐well plates and cultured in DMEM containing 10% FBS, in the absence or presence of various concentrations of rhMK (0.1, 0.3, 1.0, 3.0, 9.0 μg/ml), the inhibitor of MEK1/2, PD98059 (2.8, 8.3, and 25 μm), or the PI3K inhibitor, LY294002 (1.1, 3.3, and 10 μm) for 52 h. Bromodeoxyuridine (BrdU) reagent was added at the final 4 h of culture. BrdU is incorporated into newly synthesized DNA strands of actively proliferating chondrocytes. After partial denaturation of double stranded DNA, BrdU was detected immunochemically (Millipore, Billerica, MA, USA). Absorbance was measured at 450 nm using an ELX‐800 ELISA plate reader (Bio‐Tek Instruments, Winooski, VT, USA) according to the manufacturer’s instructions.
MTT assay of chondrocyte proliferation
Rat primary articular chondrocytes were plated at a density of 1 × 104 cells/well in 96‐well multiplates in DMEM containing 10% FBS and incubated for 48 h in a humidified atmosphere of 5% CO2 at 37 °C. Cells were washed once in DMEM and medium was subsequently replaced with DMEM containing 0.5% FBS with or without rhMK (3.0 μg/ml). After culture for different numbers of days (as indicated in Fig. 1), the effect of rhMK on chondrocyte proliferation was determined using the MTT assay (Sigma‐Aldrich, Shanghai, China). In principle, viable cell number is directly proportional to production of formazan, which after solubilization, can be measured spectrophotometrically. Briefly, 20 μl of MTT (0.5 mg/ml) in sterile phosphate‐buffered saline (PBS) was added to each well. Plates were incubated at 37 °C for 4 h, and then 120 μl of DMSO was added to each well. Absorbance was measured at 490 nm using the ELX‐800 ELISA plate reader (Bio‐Tek Instruments).
Figure 1.
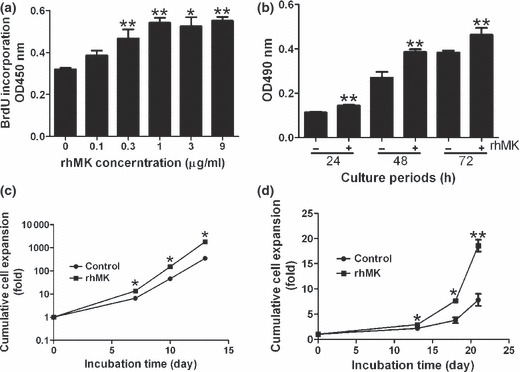
rhMK stimulates proliferation of rat articular chondrocytes in vitro. (a) rhMK promotes proliferation of rat primary articular chondrocytes in vitro in a dose‐dependent manner. Freshly isolated chondrocytes cultured in the presence of 10% FBS were treated with rhMK at indicated concentrations for 48 h. Cells were labelled with anti‐BrdU for an additional 4 h followed by quantification of BrdU incorporating cells at OD450 nm after immunochemical staining. (b) Proliferative role of rhMK was detected by MTT assay. After initial culture of rat primary articular chondrocytes in high serum concentration (10%) for 48 h, medium was changed to low serum (0.5%) supplemented with or without rhMK (3 μg/ml). The effect of rhMK on chondrocyte proliferation exposed for various time intervals was determined. (c, d) Cumulative cell expansion of rat primary articular chondrocytes cultured in 10% FBS (c), or in serum‐free medium (d) supplemented without (control) or with rhMK (3 μg/ml) was determined by direct cell counting. Cell number expansion in fold was based on direct cell counts and calculated as described in the Materials and methods section. All data are expressed as mean ± SD from triplicate cultures. Similar results were obtained in three independent experiments. *Value compared to controls without rhMK, using Student’s t‐test. *P < 0.05, **P < 0.01.
Passage of articular chondrocytes in monolayer
Rat primary articular chondrocytes were plated at a density of 1 × 104 cells/cm2 in six‐well plates and were cultured in DMEM containing 10% FBS, with or without rhMK (3.0 μg/ml), at 37 °C; media were changed every 2 days. After 7 days, primary chondrocytes (passage 0, P0) that had reached subconfluence were recovered by incubation with 0.25% trypsin solution containing 0.02% EDTA. Cells were cultured for two additional passages (P1 and P2) of 3 days culture duration each, under the same culture conditions as those of primary culture (P0). At each passage, number of cells recovered and cell viability were determined using a haemocytometer and the trypan blue exclusion test. Expansion of cell number was calculated according to the known method described previously (26). The remaining cells were lysed using TRIzol reagent (Invitrogen) for RNA extraction.
For serum‐free culture, the medium used was DMEM supplemented with ITS+1 (insulin‐transferrin‐selenium; Sigma) in the presence or absence of rhMK (3 μg/ml).
Western blot analysis
Rat primary articular chondrocytes were plated in 24‐well plates at cell density of 2 × 105 cells/cm2 and cultured in DMEM containing 10% FBS, with or without rhMK (3.0 μg/ml). After incubation, cells were lysed in RIPA buffer containing proteinase inhibitors (Beyotime, China). Total protein extracts (15 μg) were resolved by SDS–PAGE, electroblotted on to PVDF membranes, blocked with 5% non‐fat milk and probed with 1:1000 dilution of primary antibodies (ERK1/2, phospho‐ERK1/2, p38, phospho‐p38, JNK, phosphor‐JNK, Akt, and phospho‐Akt, all from Cell signaling Technology, Beverly, MA, USA) overnight at 4 °C. Antibody‐bound protein bands were detected using reagents from the enhanced chemiluminescence kit as described by the manufacturer (Pierce, Holmdel, NJ, USA), and were photographed using Kodak X‐OMAT LS film (Eastman Kodak, Rochester, NY, USA).
Analysis of chondrocyte dedifferentiation using reverse transcription‐polymerase chain reaction
Total RNA was extracted from cultured chondrocytes using TRIzol reagent (Invitrogen). Isolated RNA (1 μg) was reverse transcribed and then amplified using reagents from a commercial kit (Takara, Shanghai, China) according to the manufacturer’s instruction. After an initial denaturation step of 2 min at 95 °C, amplification consisted of 24–30 cycles of 30 s at 95 °C, 30 s at optimal annealing temperature (AT), and 30 s at 72 °C. Primers and conditions for reverse transcription‐polymerase chain reaction analyses were as follows: Col2a1 (AT 54 °C, 24 cycles), forward GAATGGCTGACCTGACCTGATA and reverse GGCGT CTGACTCACACCAGATA; Col1a1 (AT 50 °C, 24 cycles), forward GGGCAAGACAGTCATCGAATA and reverse ATGTCCATTCCGAATTCCT; Sox9 (AT 54 °C, 30 cycles), forward TGGCAGACCAGTACCCGCATCT and reverse TCTTTCTTGTGCTGCACGCGC; β‐actin (AT 54 °C, 24 cycles), forward GAGGCATCCTGAC CCTGAAG and reverse CATCACAATGCCAGTGGTACG. PCR products were separated on 1.5% (w/v) agarose gels and quantified, after staining with ethidium bromide, in comparison to internal control gene expression of β‐actin.
Systemic administration of rhMK into mice
All animal experiments were performed according to the guidelines of the Animal Care and Use Committee of School of Pharmacy of Shanghai Jiao Tong University. Eight‐week‐old male C57BL/6 mice were used for systemic administration of rhMK. Various doses including 10, 33, 100, 300, and 900 μg/kg of rhMK in 100 μl of saline were injected subcutaneously into mice twice a day for 7 days. The mice were killed at 15 days after first injection. Distal ends of femurs and tibias were harvested, dissected from surrounding soft tissue and were processed as described below in the ‘ Histological evaluation ’ section.
Intra‐articular injection of rhMK into rats
Sodium pentobarbital (65 mg/kg) was injected intraperitoneally into 3‐week‐old male Sprague–Dawley rats, and lower extremities of the anaesthetized animals were shaved and cleaned. A single dose of 175 μg rhMK/kg in 10 μl of saline was injected into knee joints using a 27‐gauge needle daily for 7 days. Rats were allowed unrestricted activity after waking from the anaesthesia, and were killed 15 days after the first injection. Distal ends of femurs and tibias were harvested, dissected from surrounding soft tissue and were processed as described below in the ‘ Histological evaluation ’ section.
Histological evaluation of cartilage tissue
All specimens were harvested, fixed in 10% neutral‐buffered formalin, decalcified and embedded in paraffin. Serial 6 μm sections were stained with haematoxylin and eosin (HE) or Safranin O for microscopic examination. For comparison of cartilage thickness in saline vehicle, articular cartilages were measured using an ocular micrometer and values were averaged for the three sections per animal. Depth to tidemark was used to express thickness of articular cartilage at 1/2 distance across the femoral condyle or tibial plateau.
To determine cellularity of the articular cartilage, cell density per defined area in the tissue section was calculated (50 ×50 μm for mouse and 100 × 100 μm for rat samples). Three sections from each paraffin wax sample were used to calculate average cell density from one animal. Mean cell density per experimental group was the average from three animals.
Immunohistochemical staining using PCNA in articular cartilages
Articular cartilage sections prepared as above were deparaffinized and dehydrated. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide in distilled water. Sections were washed three times in PBS then treated with 0.01 m citrate sodium solution for antigen retrieval. Non‐specific staining was reduced by incubation with 5% BSA for 20 min at room temperature. Sections were incubated in monoclonal antibody against proliferating cell nuclear antigen (PCNA; Santa Cruz Biotechnology, Santa Cruz, CA, USA), diluted 1:400 with PBS containing 1% BSA, at room temperature for 2 h. Antibody binding was visualized using reagents StrepAvidin–biotin–peroxidase complex (SABC) kit (Boster, Wuhan, China) in combination with diaminobenzidine (DAB) liquid substrate solution (Boster). Sections were then counterstained slightly with haematoxylin and examined microscopically.
To determine frequency of PCNA‐positive cells per tissue section, positive and negative PCNA‐stained chondrocytes were counted in a defined area under magnification of ×400 (100 × 100 μm). Three sections from each paraffin wax sample were used to calculate frequency of PCNA‐positive cells of one animal. Three animals were analysed for each experimental group.
Statistical analysis
Results are expressed as mean ± SD. Statistically significant differences among different treatment groups were determined using the two‐tailed Student’s t‐test. Statistical significance was assumed for P‐values <0.05.
Results
rhMK promoted proliferation of articular chondrocytes in vitro
Midkine is known to be a growth factor for several cell types, such as human umbilical vein endothelial cells, human fibroblasts WI‐38 and a neuronal cell line PC‐12 (27). We proposed that it may also stimulate growth of articular chondrocytes. To test this hypothesis, we expressed and purified recombinant protein as described previously (24) and tested its role in proliferation of articular chondrocyte in monolayer cultures. Addition of rhMK significantly increased chondrocyte proliferation, directly measured by BrdU incorporation into S phase cells (Fig. 1a). Maximal effect was 72% increase over control values at the highest tested dose of 9 μg/ml, and it was effective over a wide range of concentrations from 0.3 to 9 μg/ml (Fig. 1a). After determination of optimal working concentration of rhMK, its temporal influence in primary culture of the chodrocytes was evaluated. Chosen 3 μg/ml rhMK enhanced proliferation of the cells as early as 24 h and maintained its influence over the entire culture of 72 h (Fig. 1b). These data demonstrate that rhMK promotes proliferation of rat articular chondrocytes in primary monolayer cultures.
It is known that culture‐expanded articular chondrocytes lose their chondocyte phenotype quickly (28). Thus, the cells may not respond to proliferative stimulation of rhMK after a number of passages. To test this hypothesis, rhMK was added to each of the three passages of articular chondrocyte culture. Addition of rhMK resulted in sustained increase in cell numbers in all three (Fig. 1c). Cell numbers were expanded by 1801 ± 127 fold compared to 347 ± 49 fold in controls after three passages. However, percentage of increase in cell output in passage 0 (113 ± 24%), passage 1 (75 ± 30%) and passage 2 (49 ± 12%) were significantly reduced, and at passage 5, there was no difference. The data suggest that because of phenotypic changes, the cultured chondrocytes lost their ability to respond to rhMK, and that rhMK influenced growth of chondrocyte specifically.
We noticed that the chondrocytes proliferated in monolayer cultures even without rhMK, although at a slower rate (Fig. 1), which may be due to presence of 10% serum. Indeed, freshly isolated articular chondrocytes, cultured in serum‐free medium with or without rhMK, failed to attach to the surface of culture plates and formed aggregates in suspension without proliferation. We propose that attachment of cells is required to respond to rhMK stimulation. To test this hypothesis, cells were plated in serum medium for 24 h to allow their adhesion to the culture plates. Subsequently, medium was changed to serum‐free medium and cells were cultured for three passages without serum. Interestingly, initial adhesion of freshly isolated cells enabled them to be cultured and passaged in monolayer without serum. rhMK in absence of serum increased proliferation of the cultured chondrocytes. Cells were expanded by 18.6 ± 1.2 fold compared to 7.8 ± 1.2 fold of controls after three passages (Fig. 1d). However, percentage of increase in cell output in passage 0 (34 ± 19%), passage 1 (51 ± 4.5%), and passage 2 (18 ± 5.5%) were significantly lower, and at passage 4, there was no difference, which is consistent with those of cultures with serum. The data further suggest that growth of articular chondrocytes and their response to rhMK stimulation all require solid‐surface attachment, which may reflect their characteristic tissue originality.
Taken together, data of in vitro proliferation analysis strongly suggest that rhMK enhances proliferation of culture‐expanded, in addition to primary, articular chondrocytes with or without serum, and that its role is dependent on cell attachment.
ERK and Akt activation mediates proliferative role of rhMK on articular chondrocytes
To investigate molecular mechanisms underlying effects of rhMK on primary articular chondrocytes, phosphorylation status of MAPKs (ERK1/2, p38, JNK) and Akt, which are implicated in chondrocyte proliferation (5, 29), was examined using western blot analysis. rhMK stimulated phosphorylation of ERK1/2 2 h after addition of rhMK to the culture of freshly isolated articular chondrocytes, and levels of phosphorylated ERK1/2 were maintained for 24 h; in contrast, phosphorylated p38 and JNK were not detected (Fig. 2a). There were no significant changes in levels of total ERK in the same samples as demonstrated using polyclonal anti‐ERK1/2 antibody, which recognizes both activated and non‐activated ERK1/2 (data not shown). Furthermore, Akt was phosphorylated 2 h after addition of rhMK (Fig. 2a).
Figure 2.
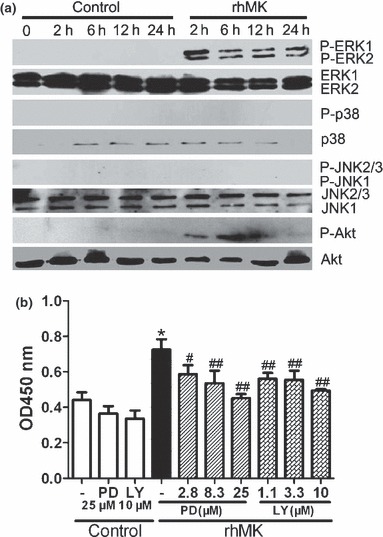
ERK and PI3K signalling pathways involved in proliferation of primary rat articular chondrocytes stimulated by rhMK. (a) Phosphorylation of ERK1/2 and Akt in articular chondrocytes cultured in the presence of rhMK were detected by western blot analysis. Freshly isolated rat articular chondrocytes were cultured in DMEM containing 10% FBS with and without rhMK (3 μg/ml) for the indicated time periods (2–24 h) and lysed. Immunoblotting with antibodies against phospho‐ERK1/2, ERK1/2, phospho‐p38, p38, phospho‐JNK, JNK, phospho‐Akt and Akt were performed. Experiments were repeated three times. (b) MEK1/2 inhibitor PD98059 (PD) and PI3K inhibitor LY294002 (LY) inhibited the proliferative role of rhMK in cultures of primary articular chondrocytes. Freshly isolated rat articular chondrocytes were cultured in DMEM containing 10% FBS supplemented with combination of rhMK (3 μg/ml), PD, or LY as indicated at various concentrations. After culture for 48 h, cells were labelled with BrdU for an additional 4 h followed by quantification of BrdU‐incorporated cells. All data are expressed as mean ± SD from triplicate cultures. Experiments were repeated three times. *Value was compared to control/group (−); #, ##Values were compared to the rhMK/group (−) using two‐tailed Student’s t‐test. *P < 0.01, #P < 0.05, ##P < 0.01.
Phosphorylation of ERK and Akt requires their upstream kinases of MEK1/2 and PI3K respectively (30, 31). To correlate rhMK‐mediated ERK and Akt activation with rhMK‐proliferative role on articular chondrocytes, we analysed effects of MEK1/2 and PI3K inhibition on proliferation of primary articular chondrocytes. MEK1/2 inhibitor, PD98059 at a dose of 25 μm, did not alter proliferation of articular chondrocytes without rhMK, but significantly attenuated its effect on proliferation of articular chondrocytes. PD98059 inhibited stimulation of BrdU incorporation by rhMK in a dose‐dependent manner (Fig. 2b). Likewise, LY294002, a PI3K inhibitor at a concentration of 10 μm, inhibited rhMK‐induced chondrocyte proliferation, but not cells without rhMK treatment. Moreover, the inhibitory role of LY294002 was dose‐dependent (Fig. 2b). Thus, we have demonstrated that both activation and inactivation of ERK and Akt phosphorylation correlated with rhMK‐stimulated chondrocyte growth. Our data strongly suggest that ERK and Akt activation mediates the proliferative role of rhMK on articular chondrocytes.
rhMK does not affect dedifferentiation of articular chondrocytes cultured in monolayer
Chondrocytes are known for their phenotypic change from chondrocytic to fibroblastic type when cultured in monolayer (28). After defining the role of rhMK in promoting proliferation of chondrocytes in monolayer culture, phenotypes of culture‐expanded articular chondrocytes were examined by semi‐quantitative PCR and immunohistochemical staining. Expression levels of Col2a1 and Col1a1 encoding collagen types II α1 and collagen type I α1, respectively, and Sox9 encoding chondrocyte‐specific transcription factor Sox9 (32) relative to the house‐keeping gene β‐actin were determined. Similar gene expression patterns were observed on cultured articular chondrocytes at each passage, for three passages with or without addition of rhMK (data not shown). Immunostaining of collagen II of cultured articular chondrocytes confirmed the results of gene expression (data not shown). Thus, rhMK had no effect on phenotype changes of the chondrocytes and did not prevent their dedifferentiation in monolayer culture.
rhMK stimulates articular cartilage augmentation after systemic delivery in mice
The direct proliferative role of rhMK on primary articular chondrocytes in vitro suggests that it may also stimulate growth of articular cartilage through enhancing cell proliferation. To test the hypothesis, rhMK was administered to adult mice, systemically. The approach is supported by the fact that mature articular chondrocytes in 8‐week‐old mice, mostly not in active cell cycle, receive their nutrients from the joint fluid, which interchanges with the blood constantly through synovial membranes (33). We examined augmentation of articular cartilage in knee joints after administration of rhMK for 7 days as described in the Materials and methods section. Histological evaluation of the cartilage tissue sections showed significant increase in articular cartilage in those that had received rhMK at 33–900, but not at 10 μg/kg, and a dose–response curve was observed with ED50 of 103.7 μg/kg (Fig. 3a). Compared to vehicle saline‐treated mice, rhMK increased thickness of knee joint cartilage by a maximum of 44% at the highest given dose of 900 μg/kg (Fig. 3a,b).
Figure 3.
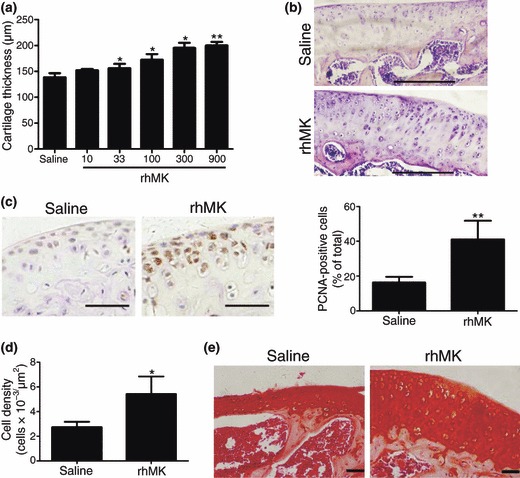
rhMK stimulated proliferation of articular chondrocytes in mice after systemic delivery. Mice received a daily subcutaneous injection of vehicle saline or rhMK at different dosages for 7 days. After 15 days from first injection, the mice were killed. Histological examination and cell analysis of articular cartilage and chondrocyte were performed as described in the Materials and methods section. (a) Tibial plateau thickness of articular cartilage of three animals per treatment group is shown. rhMK stimulated enlargement of tibial plateau cartilage in a dose‐dependent manner. (b) Representative tibial cartilage tissue sections from mice treated with saline or rhMK (300 μg/kg). HE staining, bar = 200 μm. (c) Cell proliferation marker PCNA expression analysis in condylar cartilage tissue sections from the mice treated with saline or rhMK (300 μg/kg). Left, representative tissue sections immunohistochemically stained for PCNA, bar = 50 μm; right, quantitative and statistical analysis of PCNA‐positive chondrocytes (n = 3). (d) Chondrocyte density is shown for tibial cartilage from mice treated with saline or rhMK (300 μg/kg) (n = 3). (e) Condylar cartilage sections from mice treated with saline or rhMK (300 μg/kg) were stained with cartilage matrix‐specific dye Safranin O (bar = 50 μm). Similar intensity of staining is shown. All data are presented as mean ± SD. Three independent experiments showed similar results. Values were compared to the saline group using the two‐tailed Student’s t‐test. *P < 0.05, **P < 0.01.
Augmentation of articular cartilage observed in H and E‐stained tissue sections suggests enhanced proliferation of chondrocytes and hence number of chondrocytes. To test the hypothesis, we quantified chondrocyte density and proliferation status by directly counting number of PCNA‐positive chondrocytes in cartilage sections, which were stained for the cell proliferation marker protein PCNA immunohistochemically. rhMK at 300 μg/kg induced 2.59‐fold increase in PCNA‐positive cells (Fig. 3c), accompanied by 1.98‐fold increase in chondrocyte cell density (Fig. 3d), thereby demonstrating that increase in knee cartilage was due to enhanced proliferation of chondrocytes.
The articular chondrocytes, newly produced by rhMK treatment appeared morphologically identical to normal chondrocytes located in cartilage lacunae in the HE‐stained tissue sections (Fig. 3b), which suggests that they have normal anabolic and catabolic functions synthesizing cartilage‐specific extracellular matrix. To test this hypothesis, matrix mucopolysaccharide of cartilage tissue sections was examined by Safranin O staining (34). Indeed, rhMK‐treated mice showed essentially identical intensity of dark red Safranin O staining, confined to newly formed areas of thick cartilage layers as that of control mice, with thinner layers of existing cartilage (Fig. 3e).
Taken together, results of the analysis of mouse articular cartilage demonstrate that rhMK promotes augmentation of articular cartilage when delivered systemically. Increase in articular cartilage in adult mice is due to enhanced proliferation of articular chondrocytes, and the newly produced ones secrete normal amounts of cartilage‐specific extracellular matrix molecules, such as mucopolysaccharide.
rhMK stimulates articular cartilage augmentation after intra‐articular injection in rats
The in vitro direct proliferative role of rhMK stimulating proliferation of rat primary articular chondrocytes suggests that it may promote growth of articular cartilage when delivered in direct contact with cartilage tissue itself. To test this possibility, we delivered the protein into rat knee joints and examined its role, essentially as that of the mouse experiment described above. Adopted from results of the mouse dose–effect study, 175 μg/kg rhMK was injected daily for 7 days. Fifteen days after the first injection, animals were killed and knee joints were processed for analysis as described in the Materials and methods section. Measurement of thickness of cartilage in HE‐stained tissue sections revealed significant increase in articular cartilage also in rats. rhMK increased thickness of cartilage by 1.52‐fold over controls (Fig. 4a). Furthermore, thickened cartilage was associated with a 1.44‐fold increase in chondrocyte cell density (Fig. 4b), and 3.66‐fold increase in PCNA‐positive cells (Fig. 4c). The observed rhMK effect in rats after local delivery was essentially similar to that of the mouse results, where the protein was delivered systemically. Thus, rhMK has been found to promote growth of articular chondrocytes by enhancing proliferation of rat chondrocytes in vivo.
Figure 4.
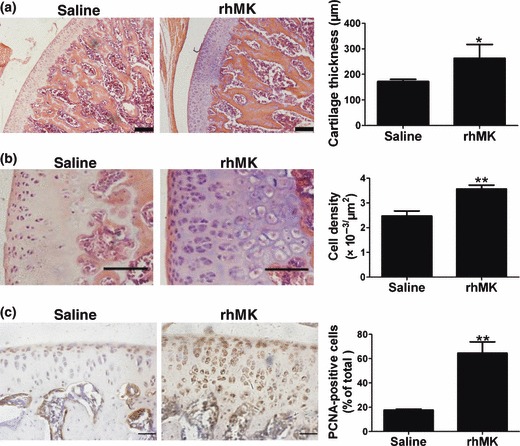
rhMK stimulated proliferation of articular chondrocytes in rats after intra‐articular injection. Rats received a daily injection of saline or rhMK (175 μg/kg) into knee joints for 7 days. After 15 days from first injection, the rats were killed. Histological examination and cell analysis of condylar cartilage and chondrocytes were performed as described in the Materials and methods section. (a) Histological analysis of thickness of femoral condylar cartilage from rats treated with saline or rhMK. Left, representative tissue sections stained for HE showing defined layer of cartilage, bar = 200 μm; right, quantitative and statistical analysis of the cartilage thickness (n = 3). (b) Chondrocyte density in condylar cartilage sections from rats treated with saline or rhMK. Left, representative tissue stained for HE showing defined morphology of chondrocytes, bar = 100 μm; right, quantitative and statistical analysis of chondrocyte cell density (n = 3). (c) Cell proliferation marker PCNA expression analysis in condylar cartilage sections from rats treated with saline or rhMK. Left, representative tissue sections immunohistochemically stained for PCNA, bar = 50 μm; right, quantitative and statistical analysis of PCNA‐positive chondrocytes (n = 3). All data are presented as mean ± SD. Experiments were repeated three times. Values were compared to the saline group using the two‐tailed Student’s t‐test. *P < 0.05, **P < 0.01.
Discussion
We have demonstrated in this study that rhMK stimulates proliferation of articular chondrocytes in vitro and in vivo. rhMK stimulated proliferation of articular chondrocytes by enhancing their DNA synthesis, which was revealed by measurement of BrdU incorporation in monolayer culture of freshly isolated articular chondrocytes from adult rats. Cell proliferative role of rhMK was apparently mediated by activation of MEK1/2 and PI3K signalling pathways, which was strongly suggested by rhMK‐dependent ERK and Akt phosphorylation, and ERK/Akt‐dependent activity of rhMK. Consistent with its role in vitro, rhMK promoted growth of native articular cartilage through enhanced proliferation of otherwise quiescent chondrocytes in vivo. Newly produced chondrocytes were functionally similar to native ones in synthesis of cartilage‐specific matrix molecules, such as mucopolysaccharide.
The role of MK in proliferation and differentiation of primary chondrocytes in vitro and in vivo has not been determined previously. Ohta et al. reported that transfection of MK cDNA into a mouse chondrogenic clonal cell line ATDC5 resulted in enhanced synthesis of cartilage ECM. However, not all the transfectants producing high levels of MK were promoted to produce cartilage ECM; MK exogenously added to culture of ATDC5 cells had no effect on synthesis of ECM (17). Similarly, Dreyfus et al. showed that exogenous MK did not enhance chondrogenesis of the whole limb mesenchymal cells isolated from stage 22 or 24 chick embryos in micromass culture (35). These results suggest that exogenous MK does not promote chondrogenic differentiation in culture of cells that harbour chondrogenic potential. Furthermore, Cockshutt et al. have reported that MK purified from chick embryos had no effect on proliferation of cultured primary chondrocytes isolated from sterna of chick embryos at concentrations of 10–500 ng/ml (36), which may be explained by our observation that optimal MK concentration required for promoting proliferation of primary articular chondrocytes, is 3 μg/ml.
MK is known to activate several cell signalling pathways in a number of different cell types. MK induced phosphorylation of ERK and PI3K in culture of tumour cell lines and human primary endothelial cells, which was responsible for its mitogenic function (27, 37). Furthermore, phosphorylation of ERK contributed to enhanced proliferation of primary chondrocytes cultured with FGF‐18 (5), and a fish chondrocyte cell line treated with vanadate (29). In this study, we have provided new evidence that rhMK also activates the phosphorylation of ERK and Akt, and utilizes the MAPK and PI3K pathways to mediate its mitogenic function in primary articular chondrocytes, similar to that shown in other cell types. The cognate MK receptor in chondrocytes remains to be determined. However, our data on activation of ERK and PI3K in chondrocytes by rhMK suggests that anaplastic lymphoma kinase (ALK) may be the functional receptor in chondrocytes, as ALK has been shown to mediate activation of both ERK and PI3K by MK in several tumour cell lines and endothelial cells (27).
We have observed that activation of ERK was sustained for 24 h, and activation of Akt was detected at 2 h after rhMK treatment. Activation of MAPKs and Akt is the common theme of other growth factors such as FGF‐2 and FGF‐18 (5, 38, 39). However, onset and sustained phosphorylation time varies amongst growth factors and also among cell types, by a specific factor. FGF‐2 induced ERK activation at 5 min, and that was maintained for 24 h in cultured human adult articular chondrocytes (38); however, its activation of ERK and Akt lasted only 1 h in cultured bone marrow mesenchymal stem cells (39). Similarly, midkine activated ERK phosphorylation in 15 min, which was sustained for 24 h, in cultured neurons (40), but its activation of ERK at 5 min was sustained for 8 h in a cancer cell line (41). Furthermore, activation of Akt by midkine in the cultured neurons took only 15 min (40), compared to that of 1 h for LNCap tumour cells (41). It is clear that the importance and mechanisms of the onset and sustained activation of MAPKs and Akt by various growth factors functioning in different cell types remain to be determined.
Pleiotrophin is another heparin‐binding growth/differentiation factor, which is 50% homologous to MK at the amino acid level and shares the genomic organization with MK and predicted protein structure (27). Previous studies have shown that exogenous addition of PTN enhanced chondrocyte differentiation of whole limb mesenchymal cells isolated from stage 22 or 24 chick embryos in micromass culture (35), which is consistent with the observation of stimulated proteoglycan synthesis in culture of mature bovine chondrocytes with PTN (42), suggesting a chondrogenic differentiation role of PNT in vitro. However, there have been conflicting reports regarding the role of PTN in chondrocyte proliferation. Tapp et al. have reported that PTN inhibited proliferation of mature bovine articular chondrocytes and had no effect on cultures of foetal and newborn bovine articular chondrocytes (42). On the contrary, Pufe et al. showed that recombinant human PTN stimulated proliferation of immortalized chondrocytes (C28/I2) and primary human chondrocytes (43). Compared to our observation of the proliferative effect of rhMK on primary articular chondrocytes, the role of PTN on proliferation of articular chondrocytes in vitro and in vivo remains to be determined.
Reducing chondrocyte dedifferentiation during monolayer culture expansion is a major challenge in cartilage tissue engineering. Previous studies have reported that growth factors stimulating the highest chondrocyte proliferation also induce strongest cell dedifferentiation in monolayer culture (44). Our work however, on the contrary while promoting cell proliferation, rhMK had no effect on dedifferentiation of cultured articular chondrocytes (data not shown). It enhanced production of the cells without accelerating their culture‐associated dedifferentiation, suggesting a potential use of the factor in preparation of demanding articular chondrocytes in cartilage tissue engineering and cell therapeutics.
In conclusion, here we have provided strong evidence that rhMK accelerates articular chondrocyte proliferation in culture as well as in vivo, in experimental animals. Our findings indicate that rhMK may have clinical applications for repair and reconstruction of diseased articular cartilage through stimulating population growth of residual articular chondrocytes. Further experiments are being conducted to evaluate its therapeutic effects on articular cartilage injuries and diseases in animal models.
Acknowledgements
We thank Dr Dangsheng Li for reviewing and editing this manuscript. This study was supported by China Ministry of Science and Technology (grant ‘863’ 2007AA02Z149), National Natural Science Foundation of China (grant 30801419), and Science and Technology Commission of Shanghai Municipality (grant 075407071).
References
- 1. Ulrich‐Vinther M, Maloney MD, Schwarz EM, Rosier R, O’Keefe RJ (2003) Articular cartilage biology. J. Am. Acad. Orthop. Surg. 11, 421–430. [DOI] [PubMed] [Google Scholar]
- 2. Chung C, Burdick JA (2008) Engineering cartilage tissue. Adv. Drug Deliv. Rev. 60, 243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Den Berg WB, Van Der Kraan PM, Scharstuhl A, Van Beuningen HM (2001) Growth factors and cartilage repair. Clin. Orthop. Relat. Res. 321S, s244–s250. [DOI] [PubMed] [Google Scholar]
- 4. Olney RC, Wang J, Sylvester JE, Mougey EB (2004) Growth factor regulation of human growth plate chondrocyte proliferation in vitro . Biochem. Biophys. Res. Commun. 317, 1171–1182. [DOI] [PubMed] [Google Scholar]
- 5. Shimoaka T, Ogasawara T, Yonamine A, Chikazu D, Kawano H, Nakamura K et al. (2002) Regulation of osteoblast, chondrocyte, and osteoclast functions by fibroblast growth factor (FGF)‐18 in comparison with FGF‐2 and FGF‐10. J. Biol. Chem. 277, 7493–7500. [DOI] [PubMed] [Google Scholar]
- 6. Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S et al. (2002) Fibroblast growth factor‐18 is a trophic factor for mature chondrocyte and their progenitors. Osteoarthritis Cartilage 10, 308–320. [DOI] [PubMed] [Google Scholar]
- 7. Oh CD, Chun JS (2003) Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin‐like growth factor‐1. J. Biol. Chem. 278, 36563–36571. [DOI] [PubMed] [Google Scholar]
- 8. Worster AA (2001) Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor‐beta1 in monolayer and insulin‐like growth factor‐1 in a three‐dimensional matrix. J. Orthop. Res. 19, 738–749. [DOI] [PubMed] [Google Scholar]
- 9. Phornphutku C, Wu KY, Yang X, Chen Q, Gruppuso PA (2004) Insulin‐like growth factor‐1 signaling is modified during chondrocyte differentiation. J. Endocrinol. 183, 477–486. [DOI] [PubMed] [Google Scholar]
- 10. Nishida T, Kubota S, Nakanishi T, Kuboki T, Yosimichi G, Kondo S et al. (2002) CTGF/Hcs24, a product of a hypertrophic chondrocyte‐specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes. J. Cell. Physiol. 192, 55–63. [DOI] [PubMed] [Google Scholar]
- 11. Fujisawa T, Hattori T, Ono M, Uehara J, Kubota S, Kuboki T et al. (2008) CCN family 2/connective tissue growth factor (CCN2/CTGF) stimulates proliferation and differentiation of auricular chondrocytes. Osteoarthritis Cartilage 16, 787–795. [DOI] [PubMed] [Google Scholar]
- 12. Shida J, Jingushi S, Izumi T, Iwaki A, Sugioka Y (1996) Basic fibroblast growth factor stimulates articular cartilage enlargement in young rats in vivo . J. Orthop. Res. 14, 265–272. [DOI] [PubMed] [Google Scholar]
- 13. Loeser MF, Erickson EA, Long DL (2008) Mitogen‐activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 20, 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsubara S, Tomomura M, Kadomatsu K, Muramatsu T (1990) Structure of a retinoic acid‐responsive gene, MK, which is transiently activated during the differentiation of embryonal carcinoma cells and the mid‐gestation period of mouse embryogenesis. J. Biol. Chem. 265, 9441–9443. [PubMed] [Google Scholar]
- 15. Tomomura M, Kadomatsu K, Matsubara S, Muramatsu T (1990) A retinoic acid‐responsive gene, MK, found in the teratocarcinoma system. Heterogeneity of the transcript and the nature of the translation product. J. Biol. Chem. 265, 10765–10770. [PubMed] [Google Scholar]
- 16. Tsutsui J, Kadomatsu K, Matsubara S, Nakagawara A, Hamanoue M, Takao S et al. (1993) A new family of heparin‐binding growth/differentiation factors: increased midkine expression in Wilms’ tumor and other human carcinomas. Cancer Res. 53, 1281–1285. [PubMed] [Google Scholar]
- 17. Ohta S, Muramatsu H, Senda T, Zou K, Iwata H, Muramatsu T (1999) Midkine is expressed during repair of bone fracture and promotes chondrogenesis. J. Bone Miner. Res. 14, 1132–1144. [DOI] [PubMed] [Google Scholar]
- 18. Kretschmer PJ, Fairhurst JL, Decker MM, Chan CP, Gluzman Y, Bohlen P (1991) Cloning, characterization and developmental regulation of two members of a novel human gene family of neurite outgrowth‐promoting proteins. Growth Factors 5, 99–114. [DOI] [PubMed] [Google Scholar]
- 19. Maruyama K, Muramatsu H, Ishiguro N, Muramatsu T (2004) Midkine, a heparin‐binding growth factor, is fundamentally involved in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 50, 1420–1429. [DOI] [PubMed] [Google Scholar]
- 20. Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R (1997) An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 57, 1814–1819. [PubMed] [Google Scholar]
- 21. Yoshida Y, Goto M, Tsutsui J, Ozawa M, Sato E, Osame M (1995) Midkine is present in the early stage of cerebral infarct. Dev. Brain Res. 85, 25–30. [DOI] [PubMed] [Google Scholar]
- 22. Horiba M, Kadomatsu K, Yasui K, Lee JK, Takenaka H, Sumida A (2006) Midkine plays a protective role against cardiac ischemia/reperfusion injury through a reduction of apoptotic reaction. Circulation 114, 1713–1720. [DOI] [PubMed] [Google Scholar]
- 23. Ochiai K, Muramatsu H, Yamamoto S, Ando H, Muramatsu T (2004) The role of midkine and pleiotrophin in liver regeneration. Liver Int. 24, 484–491. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Z, Du L, Xiang D, Zhu S, Wu M, Lu H et al. (2009) Expression and purification of bioactive high‐purity human midkine in Escherichia coli . J. Zhejiang Univ. Sci. B 10, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE (2000) Reduction in the chondrocyte response to insulin‐like growth factor 1 in aging and osteoarthritis: studies in a non‐human primate model of naturally occurring disease. Arthritis Rheum. 43, 2110–2120. [DOI] [PubMed] [Google Scholar]
- 26. Lee DA, Reisler T, Bader DL (2003) Expansion of chondrocytes for tissue engineering in alginate beads enhances chondrocytic phenotype compared to conventional monolayer techniques. Acta Orthop. Scand. 74, 6–15. [DOI] [PubMed] [Google Scholar]
- 27. Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT (2002) Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 277, 35990–35998. [DOI] [PubMed] [Google Scholar]
- 28. Darling EM, Athanasiou KA (2005) Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 23, 425–432. [DOI] [PubMed] [Google Scholar]
- 29. Tiago DM, Cancela ML, Aureliano M, Laize V (2008) Vanadate proliferative and anti‐mineralogenic effects are mediated by MAPK and PI‐3K/Ras/Erk pathways in a fish chondrocyte cell line. FEBS Lett. 582, 1381–1385. [DOI] [PubMed] [Google Scholar]
- 30. Rescan C, Coutant A, Talarmin H, Theret N, Glaise D, Guguen‐Guillouzo C et al. (2001) Mechanism in the sequential control of cell morphology and s phase entry by epidermal growth factor involves distinct MEK/ERK activations. Mol. Biol. Cell 12, 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang BH, Zheng JZ, Aoki M, Vogt PK (2000) Phosphatidylinositol 3‐kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 97, 1749–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W (2000) Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 19, 389–394. [DOI] [PubMed] [Google Scholar]
- 33. O’Hara BP, Urban JPG, Maroudas A (1990) Influence of cyclic loading on the nutrition of articular cartilage. Ann. Rheum. Dis. 49, 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenberg L (1971) Chemical basis for the histological use of safranin O in the study of articular cartilage. J. Bone Joint Surg. (Am) 53, 69–82. [PubMed] [Google Scholar]
- 35. Dreyfus J, Carvalho NB, Duprez D, Raulais D, Vigny M (1998) HB‐GAM/pleiotrophin but not RIHB/midkine enhances chondrogenesis in micromass culture. Exp. Cell Res. 241, 171–180. [DOI] [PubMed] [Google Scholar]
- 36. Cockshutt AM, Régnier F, Vigny M, Raulais D, Chany‐Fournier F (1993) Retinoic acid induced heparin‐binding factor (RIHB) mRNA and protein are strongly induced in chick embryo chondrocytes treated with retinoic acid. Exp. Cell Res. 207, 430–438. [DOI] [PubMed] [Google Scholar]
- 37. Sandra F, Harada H, Nakamura N, Ohishi M (2004) Midkine induced growth of ameloblastoma through MAPK and Akt pathways. Oral Oncol. 40, 274–280. [DOI] [PubMed] [Google Scholar]
- 38. Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F et al. (2007) Basic fibroblast growth factor stimulates matrix metalloproteinase‐13 via the molecular cross‐talk between the mitogen‐activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J. Biol. Chem. 282, 11110–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi SC, Kim SJ, Choi JH, Park CY, Shim WJ, Lim DS (2008) Fibroblast growth factor‐2 and ‐4 promote the proliferation of bone marrow mesenchymal stem cells by the activation of the PI3K‐Akt and ERK1/2 signaling pathways. Stem Cells Dev. 17, 725–736. [DOI] [PubMed] [Google Scholar]
- 40. Owada K, Sanjo N, Kobayashi T, Mizusawa H, Muramatsu H, Muramatsu T et al. (1999) Midkine inhibits caspase‐dependent apoptosis via the activation of mitogen‐activated protein kinase and phosphatidylinositol 3‐kinase in cultured neurons. J. Neurochem. 73, 2084–2092. [PubMed] [Google Scholar]
- 41. You Z, Dong Y, Kong X, Beckett LA, Gandour‐Edwards R, Melamed J (2008) Midkiine is a NF‐kappaB‐inducible gene that supports prostate cancer cell survival. BMC Med. Genomics 1, 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tapp H, Hernandez DJ, Neame PJ, Koob TJ (1999) Pleiotrophin inhibits chondrocyte proliferation and stimulates proteoglycan synthesis in mature bovine cartilage. Matrix Biol. 18, 543–556. [DOI] [PubMed] [Google Scholar]
- 43. Pufe T, Groth G, Goldring MB, Tillmann B, Mentlein R (2007) Effects of pleiotrophin, a heparin‐binding growth factor, on human primary and immortalized chondrocytes. Osteoarthritis Cartilage 15, 155–162. [DOI] [PubMed] [Google Scholar]
- 44. Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M et al. (2001) Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro . J. Cell. Biochem. 81, 368–377. [DOI] [PubMed] [Google Scholar]


