Abstract
The ex utero intrapartum treatment (EXIT) procedure is performed in cases of fetal congenital malformation. The anesthetic management is much more challenging and involves providing profound uterine relaxation, maintenance of Uteroplacental blood flow and fetal anesthesia. The aim of the article is to review the literature and compare the efficacy of both the anesthetic techniques with respect to maternal and fetal outcomes. The literature source for this review was obtained via PubMed, Medline, Google scholar and Cochrane database of systematic reviews until January 2017. In our literature review we found that both GA and Regional anesthesia were successfully described for EXIT procedure but GA was performed in the majority of cases. Consideration for anesthetic technique should be done on a case-by-case basis.
Keywords: Anesthesia, cesarean delivery, Ex-uterus intrapartum treatment, fetal malformation
Introduction
The ex-utero intrapartum treatment (EXIT) is a rare surgical procedure to manage fetal airway anomalies. It was first described in 1989 to manage a fetus with complete tracheal obstruction secondary to a prenatal diagnosed cervical teratoma.[1]
Since then, the indications for EXIT procedures have expanded to include a variety of fetal congenital abnormalities such as congenital high airway obstruction syndrome (CHAOS), laryngeal or tracheal atresia/stenosis, intrathoracic lesions (lung and mediastinal masses, pulmonary arteriovenous malformation with pulmonary hypoplasia), as well as congenital diaphragmatic hernias.[2,3,4]
It is a specialized surgical procedure that involves partial delivery of fetus during a cesarean section allowing the fetal airway to be secured while fetal oxygenation is maintained by placental perfusion. It has also been described as “operation on a placental support (OOPS).”[5,6]
Anesthetic management of the EXIT procedure is challenging and different from a standard cesarean section and involves providing profound uterine relaxation, maintenance of uteroplacental blood flow, and fetal anesthesia.[2,3,4,7,8,9]
Traditionally, general anesthesia (GA) has been the technique of choice for EXIT procedures due to the ease of titration of inhalational agents to achieve satisfactory uterine relaxation and to provide fetal anesthesia. In recent years, several reports have described neuraxial anesthesia use in combination with uterine relaxants to achieve satisfactory operating conditions for EXIT. The aim of this article is to review the literature and compare the efficacy of both anesthetic techniques with respect to maternal and fetal outcomes.
Material and Methods
We searched PubMed, Medline, Google scholar, and Cochrane Database of Systematic Reviews till January 2017 using the terms “Ex-utero intrapartum treatment” and “Anesthesia/anaesthesia.” The search was limited to the English language. The reference lists of retrieved articles were examined to identify further articles. Articles were reviewed by the authors independently and evaluated for eligibility. This review includes all reports describing anesthetic techniques as well as maternal and fetal outcomes.
Results
In the literature search [Figure 1], we found 224 articles, from which 167 were excluded due to duplication or because sufficient details were not available regarding anesthetic management. A total of 43 eligible articles of 224 patients were considered for analysis. Thirty-seven reports described the use of GA in 214 patients and six reports described regional anesthesia (RA) as the primary anesthetic technique in 10 patients. Primary anesthetic techniques are described in Table 1.
Figure 1.
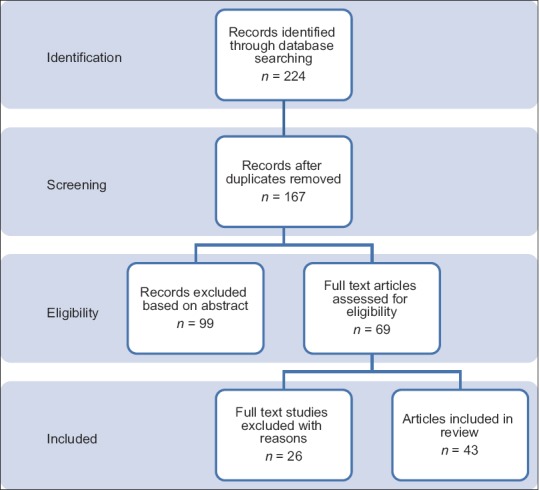
Methods and Results
Table 1.
Maternal Outcome
| Anesthesia technique | GA | Regional |
|---|---|---|
| Total number of patients | 214 | 10 |
| Mean placental bypass time | 14.37 min (n=117) | 7.6 min (n=10) |
| Mean estimated blood loss (ml) | 1128.34 (n=174) | 918 (n=8) |
| Estimated blood loss >1000ml | 18 (n=214) | 1 (n=10) |
| Blood transfusions | 17 (n=214) | 0 (n=10) |
| Use of multiple uterotonics | 23 (n=214) | 4 (n=10) |
| Invasive monitoring | 121 (n=214) | 1 (n=10) |
| Use of vasopressors (Ephedrine, phenylephrine, dopamine) | 139 (n=214) | 7 (n=10) |
| ICU transfer | 1 (n=214) | 0 (n=10) |
| Maternal deaths | 0 (n=214) | 0 (n=10) |
| Conversion of anesthetic techniques | 0 (n=214) | 0 (n=10) |
| Conversion of EXIT | 5 (n=214) | 0 (n=10) |
All parturients were monitored with standard monitors. Seventy-four parturients received arterial lines for continuous blood pressure monitoring in the GA group and one in the RA group. Fetal monitoring was achieved with SpO2 monitoring in most cases, whereas fetal ultrasonography was used by thirteen authors.
General anesthesia
All parturients receiving GA were given aspiration prophylaxis and placed in the left lateral decubitus prior to induction. Rapid sequence induction was performed for endotracheal intubation.
Thiopentone or Propofol was used as induction agents and in a majority of patients; succinylcholine was the muscle relaxant of choice except for the two Malignant hyperthermia susceptible patients.
GA was the main anesthetic technique in 37 of the 43 reports, involving a total of 214 patients. Epidural anesthesia was used for 72 patients for postoperative pain control[1,2,8,13,14] and one patient received intrathecal morphine. Thirty-five reports described inhalational anesthesia for maintenance in 212 patients with a target MAC of >2. Isoflurane, sevoflurane, and desflurane were used with good success. Desflurane was the most commonly used halogenated agent (141/212 patients) [Figure 2].
Figure 2.

Anesthesia technique distribution by percentage
Three authors described Total intravenous anesthesia (TIVA in 20 patients, 18 of which were combined TIVA and desflurane.[10,11,12] Remifentanil and propofol were the most common combination with only one patient receiving TIVA with propofol alone and one receiving a remifentanil and N2O combination; of these patients, two were MH susceptible.[11,12] Duration of placental support under GA ranged from 3 to 93 minutes.
Regional anesthesia
Gagnon et al.[13] published the initial case report using RA as the primary anesthetic technique in 1998. They successfully used epidural anesthesia with IV nitroglycerin (NTG) to achieve adequate surgical conditions. Subsequently, five other reports of RA have been published[14,15,16,17,18] involving 9 patients [Figure 2]. Eight patients received a combined spinal-epidural (CSE) anesthetic with bupivacaine in doses ranging from 10 to 12 mg and intrathecal fentanyl and epimorphine in doses of 15–25 mcg and 150–300 mcg, respectively. Benonis et al.[18] described the successful use of a subarachnoid catheter following a failed CSE in one patient with a history of failed spinal for the previous cesarean section. This patient achieved a T4 sensory level and adequate surgical anesthesia with a total bupivacaine of 11.25 mg, epimorphine 150 mcg, and fentanyl 10 mcg. All patients received IV NTG for uterine relaxation. Duration of placental support under RA ranged from 1 to 21 min. None of the patients with RA required a conversion to GA.
Uterine relaxation
NTG IV was the uterine relaxant of choice in all RA and TIVA[10,11,12,14,15,16,17,18,19] cases; it was required as an adjunct to inhalational agents in 25 cases.[1,5,8,20,22,23]
We found that NTG was most commonly used as a loading dose, ranging from 25–100 mcg followed by an infusion at a rate of 1–20 mcg/kg/min to keep the uterus adequately relaxed. Pharmacokinetic studies have demonstrated that placental transfer of NTG has no significant fetal hemodynamic effects. This is most likely due to the short half-life and rapid placental metabolism of the drug.[17,18]
In a majority of the patients, maternal hypotension was treated with intermittent boluses of phenylephrine and ephedrine as well as with judicious fluid management. Four patients in the RA group[15,16] required phenylephrine infusions of 50 mcg/min. Three patients in the GA group[20,21,22,23] required dopamine infusions to maintain adequate mean arterial pressures while receiving nitroglycerine (NG). Shih et al.[23] used an angiotensin II infusion ranging from 10–55 ng/kg/min to maintain a systolic BP >100 mmHg without concurrent use of NG.
Uterine tone
Oxytocin was the primary uterotonic in all cases, ranging from boluses of 5 U IV to infusions of 10 U–50/L [Figure 3].
Figure 3.

Distribution of uterotonic in general anesthesia and regional anesthesia groups
Carboprost 200–250 mcg IM was used in 9 patients in the GA group[1,20,21,24,25,26] (4.2%) and 3 patients (30%) in the RA group.[17]
Methylergonovine 200–250 mcg IM was used in 14 patients (6.5%) in the GA group[1,26,27,28] and 3 patients (30%) in the RA group.[15,16,17,18,19]
Two patients in the RA group[15] and one patient in the GA group[26] required oxytocin, carboprost, and methylergonovine concurrently.
None of the patients receiving more than one uterotonic received any blood transfusions.
Maternal outcome
Eight maternal postpartum hemorrhages (blood loss >2000ml) were reported in the GA group.[1,5,13,29,30,31,32,33] Of these, 5 required blood transfusion and 1 patient required ICU admission overnight.[33] Five EXIT procedures were converted to cesarean section secondary to significant bleeding and inadequate placental perfusion.[1,33] There were no postpartum hemorrhages in the RA group. There were no maternal deaths in either the RA or GA group [Table 1].
Fetal outcomes
The overall fetal/neonatal mortality rate was 10.31% (23/223). Five out of the 23 fetuses died intraoperatively[1,29,30,34] due to various causes which included failure to oxygenate and intubate, parents refusal for tracheostomy, and intraoperative bleeding. Eleven neonates who required complex surgery died due to pulmonary hypoplasia in the neonatal intensive care unit.[1,5,13,29,35,36] Five had complex congenital heart disease and died due to sever PHT and congestive heart failure.[1,5,29,30] One died because of postoperative bleeding and of unknown causes.[1,34]
There were no maternal or fetal complications related to anesthesia in either GA or RA groups [Table 2].
Table 2.
Fetal outcomes
| Anesthesia techniques | GA | Regional |
|---|---|---|
| Total number of patients | 214 | 10 |
| APGAR score at 1 and 5 min | 5.9 and 8.3 (n=8) | 3.9 and 7.2 (n=9) |
| Mean umbilical cord pH (arterial) | 7.21 (n=54) | 7.23 (n=3) |
| Neonatal death | 21 (n=214) | 2 |
| Need for supplemental anesthesia | 149 (n=214) | 1 |
Fetal monitoring and anesthesia
Fetuses were monitored using different techniques at various times during the surgical procedure. Sterile SpO2 probes were the most common fetal monitor described fetal sonography, and ECG were used additionally by some authors. Sixteen of the 42 authors used additional medications for infants, including atropine, analgesics, and NDMB. Fentanyl was the most commonly used narcotic.[1,5,7,8,16,25,26,29,31,34,37,38,39,40,41,42,43,44]
Discussion
Our literature review has shown that successful anesthetic management of the EXIT is possible with both GA and RA. However, there is a significant preponderance towards GA. The preference for GA for EXIT procedures can be explained by a number of reasons. A major goal of EXIT is profound uterine relaxation. Inhalational anesthetics provide reliable and titratable uterine relaxation at MAC >2 with relatively quick reversibility. Neuraxial techniques require additional IV NTG use for uterine relaxation that can lead to problematic hypotension and uterine hypoperfusion. We found increased use of vasopressors in the RA group as evidenced by the use of phenylephrine infusions in 50% of the patients receiving RA. We found few descriptions of vasopressor infusions in the GA group.
Fetal anesthesia is an important component of the anesthetic goals for EXIT and can usually be achieved by transplacental transfer of inhalational anesthetics. Remifentanil has been used successfully to achieve fetal anesthesia and should be considered in parturients receiving RA. A retrospective review of medical records by Boat et al.[10] comparing maintenance with desflurane alone to desflurane and TIVA with propofol and remifentanil in women having both open fetal procedures or EXIT showed a reduction in acute fetal cardiac dysfunction in the desflurane plus TIVA group as well as an overall decrease in fetal interventions. There are no other studies comparing TIVA and inhalational agents.
Remifentanil rapidly crosses the placenta providing excellent fetal immobilization and is rapidly metabolized by fetal nonspecific blood and tissue esterase. It also acts as a good anxiolytic for an awake parturient, and is easily titratable to avoid excessive sedation in the presence of a high risk of aspiration.
Although most procedures described lasted between 5 and 25 min with relatively uncomplicated airway manipulations, either direct laryngoscopy or tracheostomy, there were some procedures that lasted over 50 min. Calculation of the mean duration of EXIT was difficult because some case reports lacked an explicit time of placental bypass. Due to this potentially unpredictable length of time required for placental support coupled with the emotional stress of witnessing airway manipulations on the baby, it may be more difficult for an awake parturient to tolerate the procedure.
Postpartum atony and hemorrhage (PPH) is an important intraoperative complication. There were eight PPH cases reported in our review, and of these five patients who received blood transfusions all were in the GA group. However, given the large difference in the number of GA vs RA patients, it is hard to draw any definitive conclusion regarding the effect of anesthetic technique on the incidence of PPH in EXIT. It is also interesting to note that the percentage of patients receiving more than one uterotonic was much higher in the RA group, which may have a direct impact on PPH incidence.
Unfortunately, APGAR scores were not available for infants, especially as some received NDMB and narcotics IM. Hence, it was impossible to use this method to assess fetal outcomes. Similarly, cord pH was not available for all patients and could not be used reliably.
Other potential shortfalls of our review include the disparity between the level of detail provided by each author regarding drugs used, including inhalational agents, vasopressors, uterotonics, and blood loss, making it more difficult to draw definitive conclusions.
Conclusion
In summary, we found in our literature review that both GA and RA were successfully described for EXIT procedures, but GA was performed in a majority of cases. The goals of uterine relaxation, maintenance of placental perfusion, and fetal anesthesia must always be considered when choosing the anesthetic technique. Consideration for anesthetic technique should be done on a case-by-case basis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lin EE, Moldenhauer JS, Tran KM, Cohen DE, Adzick NS. Anesthetic management of 65 cases of ex utero intrapartum therapy: A 13-year single-center experience. Anesth Analg. 2016;123:411–7. doi: 10.1213/ANE.0000000000001385. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira E, Pereira P, Retroz C, Mártires E. Anesthesia for EXIT procedure in congenital cervical malformation--a challenge to the anesthesiologist. Braz J Anesthesiol. 2015;65:529–33. doi: 10.1016/j.bjane.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Helfer DC, Clivatti J, Yamashita AM, Moron AF. Anesthesia for ex utero intrapartum treatment (EXIT procedure) in fetus with prenatal diagnosis of oral and cervical malformations: Case reports. Rev Bras Anestesiol. 2012;62:411–23. doi: 10.1016/S0034-7094(12)70141-1. [DOI] [PubMed] [Google Scholar]
- 4.Norris MC, Joseph J, Leighton BL. Anesthesia for perinatal surgery. Am J Perinatol. 1989;6:39–40. doi: 10.1055/s-2007-999541. [DOI] [PubMed] [Google Scholar]
- 5.Mychaliska GB, Bealer JF, Graf JL, Rosen MA, Adzick NS, Harrison MR. Operating on placental support: The ex uterointrapartum treatment procedure. J Pediatr Surg. 1997;32:227–31. doi: 10.1016/s0022-3468(97)90184-6. [DOI] [PubMed] [Google Scholar]
- 6.Skarsgard ED, Chitkara U, Krane EJ, Riley ET, Halamek LP, Dedo HH. The OOPS procedure (operation on placental support): In utero airway management of the fetus with prenatally diagnosed tracheal obstruction. J Pediatr Surg. 1996;31:826–8. doi: 10.1016/s0022-3468(96)90144-x. [DOI] [PubMed] [Google Scholar]
- 7.Luo D, Wu L, Wu H, Huang W, Huang H. Anesthetic management of a neonate receiving prenatal repair of gastroschisis. Int J Clin Exp Med. 2015;8:8234–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Braden A, Maani C, Nagy C. Anesthetic management of ex utero intrapartum treatment procedure: A novel approach. J Clin Anesth. 2016;31:60–3. doi: 10.1016/j.jclinane.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Marques MV, Carneiro J, Adriano M, Lança F. Anesthesia for ex utero intrapartum treatment: Renewed insight on a rare procedure. Rev Bras Anestesiol. 2015;65:525–8. doi: 10.1016/j.bjan.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Boat A, Mahmoud M, Michelfelder EC, Lin E, Ngamprasertwong P, Schnell B, et al. Supplementing desflurane with intravenous anesthesia reduces fetal cardiac dysfunction during open fetal surgery. Paediatr Anaesth. 2010;20:748–56. doi: 10.1111/j.1460-9592.2010.03350.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosen MA, Andreae MH, Cameron AG. Nitroglycerin for fetal surgery: Fetoscopy and ex utero intrapartum treatment procedure with malignant hyperthermia precautions. Anesth Analg. 2003;96:698–700. doi: 10.1213/01.ANE.0000049686.20464.3B. [DOI] [PubMed] [Google Scholar]
- 12.Hofer IS, Mahoney B, Rebarber A, Beilin Y. An ex utero intrapartum treatment procedure in a patient with a family history of malignant hyperthermia. Int J Obstet Anesth. 2013;22:146–8. doi: 10.1016/j.ijoa.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Laje P, Johnson MP, Howell LJ, Bebbington MW, Hedrick HL, Flake AW, et al. Ex utero intrapartum treatment in the management of giant cervical teratomas. J Pediatr Surg. 2012;47:1208–16. doi: 10.1016/j.jpedsurg.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Osborn AJ, Baud D, Macarthur AJ, Propst EJ, Forte V, Blaser SM, et al. Multidisciplinary perinatal management of the compromised airway on placental support: Lessons learned. Prenat Diagn. 2013;33:1080–7. doi: 10.1002/pd.4200. [DOI] [PubMed] [Google Scholar]
- 15.Gagnon AL, Bebbington MW, Kamani A, Solimano A. Prenatally diagnosed fetal neck teratoma: Case report and novel management options. Fetal Diagn Ther. 1998;13:266–70. doi: 10.1159/000020852. [DOI] [PubMed] [Google Scholar]
- 16.Clark KD, Viscomi CM, Lowell J, Chien EK. Nitroglycerin for relaxation to establish a fetal airway (EXIT procedure) Obstet Gynecol. 2004;103:1113–5. doi: 10.1097/01.AOG.0000125158.61232.b3. [DOI] [PubMed] [Google Scholar]
- 17.Fink RJ, Allen TK, Habib AS. Remifentanil for fetal immobilization and analgesia during the ex utero intrapartum treatment procedure under combined spinal-epidural anaesthesia. Br J Anaesth. 2011;106:851–5. doi: 10.1093/bja/aer097. [DOI] [PubMed] [Google Scholar]
- 18.Benonis JG, Habib AS. Ex utero intrapartum treatment procedure in a patient with arthrogryposis multiplex congenita, using continuous spinal anesthesia and intravenous nitroglycerin for uterine relaxation. Int J Obstet Anesth. 2008;17:53–6. doi: 10.1016/j.ijoa.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 19.George RB, Melnick AH, Rose EC, Habib AS. Case series: Combined spinal epidural anesthesia for cesarean delivery and ex utero intrapartum treatment procedure. Can J Anaesth. 2007;54:218–22. doi: 10.1007/BF03022643. [DOI] [PubMed] [Google Scholar]
- 20.Govindarajan A, Atkinson S. Combined spinal epidural anaesthesia for ex utero intrapartum treatment (EXIT) procedure. Anaesthesia, 2009;64:793–813. [Google Scholar]
- 21.Atalay C, Aykan S, Bayram E, Dogan N. Natal and postnatal approach in a fetus with intrauterine cystic hygroma: Case report. Turk J Med Sci. 2008;38:609–12. [Google Scholar]
- 22.Chang LC, Kuczkowski KM. The ex utero intrapartum treatment procedure: Anesthetic considerations. Arch Gynecol Obstet. 2008;277:83–5. doi: 10.1007/s00404-007-0402-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Ryu JW, Kim DY, Lee GY. Anesthetic management of the ex utero intrapartum treatment (EXIT) procedure - A case report. Korean J Anesthesiol. 2010;59(Suppl):S154–7. doi: 10.4097/kjae.2010.59.S.S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott R, Vallera C, Heitmiller ES, Isaac G, Lee M, Crino J, et al. Ex utero intrapartum treatment procedure for management of congenital high airway obstruction syndrome in a vertex/breech twin gestation. Int J Pediatr Otorhinolaryngol. 2013;77:439–42. doi: 10.1016/j.ijporl.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Shih GH, Boyd GL, Vincent RD, Jr, Long GW, Hauth JC, Georgeson KE. The EXIT procedure facilitates delivery of an infant with a pretracheal teratoma. Anesthesiology. 1998;89:1573–5. doi: 10.1097/00000542-199812000-00039. [DOI] [PubMed] [Google Scholar]
- 26.Dahlgren G, Tornberg DC, Pregner K, Irestedt L. Four cases of the ex utero intrapartum treatment (EXIT) procedure: Anesthetic implications. Int J Obstet Anesth. 2004;13:178–82. doi: 10.1016/j.ijoa.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Stevens GH, Schoot BC, Smets MJ, Kremer B, Manni JJ, Gavilanes AW, et al. The ex utero intrapartum treatment (EXIT) procedure in fetal neck masses: A case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2002;100:246–50. doi: 10.1016/s0301-2115(01)00467-5. [DOI] [PubMed] [Google Scholar]
- 28.De Assuncao Braga ADF, Frias JAF, Da Silva Braga FS, Rousselet MS, Barini R, Sbragia L, et al. Anesthesia for ex utero intrapartum treatment of fetus with prenatal diagnosis of cervical hygroma. Case report. Rev Bras Anestesiol. 2006;56:278–86. doi: 10.1590/s0034-70942006000300008. [DOI] [PubMed] [Google Scholar]
- 29.Bilgin F, Cekmen N, Ugur Y, Kurt E, Gungor S, Atabek C. Congenital Cervical Teratoma: Anaesthetic Management (The EXIT Procedure) Indian J Anaesth. 2009;53:678–82. [PMC free article] [PubMed] [Google Scholar]
- 30.Liechty KW, Crombleholme TM, Flake AW, Morgan MA, Kurth CD, Hubbard AM, et al. Intrapartum airway management for giant fetal neck masses: The EXIT (ex utero intrapartum treatment) procedure. Am J Obstet Gynecol. 1997;177:870–4. doi: 10.1016/s0002-9378(97)70285-0. [DOI] [PubMed] [Google Scholar]
- 31.Bouchard S, Johnson MP, Flake AW. The EXIT procedure: experience and outcome in 31 cases. J Pediatr Surg. 2002;37(3):418–26. doi: 10.1053/jpsu.2002.30839. [DOI] [PubMed] [Google Scholar]
- 32.Butwick A, Aleshi P, Yamout I. Obstetric hemorrhage during an EXIT procedure for severe fetal airway obstruction. Can J Anaesth. 2009;56:437–42. doi: 10.1007/s12630-009-9092-z. [DOI] [PubMed] [Google Scholar]
- 33.Pascoli I, Gritti A, Cutrone C, Presotto S, Bendini M, Bordignon L, et al. EXIT (Ex Utero Intrapartum Treatment) technique - Management of a giant fetal lymphangioma. J Matern Fetal Neonatal Med. 2010;23:190–2. doi: 10.3109/14767050903121423. [DOI] [PubMed] [Google Scholar]
- 34.Lazar DA, Olutoye OO, Moise KJ, Jr, Ivey RT, Johnson A, Ayres N, et al. Ex-utero intrapartum treatment procedure for giant neck masses--fetal and maternal outcomes. J Pediatr Surg. 2011;46:817–22. doi: 10.1016/j.jpedsurg.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Gaiser RR, Cheek TG, Kurth CD. Anesthetic management of cesarean delivery complicated by ex utero intrapartum treatment of the fetus. Anesth Analg. 1997;84:1150–3. doi: 10.1097/00000539-199705000-00039. [DOI] [PubMed] [Google Scholar]
- 36.Ioscovich A, Shen O, Sichel JY, Lajos Y, Orkin D, Bromiker R, et al. Remifentanil-nitroglycerin combination as an anesthetic support for ex utero intrapartum treatment (EXIT) procedure. J Clin Anesth. 2011;23:142–4. doi: 10.1016/j.jclinane.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Kern C, Ange M, Morales, Peiry B, Pfister RE. Ex utero intrapartum treatment (EXIT), a resuscitation option for intra-thoracic foetal pathologies. Swiss Med Wkly. 2007;137:279–85. doi: 10.4414/smw.2007.11526. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz DA, Moriarty KP, Tashjian DB, Wool RS, Parker RK, Markenson GR, et al. Anesthetic management of the exit procedure. J Clin Anesth. 2001;13:387–91. doi: 10.1016/s0952-8180(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 39.Baker PA, Aftimos S, Anderson BJ. Airway management during an EXIT procedure for a fetus with dysgnathia complex. Paediatr Anaesth. 2004;14:781–6. doi: 10.1111/j.1460-9592.2004.01284.x. [DOI] [PubMed] [Google Scholar]
- 40.Choleva AJ. Anesthetic management of a patient undergoing an ex utero intrapartum treatment (EXIT) procedure: A case report. AANA J. 2011;79:497–503. [PubMed] [Google Scholar]
- 41.Suenaga M, Hidaka N, Kido S, Otera Y, Fukushima K, Kato K. Successful ex utero intrapartum treatment procedure for prenatally diagnosed severe micrognathia: A case report. J Obstet Gynaecol Res. 2014;40:2005–9. doi: 10.1111/jog.12423. [DOI] [PubMed] [Google Scholar]
- 42.Udayakumar P, Arunachalam P, Vijayakumar V, Kandappan G. Ex-utero intrapartum treatment in the Indian scenario: Anesthetic challenges and positioning. J Indian Assoc Pediatr Surg. 2014;19:106–8. doi: 10.4103/0971-9261.129608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavares J, Faria A, Fonseca C, Sampaio C, Abreu F. Ex utero intrapartum procedure for delivery of a fetus with a large cervical mass. Eur J Anaesthesiol. 2005;22:642–3. doi: 10.1017/s0265021505251062. [DOI] [PubMed] [Google Scholar]
- 44.Eschertzhuber S, Keller C, Mitterschiffthaler G, Jochberger S, Kuhbacher G. Verifying correct endotracheal intubation by measurement of end-tidal carbon dioxide during an ex utero intrapartum treatment procedure. Anesth Analg. 2005;101:658–60. doi: 10.1213/01.ANE.0000175206.91231.77. [DOI] [PubMed] [Google Scholar]


