Abstract
Four proteins, DpgA–D, required for the biosynthesis by actinomycetes of the nonproteinogenic amino acid monomer (S)-3,5-dihydroxyphenylglycine (Dpg), that is a crosslinking site in the maturation of vancomycin and teicoplanin antibiotic scaffolds, were expressed in Escherichia coli, purified in soluble form, and assayed for enzymatic activity. DpgA is a type III polyketide synthase, converting four molecules of malonyl-CoA to 3,5-dihydroxyphenylacetyl-CoA (DPA-CoA) and three free coenzyme A (CoASH) products. Almost no turnover was observed for DpgA until DpgB was added, producing a net kcat of 1–2 min−1 at a 3:1 ratio of DpgB:DpgA. Addition of DpgD gave a further 2-fold rate increase. DpgC had the unusual catalytic capacity to convert DPA-CoA to 3,5-dihydroxyphenylglyoxylate, which is a transamination away from Dpg. DpgC performed a net CH2 to C⩵O four-electron oxidation on the Cα of DPA-CoA and hydrolyzed the thioester linkage with a kcat of 10 min−1. Phenylacetyl-CoA was also processed, to phenylglyoxylate, but with about 500-fold lower kcat/KM. DpgC showed no activity in anaerobic incubations, suggesting an oxygenase function, but had no detectable bound organic cofactors or metals. A weak enoyl-CoA hydratase activity was detected for both DpgB and DpgD.
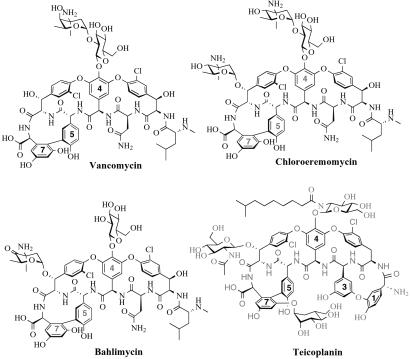
One of the key features of the vancomycin and teicoplanin classes of glycopeptide antibiotics, used to treat life-threatening Gram-positive bacterial infections (1, 2), are the rigidifying crosslinks in the side chains of the heptapeptide backbone. These crosslinks create the cup-shaped architecture in the antibiotic that sets the geometry for interaction with the targets: N-acyl-D-Ala-D-Ala termini of cell wall biosynthetic intermediates of the bacterial pathogens (3–5). The crosslinks are between the electron-rich aryl side chains of tyrosines and two variants of nonproteinogenic amino acids, (S)-4-hydroxyphenylglycine (Hpg) (residues 4 and 5 of vancomycin and teicoplanin) and (S)-3,5-dihydroxyphenylglycine (Dpg) (residue 7 of vancomycin, 3 and 7 of teicoplanin) (Fig. 1). These two unusual amino acid monomers are encoded by two sets of four orfs each in the biosynthetic clusters for chloroeremomycin (6) and bahlimycin (7), which have the identical heptapeptide aglycone as vancomycin (8), and in the biosynthetic cluster for a teicoplanin family member, A47934 (Gerard Wright, personal communication). The four-enzyme pathway for Hpg (6) has recently been reconstituted from purified proteins in vitro and shunts the common aromatic amino acid precursor chorismate to the Hpg skeleton (9).
Figure 1.
Vancomycin and teicoplanin family members containing the nonproteinogenic amino acids Hpg and Dpg. The Hpg and Dpg residues in each structure are indicated by their residue numbers.
By contrast, the carbon skeleton of the isomeric Dpg is assembled from malonyl-CoA by a polyketide synthase-type pathway (10). Much clarification on the biosynthesis of this amino acid has recently been achieved by studies on the bahlimycin dpgA, -B, -C, and -D genes (11), where a knockout in dpgA could be complemented by exogenous 3,5-dihydroxyphenylacetate to restore antibiotic production. This is consistent with the results of feeding experiments using 13C-labeled precursors in Amycolatopsis orientalis (12). Expression of the four-gene bahlimycin cassette in Streptomyces lividans led to accumulation of 3,5-dihydroxyphenylglyoxylate in ethyl acetate extracts, and a knockout of the transaminase gene, hpgT (9, 13), from the Hpg pathway in Amycolatopsis mediterranei led to the accumulation of 3,5-dihydroxyphenyglyoxylate, clearly indicating the participation of hpgT in Dpg biosynthesis (11). The role of hpgT was also determined by in vitro biochemical studies using the purified enzyme from A. orientalis (12).
Pfeifer et al. (11) noted that they were unable to get soluble DpgA via heterologous expression in E. coli, and so reported no studies on the four enzymes to establish their individual roles and reaction sequences, although they clearly indicated that DpgA would be a type III polyketide synthase. In this work, we report successful heterologous expression in E. coli of all four proteins, DpgA–D (originally listed as ORFs 27–30), from the A. orientalis chloroeremomycin biosynthetic cluster (6), and purification of the proteins in soluble form as His-tagged enzymes. We describe enzymatic activities for DpgA–D, validating that these enzymes suffice for conversion of four malonyl-CoAs to 3,5-dihydroxyphenylacetyl-CoA (DPA-CoA, by DpgA plus DpgB/DpgD) and then on to 3,5-dihydroxyphenylglyoxylate (by DpgC), the direct precursor by transamination to the amino acid Dpg.
Materials and Methods
Materials.
All chemicals were purchased from Sigma, with the exception of the synthesized CoA compounds noted below. [2-14C]Malonyl-CoA was purchased from New England Nuclear. DNA oligomers were purchased from Integrated DNA Technologies (Coralville, IA). Ni-NTA Superflow resin was purchased from Qiagen (Chatsworth, CA).
Cloning, Overexpression, and Purification of DpgA–D.
Each of the four genes was amplified from A. orientalis NRRL 18098 genomic DNA by PCR, using the following primers (introduced restriction sites are underlined): DpgANterm, 5′-GGGAATTCCATATGGGGGTGGATGTATCGATGACG-3′; DpgACterm, 5′-AGCTTTGAATTCTCACCATTGGATCAGCGCCATTT-3′; DpgBNterm, 5′-GGGAATTCCATATGAACAACGAACTTGTGCTGCGT-3′; DpgBCterm, 5′-AATCCGCTCGAGTCACGACGCGGCTTCCCGGCGCAA-3′; DpgCNterm, 5′-GAGAATTCCATATGACAACGGATTCCGCGACGCTG-3′; DpgCCterm, 5′-AACAACTCGAGTCATGCCGATCCCGCCGCGAACCT-3′; DpgDNterm 5′-GAATTCCATATGAGCTACACCCGGGTGC-3′; and DpgDCterm 5′-CCCAAGCTTTCACATGTAATGCCCTGTCCA-3′. DpgA was cloned into the NdeI/EcoRI sites of the pET16b vector, which encodes an N-terminal His10-tag; DpgB was cloned into the NdeI/XhoI sites of the pET22b vector, which encodes an N-terminal His6-tag; DpgC was cloned into the NdeI/XhoI sites of the pET16b vector; and DpgD was cloned into the NdeI/HindIII sites of the pET22b vector. Each plasmid was transformed into E. coli strain BL21(DE3). Cultures of the cells were grown at 37°C until mid-log phase, induced with 60 μM isopropyl β-D-thiogalactopyranoside, and then grown at 25°C for 4 h. Cells were harvested by centrifugation and purified by nickel affinity chromatography using Ni-NTA Superflow resin. The purified proteins were dialyzed into 20 mM Tris-Cl, pH 7.5/100 mM NaCl/10% glycerol/500 μM DTT, flash frozen, and stored at −80°C.
Chemical Synthesis of CoA Esters.
DPA-CoA, 3-hydroxyphenylacetyl-CoA (HPA-CoA), (R)-mandelyl-CoA, and (S)-mandelyl-CoA were synthesized following the protocol by Belshaw et al. (14), with two modifications: (i) the reaction mixture was acidified with 50% trifluoroacetic acid (TFA), dried, and redissolved in H2O before purification by HPLC; and (ii) the CoA esters were purified using a semipreparative Vydac peptide and protein C18 column, monitoring at 260 nm (10 ml/min; 0–5 min, 0% B; 5–30 min, 0–40% B; A = H2O, 0.1% TFA; B = acetonitrile; the same HPLC solvents are used throughout this study).
Assays for DpgA/DpgB/DpgD.
Incubation with [14C]malonyl-CoA.
Reaction mixtures (200 μl, pH 7.5) containing [14C]malonyl-CoA (0.5 mM, 0.85 Ci/mol) and DpgA (5 μM) were incubated at 24°C for 1 h and quenched by adding 4 μl of 50% TFA. Parallel incubations with DpgB, both DpgA and DpgB, and without any enzyme were also carried out. Precipitated protein was removed by centrifugation, and the supernatant was analyzed by HPLC using a Vydac C18 small pore (5 μm, 4.6 × 250 mm) column (1 ml/min; 0–25 min, 0–40% B). Dual online UV (at 260 nm) and radioactivity detectors were used to monitor product formation.
Kinetic analysis.
To determine the minimal ratio of DpgB that would result in maximal activity by DpgA, malonyl-CoA (2 mM) was incubated with DpgA (5 μM) and increasing concentrations of DpgB at 24°C (100 μl, pH 7.5) for 15 min. The reaction mixtures were quenched with 50 μl of 4% TFA and analyzed by HPLC using a Vydac C18 small pore column, monitoring at 260 nm (1 ml/min; 0–3 min, 0% B; 3–28 min, 0–50% B).
To quantify the increase in activity of DpgA in the presence of DpgB, malonyl-CoA (2 mM) was incubated with DpgA (5 μM) either in the presence or absence of DpgB (15 μM) at 24°C (310 μl, pH 7.5). At 2, 15, and 30 min, 100-μl aliquots of the reaction mixtures were quenched and analyzed by HPLC as above. The quantification of the increase in activity of DpgA/DpgB in the presence of DpgD was determined similarly, with an incubation of malonyl-CoA (2 mM), DpgA (5 μM), and DpgB (15 μM) either in the presence or absence of DpgD (15 μM).
To determine the kinetic parameters of DpgA on malonyl-CoA in the presence of DpgB, increasing concentrations of malonyl-CoA were incubated with DpgA (20 nM) and DpgB (60 nM) at 24°C (210 μl, pH 7.5). At 15 s and 15 min, 100-μl aliquots of the reaction mixtures were quenched and analyzed by HPLC as above, and the observed rates of increase of CoASH determined from the time courses were fit to the Michaelis–Menten equation to obtain values of kcat and KM.
Assays for DpgC.
Activity assays.
Reactions (200 μl, pH 7.5) containing 2 mM malonyl-CoA, 6 μM DpgA, 8 μM DpgB, and 8 μM DpgC were incubated at 24°C for 1 h. Similar reactions were also performed with 2 mM DPA-CoA, HPA-CoA, or phenylacetyl-CoA (PA-CoA) and only DpgC. Reactions were quenched and analyzed by HPLC as above.
Reactions (200 μl, pH 7.5) containing 2 mM (R)- or (S)-mandelyl-CoA were incubated in the presence or absence of 10 μM DpgC at 24°C for 4 h. Reactions were quenched as above and analyzed by HPLC using a Vydac C18 small pore column, monitoring at 220 nm (1 ml/min; 0–3 min, 2% B; 3–43 min, 2–22% B).
NMR and mass analysis.
DPA-CoA (10 mg) was incubated with DpgC (27 μM) at 24°C (3 ml, pH 7.5) for 4 h. The protein was removed using a Centricon 10 microconcentrator (Amicon), and the filtrate was purified as described above for the CoA esters. A peak at 13 min was collected, lyophilized, and subjected to matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF MS) and proton NMR analysis: 1H NMR (D2O) δ 6.59 (1H, t, J = 2.2 Hz), 6.85 (2H, d, J = 2.2 Hz).
Kinetic analysis by DTNB assay.
A fraction of the purified proteins was dialyzed into 20 mM Tris-Cl, pH 7.5/100 mM NaCl/10% glycerol to remove DTT for use in DTNB [5,5′-dithiobis(2-nitrobenzoic acid)] assays. To determine the kinetic parameters of DpgC on DPA-CoA, a preincubated solution of DTNB and increasing concentrations of DPA-CoA was mixed with DpgC (40 nM) at 24°C (600 μl, pH 7.5), and the reactions were monitored for 5 min at 412 nm. The observed rates of increase in absorption at 412 nm were fit to the Michaelis–Menten equation to obtain values of kcat and KM. DpgB and/or DpgD (40 nM) were added to these reactions to measure whether they had an effect on the observed rates.
Kinetic analysis by HPLC assay.
The kinetic parameters of DpgC on PA-CoA were determined as described for DpgA/DpgB on malonyl-CoA, using increasing concentrations of PA-CoA mixed with DpgC (1.2 μM).
Anaerobic experiments.
Two vials, one containing DPA-CoA (1 mM, 1 ml, pH 7.5) and one containing DpgC (40 μM, 600 μl, pH 7.5), were made anaerobic via repeated cycles of degassing by application of a vacuum followed by treatment with argon gas over 15 min. Five hundred microliters of the anaerobic DpgC solution was transferred to the substrate vial and mixed. At 5, 30, and 60 min, 100-μl aliquots were quenched with 4 μl of 50% TFA and frozen on dry ice. Then, the reaction vial was exposed to air and swirled for 3 min. At 5, 60, and 90 min after exposure to air, 100-μl aliquots were quenched and frozen as before. The aliquots of the various time points were then thawed and centrifuged, and the supernatants were analyzed by HPLC as for the DpgA assays. Similar experiments were carried out with (R)-mandelyl-CoA and PA-CoA.
Metal analysis.
Samples of DpgC and DpgA (as a control for the metal-binding properties of the His10-tag) were prepared for metal analysis following the protocol of Zamble et al. (15).
Results
Expression and Purification of DpgA, DpgB, DpgC, and DpgD from the Chloroeremomycin-Producing Strain of A. orientalis.
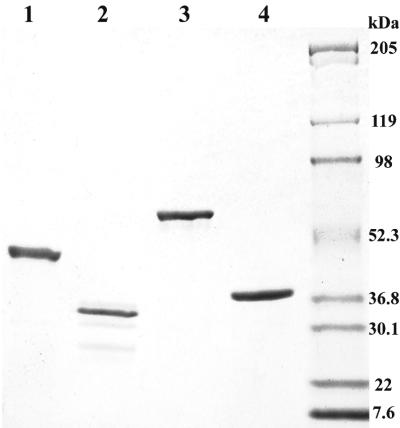
We undertook the subcloning and expression of the dpgA–D genes from the chloroeremomycin producer because we have had good success with E. coli expression of several other genes in this cluster, including those encoding the four Hpg biosynthetic enzymes (9), the five dTDP-L-β-epivancosamine biosynthetic enzymes (16), and the three glycosyl transferases (17, 18). After several problems, including optimization of induction times, temperatures, inducer concentrations, and subcloning strategies that will not be detailed here, we were successful in affinity purification of N-terminally His-tagged versions of DpgA, DpgB, DpgC, and DpgD in soluble form, as shown in the SDS gel profiles of Fig. 2. This enabled the enzyme activity and product identification experiments described below.
Figure 2.
Coomassie-stained 4–15% SDS-polyacrylamide gradient gel of purified DpgA–D. Lane 1, DpgA; lane 2, DpgB; lane 3, DpgC; lane 4, DpgD.
DpgA Is a Type III Polyketide Synthase, Converting Malonyl-CoA to 3,5-Dihydroxyphenylacetyl-CoA in a Reaction Stimulated by DpgB and DpgD.
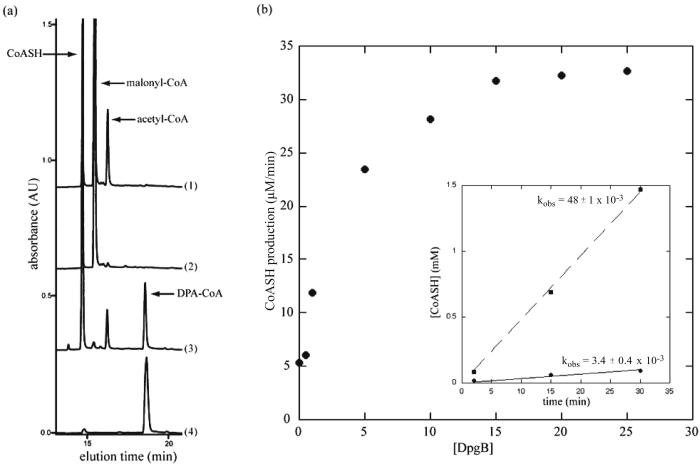
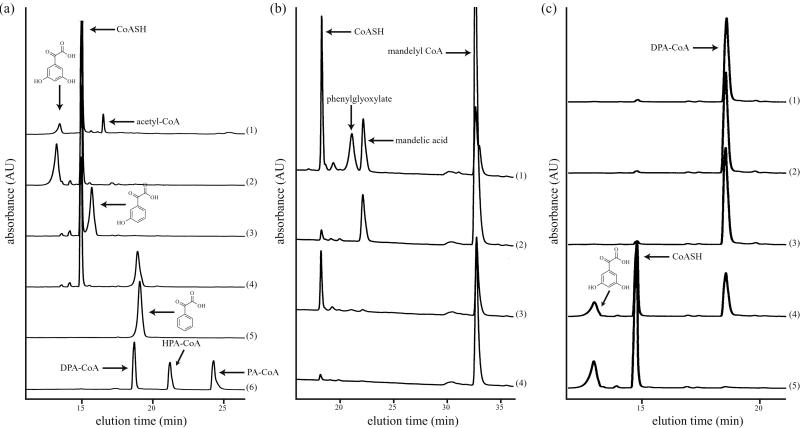
In accord with the bioinformatics predictions from homologies to chalcone synthases (6, 19) and the in vivo data of Pfeifer et al. in S. lividans (11), pure DpgA acts as a Claisen condensation catalyst with malonyl-CoA. The products in incubations with either unlabeled or [14C]malonyl-CoA, as detected by HPLC analysis, are CoASH and a peak that cochromatographed with authentic DPA-CoA. Variable amounts of acetyl-CoA as a minor product are detected, presumably resulting from uncoupled decarboxylation of malonyl-CoA and protonation of the resulting acetyl carbanion before chain extension occurs in the DpgA active site. However, the rate of reaction of DpgA was extremely sluggish and barely detectable in the absence of DpgB. Addition of DpgB led to a dramatic acceleration in the rate of formation of DPA-CoA (Fig. 3). When the acceleration produced by DpgB was measured in terms of CoASH production, 17-fold acceleration was detected. DpgB by itself had no detectable activity on the malonyl-CoA substrate. When DpgD was added to DpgA alone, there was no effect on the activity of DpgA, but when DpgD was added to DpgA/DpgB incubations, a further 2-fold increase in the rate of formation of DPA-CoA was detected by HPLC. MALDI-TOF MS confirmed the expected molecular weight for DPA-CoA [C29H42N7O19P3S (M-H+); calculated 916.2; experimental 916.1].
Figure 3.
Acceleration in rate of formation of DPA-CoA by DpgA upon addition of DpgB. (a) HPLC traces of reactants and products upon incubation of malonyl-CoA with various combinations of DpgA and DpgB for 1 h. Enzymes in each incubation: 1, DpgA; 2, DpgB; 3, DpgA/DpgB; 4, DPA-CoA standard. (b) Rate of CoASH production by DpgA (5 μM) at various concentrations of DpgB. (Inset) Rates of CoASH production by DpgA (5 μM) in the presence (dashed line) or absence (solid line) of DpgB (15 μM). kobs = mM/min.
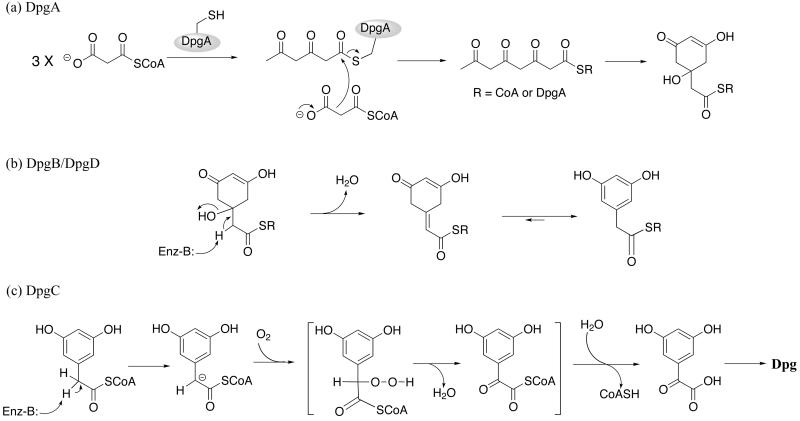
We then used an HPLC assay to obtain kcat and KM values for malonyl-CoA utilization by DpgA/DpgB, with DpgA as the limiting component, by monitoring the rate of CoASH production. Using the rationale that four malonyl-CoA substrate molecules lead to three CoASH and one DPA-CoA product molecules (see Fig. 5a), the kcat for DPA-CoA formation is 1–2 min−1, and the KM is ≈5 μM.
Figure 5.
Schemes of proposed in vivo activities of DpgA–D. (a) DpgA. In this scheme, the consumption of four malonyl-CoA molecules by DpgA leads to the production of three CoASH molecules and one hydrated DPA-CoA precursor. (b) DpgB/DpgD. This scheme depicts DpgB and DpgD as dehydratases that act on the product of DpgA to produce DPA-CoA. (c) DpgC. This scheme depicts a possible mechanism through a peroxide intermediate for the novel oxidase activity of DpgC. The lack of DPA-CoA consumption/CoASH production by DpgC in the absence of O2 suggests that the thioesterase activity occurs after the oxidase activity, as shown in the scheme.
DpgC Converts 3,5-Dihydroxyphenylacetyl-CoA to 3,5-Dihydroxyphenylglyoxylate.
When DpgC was added to DpgA/DpgB incubation mixtures, the DPA-CoA product was rerouted to a new product, as detected by HPLC analysis (Fig. 4a, line 1). This same product could be obtained when synthetic DPA-CoA was added to DpgC alone (Fig. 4a, line 2). In addition to the new product, which was identified as 3,5-dihydroxyphenylglyoxylate as noted below, CoASH was released in stoichiometric amounts. Sufficient product could be accumulated from the DpgC incubations for proton NMR to determine that the methylene hydrogens at C2 of DPA-CoA were totally absent in the product, and the downfield shift of the aromatic ring protons in the product was consistent with oxidation to an α-keto group. Confirmation of the 3,5-dihydroxyglyoxylate structure came from MALDI-TOF MS [C8H6O5 (M-H+): calculated, 182.1; experimental, 181.0]. A further validation of this unusual enzyme activity was obtained when the unsubstituted PA-CoA was incubated with pure DpgC, and the known phenylglyoxylate was produced, comigrating with authentic standard (Fig. 4a, lines 4–5).
Figure 4.
Conversion of DPA-CoA to 3,5-dihydroxyphenylglyoxylate by DpgC. (a) HPLC traces of reactants and products upon incubation of various substrates with DpgC for 1 h. Substrates in each incubation: 1, malonyl-CoA and DpgA/DpgB; 2, DPA-CoA; 3, HPA-CoA; 4, PA-CoA; 5, phenylglyoxylate standard; 6, DPA-CoA/HPA-CoA/PA-CoA standards. (b) HPLC traces of reactants and products upon incubation of (R)- and (S)-mandelyl-CoA in the presence or absence of DpgC for 1 h. 1, (R)-mandelyl-CoA with DpgC; 2, (R)-mandelyl-CoA; 3, (S)-mandelyl-CoA with DpgC; 4, (S)-mandelyl-CoA. (c) HPLC traces of time points of incubation of DPA-CoA with DpgC in the absence (traces 1–3) and then presence (traces 4–5) of O2. 1, 5 min; 2, 30 min; 3, 1 h; 4, 5 min after exposure to O2; 5, 1 h after exposure.
We then used either a DTNB assay or HPLC assay to monitor the rate of CoASH production to obtain kinetic constants for both substrates. The unsubstituted PA-CoA had a 50-fold higher KM (300 μM vs. 6 μM) and a 10-fold reduction in kcat from the authentic substrate (1 min−1 vs. 10 min−1). When DpgB and/or DpgD was added to the DpgC incubation with DPA-CoA, no effects were observed on the rate of CoASH formation.
The source of the keto oxygen introduced into 3,5-dihydroxyphenylglyoxylate could be either water or molecular oxygen. In a first effort to decipher this point, DpgC incubations were conducted anaerobically, with no product formation or loss of substrate detected. On admission of air to the reaction vials, the reaction proceeded (Fig. 4c), proving that DpgC is formally an oxygenase and that the enzyme in the anaerobic incubations was active when O2 became available. This requirement for O2 in turnover was confirmed with conversion of PA-CoA to phenylglyoxylate by DpgC (data not shown). As a further test for possible intermediacy of an α-hydroxy acid or α-hydroxyacyl-CoA in the DpgC reactions, (R)- and (S)-mandelyl-CoA were synthesized and evaluated as substrates both for hydrolysis of the thioester linkage to mandelate and for oxygenative processing to the phenylglyoxylate product. No activity was detected with (S)-mandelyl-CoA, but HPLC analysis revealed that DpgC produced the phenylglyoxylate product with (R)-mandelyl-CoA (Fig. 4b). The kinetics of PA-CoA vs. (R)-mandelyl-CoA oxidative processing indicated that the α-OH-acyl-CoA was not kinetically competent, reacting some 6-fold more slowly than PA-CoA. Further, there was no detectable buildup of mandelyl-CoA by HPLC analysis of PA-CoA incubations with DpgC. Anaerobic incubations did indicate an absolute requirement for O2 in mandelyl-CoA turnover to phenylglyoxylate, as well (data not shown).
DpgC solutions at 2 mg/ml (40 nM) showed no UV/visible absorbance, inconsistent with a bound cofactor such as a flavin, pterin, or iron (Fe/S cluster or heme). Also, atomic absorption analysis failed to reveal any tightly bound iron or copper, two potential redox-active metal ions in oxidoreductase catalysis, at levels above 2 mol %. When EDTA was included in reaction buffers at up to 50 mM, there was no effect on rate, arguing against an easily removed metal cofactor.
DpgB and DpgD Have Weak but Detectable Enoyl-CoA Hydratase Activity.
Bioinformatic analysis reveals that both DpgB and DpgD have similarities to members of the crotonase superfamily (20), enzymes that use β-hydroxy acyl-CoA substrates and catalyze reversible dehydration to the α,β-enoyl-CoAs. When pure DpgB and DpgD were tested with several available enoyl-CoA and β-OH acyl-CoA substrates, clear hydratase activity was detected on crotonyl- and β-methylcrotonyl-CoA, as determined by HPLC analysis and coelution with authentic standards (data not shown). DpgD was further evaluated and displayed saturation kinetics. Whereas the KM values were reasonable, 20 μM for crotonyl-CoA and 40 μM for β-methylcrotonyl-CoA, the kcat values were low, 1.7 min−1 for each substrate, indicating that the activity was real, but that these simple acyclic enoyl-CoAs were not the physiological substrates.
Discussion
The related vancomycin and teicoplanin families of glycopeptide antibiotics are effective antibiotics by interdiction of bacterial cell wall biosynthesis in Gram-positive pathogens, such as staphylococci, streptococci, and enterococci (1), because they complex with the N-acyl-D-Ala-D-Ala ends of peptidoglycan strands before they can become crosslinked into the peptidoglycan meshwork (3, 8). The D-D-dipeptidyl terminus is read by a series of five hydrogen bonds to and from the underside of the antibiotic (4, 5), enabled by the rigid cup-shaped architecture of the crosslinked glycopeptide. The two nonproteinogenic amino acids with electron-rich aryl side chains, Hpg and Dpg (Fig. 1), are substrates for heme protein-mediated oxidative crosslinking by cytochrome P450-type enzymes also encoded in the clusters (6, 13), to set the enabling architecture of the antibiotic. Specifically, this involves Hpg5-Dpg7 crosslinks in both vancomycin and teicoplanin, and an additional Hpg1-Dpg3 aryl ether link in the teicoplanin family. Therefore, Hpg and Dpg are crucial constituent monomers, and their de novo biosynthesis is encoded by two sets of four orfs in all four glycopeptide biosynthetic clusters known to date [chloroeremomycin (6), complestatin (13), bahlimycin (7), and A47934 (Gerard Wright, personal communication)]. At first glance, it is remarkable that the phenylglycine skeletons of Hpg and Dpg are assembled by such different metabolic logic, Hpg from chorismate via an oxygenase of unusual regiospecificity (9, 21) and Dpg by an iterative polyketide synthase reaction, using four malonyl-CoAs to make the eight-carbon skeleton of phenylglycine (22).
As Pfeifer et al. (11) have recently noted, DpgA joins a small set of type III polyketide synthases in bacteria [they are much better known in plant phenylpropanoid metabolism (23, 24)], including tetrahydronaphthalene synthase (25). Given the ability to produce substantial quantities of pure A. orientalis DpgA and its partner protein DpgB in E. coli described here, it will be possible to undertake mechanistic and structural studies to evaluate such fundamental questions of specificity and mechanism as how the C8 (nucleophile) to C3 (electrophile) regiospecificity of cyclization to DPA-CoA, unique to DpgA, is effected. In the likely reaction sequence, a series of elongating diketidyl- and triketidyl-acyl chains, all derived from malonyl-CoA, is shuttled between the active site cysteine thiolate and the thiolate of CoASH in a reciprocating mechanism (26, 27). The nucleophilic attack of the fourth malonyl-CoA during decarboxylation on the triketidyl-S-enzyme is presumed to produce the acyclic tetraketidyl-S-DpgA and then the tetraketidyl-S-CoA. Either linear tetraketidyl thioester in the DpgA active site could be cyclized, C8 onto C3, leading to a hydrated precursor of the dihydroxyphenylacetyl thioester (Fig. 5a). The catalytic turnover of DpgA to produce DPA-CoA was tremendously stimulated by DpgB, some 17-fold under the conditions of Fig. 3 for CoASH formation from malonyl-CoA. DpgD did not have such an effect on DpgA but did provide a further 2-fold increase in DPA-CoA formation rates in three-component DpgA/DpgB/DpgD incubations. These results raise the question of the catalytic activities of DpgB and DpgD. With model enoyl-CoA substrates, we showed that both enzymes act as hydratases. Physiologically, they may act in the other direction, acting as dehydratases, facilitating the aromatization to DPA-SR (R = DpgA or CoA) (Fig. 5b). The putative dihydroaromatic β-OH acyl-CoA nascent product is not released from DpgA alone at appreciable rates, as there is no substantial loss of malonyl-CoA substrate in the absence of DpgB. Over long incubation times (overnight), DPA-CoA does accumulate from DpgA alone. How DpgB and DpgD, crotonase superfamily enzymes, form a complex with DpgA, a type III polyketide synthase, will be a subject of future study.
Even more surprising is the enzymatic activity uncovered for DpgC. Bioinformatic analysis suggests homology (6, 11) of the C terminus to crotonase superfamily members and to β-hydroxy acid dehydrogenases, with no prediction about the N-terminal 20-kDa portion of the enzyme. No classical crotonase-type enoyl-CoA hydratase/β-OH acyl-CoA dehydratase activity was detected, but thioesterase activity was measurable. More intriguingly, the pure DpgC takes DPA-CoA smoothly to 3,5-dihydroxyglyoxylate and CoASH, a hydrolysis of the thioester and, remarkably, a net four-electron oxidation of the α-CH2 to C⩵O. This type of transformation is classically mediated in polyketide synthase and fatty acid synthase assembly lines by the tandem action of three enzymes: flavoprotein desaturases, crotonase-type enoyl thioester hydratases, and alcohol to ketone dehydrogenases. Whereas those enzymes all act at the beta carbon of thioester substrates, this CH2 to C⩵O four-electron oxidation is occurring at the alpha carbon. Pfeifer et al. have proposed a hydratase mechanism of unexpected regioselectivity (11), but our results instead indicate a strict requirement for oxygen in turnover, indicating that DpgC has monooxygenase function. There are no bound organic redox cofactors or redox metals in DpgC preparations, which would put DpgC into a very small subset of enzymes. There are possible precedents for metal-free enzymes that use O2 to oxidize enzyme-bound carbanion equivalents, of which the best characterized is ribulose bisphosphate carboxylase, acting in oxygenase mode (28, 29). In this mode, the electron-rich enediolate form of the substrate may react in a one-electron pathway with O2 to produce a peroxide intermediate, which is then cleaved with intramolecular assistance to yield the phosphoglycolate product (30). A comparable kinetically accessible Cα-carbanion in DPA-CoA could lead to an α-peroxy intermediate that would have to be decomposed to the ketone (Fig. 5b) by intramolecular fragmentation (31), but further study will be needed to delineate mechanism. For the alternate substrate PA-CoA, a possible intermediate could be the α-OH acyl-CoA, mandelyl-CoA. The (R)-, but not the (S)-, isomer of mandelyl-CoA could be converted on to products, showing oxidation of alcohol to ketone and thioester cleavage to yield phenylglyoxylate. It appears that (R)-mandelyl-CoA is not on the reaction pathway from PA-CoA, given its kinetic incompetence. However, its conversion to the same keto acid product by DpgC requires molecular oxygen, is stereospecific, could involve similar peroxy intermediates, and is likely to give insight into the mechanism of this remarkable DpgC catalyst.
The availability of the vancomycin/teicoplanin biosynthetic gene clusters has allowed insights into the metabolic pathways and enzymatic steps of glycopeptide antibiotic assembly. This work on the chloroeremomycin DpgA–D proteins, coupled with the balhimycin dpgA–D studies (11), completes delineation of the enzymatic logic for provision of the dedicated monomers, (S)-4-hydroxyphenylglycine (9), (S)-3,5-dihydroxyphenylglycine, and dTDP-L-vancosamine (16), accounting for 13 of the orfs in these gene clusters. When the amino acid monomers have become available in the producing cell, the nonribosomal peptide synthetase assembly line makes the heptapeptide as a cascade of elongating peptidyl-S-enzyme intermediates in 25 steps on a 24-domain assembly line (32). Then the post-nonribosomal peptide synthetase tailoring steps occur, including the aryl-aryl and aryl-ether crosslinks, e.g., Hpg5-Dpg7, to rigidify the heptapeptide and create the high affinity site for the cell wall target, the end purpose of all of the enzymatic effort to make Hpg and Dpg and to incorporate them.
Acknowledgments
We thank Rahul Kohli for his help in cloning DpgD, and the members of the Walsh lab for many helpful discussions. This work was supported in part by National Institutes of Health Grant GM 49338. C.C.T. is a National Defense Science and Engineering Graduate Fellow.
Abbreviations
- DPA-CoA
3,5-dihydroxyphenylacetyl-CoA
- Dpg
(S)-3,5-dihydroxyphenylglycine
- Hpg
(S)-4-hydroxyphenylglycine
- PA-CoA
phenylacetyl-CoA
- TFA
trifluoroacetic acid
- CoASH
coenzyme A
References
- 1.Moellering R C., Jr Am J Med. 1998;104:3S–6S. doi: 10.1016/s0002-9343(98)00148-x. [DOI] [PubMed] [Google Scholar]
- 2.Bardone M R, Paternoster M, Coronelli C. J Antibiot. 1978;31:170–177. doi: 10.7164/antibiotics.31.170. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds P E. Eur J Clin Microbiol Infect Dis. 1989;8:943–950. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- 4.Williams D H, Williamson M P, Butcher D W, Hammond S J. J Am Chem Soc. 1983;105:1332–1339. [Google Scholar]
- 5.Barna J C J, Williams D H, Williamson M P. J. Chem. Soc. Chem. Commun. 1985. 254–256. [Google Scholar]
- 6.van Wageningen A M, Kirkpatrick P N, Williams D H, Harris B R, Kershaw J K, Lennard N J, Jones M, Jones S J, Solenberg P J. Chem Biol. 1998;5:155–162. doi: 10.1016/s1074-5521(98)90060-6. [DOI] [PubMed] [Google Scholar]
- 7.Pelzer S, Huber P, Süssmuth R D, Recktenwald J, Heckmann D, Wohlleben W. Intern. Patent. WO: A1; 2000. p. 77182. [Google Scholar]
- 8.Sheldrick G M, Jones P G, Kennard O, Williams D H, Smith G A. Nature (London) 1978;271:223–225. doi: 10.1038/271223a0. [DOI] [PubMed] [Google Scholar]
- 9.Hubbard B K, Thomas M G, Walsh C T. Chem Biol. 2000;7:931–942. doi: 10.1016/s1074-5521(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 10.Hammond S J, Williamson M P, Williams D H, Boeck L D, Marconi G G. J. Chem. Soc. Chem. Commun. 1982. 344–346. [Google Scholar]
- 11.Pfeifer V, Nicholson G J, Ries J, Recktenwald J, Schefer A B, Shawky R M, Schröder J, Wohlleben W, Pelzer S. J Biol Chem. 2001;276:38370–38377. doi: 10.1074/jbc.M106580200. [DOI] [PubMed] [Google Scholar]
- 12.Sandercock A M, Charles E H, Scaife W, Kirkpatrick P N, O'Brien S W, Papageorgiou E A, Spencer J B, Williams D H. Chem. Commun. 2001. , 1252–1253. [Google Scholar]
- 13.Chiu H T, Hubbard B K, Shah A N, Eide J, Fredenburg R A, Walsh C T, Khosla C. Proc Natl Acad Sci USA. 2001;98:8548–8553. doi: 10.1073/pnas.151246498. . (First Published July 10, 2001; 10.1073/pnas.151246498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belshaw P J, Walsh C T, Stachelhaus T. Science. 1999;284:486–489. doi: 10.1126/science.284.5413.486. [DOI] [PubMed] [Google Scholar]
- 15.Zamble D B, McClure C P, Penner-Hahn J E, Walsh C T. Biochemistry. 2000;39:16190–16199. doi: 10.1021/bi001398e. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Thomas M G, Hubbard B K, Losey H C, Walsh C T, Burkart M D. Proc Natl Acad Sci USA. 2000;97:11942–11951. doi: 10.1073/pnas.210395097. . (First Published October 17, 2000; 10.1073/pnas.210395097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulichak A, Losey H, Walsh C T, Garavito M. Structure (Cambridge, UK) 2001;9:547–557. doi: 10.1016/s0969-2126(01)00616-5. [DOI] [PubMed] [Google Scholar]
- 18.Losey H, Peczuh M W, Chen Z, Eggert U S, Dong S D, Pelczer I, Kahne D, Walsh C T. Biochemistry. 2000;40:4745–4755. doi: 10.1021/bi010050w. [DOI] [PubMed] [Google Scholar]
- 19.Moore B S, Hopke J N. Chem Biochem. 2001;2:35–38. doi: 10.1002/1439-7633(20010105)2:1<35::AID-CBIC35>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Holden H M, Benning M M, Haller T, Gerlt J A. Acc Chem Res. 2001;34:145–157. doi: 10.1021/ar000053l. [DOI] [PubMed] [Google Scholar]
- 21.Choroba O W, Williams D H, Spencer J B. J Am Chem Soc. 2000;122:5389–5390. [Google Scholar]
- 22.Li T-L, Choroba O W, Hong H, Williams D H, Spencer J B. Chem. Commun. 2001. , 2156–2157. [DOI] [PubMed] [Google Scholar]
- 23.Schröder J. Nat Struct Biol. 1999;6:714–716. doi: 10.1038/11472. [DOI] [PubMed] [Google Scholar]
- 24.Schröder J. Trends Plant Sci. 1997;2:373–378. [Google Scholar]
- 25.Funa N, Ohnishi Y, Fujii I, Shibuya M, Ebizuka Y, Horinouchi S. Nature (London) 1999;400:897–899. doi: 10.1038/23748. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer J-L, Jez J M, Bowman M E, Dixon R A, Noel J P. Nat Struct Biol. 1999;6:775–784. doi: 10.1038/11553. [DOI] [PubMed] [Google Scholar]
- 27.Jez J M, Ferrer J-L, Bowman M E, Dixon R A, Noel J P. Biochemistry. 2000;39:890–902. doi: 10.1021/bi991489f. [DOI] [PubMed] [Google Scholar]
- 28.Cleland W W, Andrews T J, Gutteridge S, Hartman F C, Lorimer G H. Chem Rev. 1998;98:549–561. doi: 10.1021/cr970010r. [DOI] [PubMed] [Google Scholar]
- 29.Hartman F C, Harpel M R. Annu Rev Biochem. 1994;63:197–234. doi: 10.1146/annurev.bi.63.070194.001213. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y-R, Hartmann F C. J Biol Chem. 1995;270:11741–11744. doi: 10.1074/jbc.270.20.11741. [DOI] [PubMed] [Google Scholar]
- 31.March J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. New York: Wiley; 1992. [Google Scholar]
- 32.Hubbard, B. K. & Walsh, C. T. (2002) Angew. Chem. Int. Ed., in press.