Abstract
Background and Aims:
Although no pain control following hip and knee replacement surgeries has attained gold standard, it is clear that patients should have optimum pain control after total knee arthroplasty and total hip arthroplasty for enhanced satisfaction and function. We conducted this study to evaluate the effect of preoperative application of buprenorphine transdermal patch on analgesic requirement in perioperative period after knee and hip replacement surgeries
Material and Methods:
Following institutional ethical committee approval and written informed consent, a prospective study was conducted in 50 patients of either gender belonging to ASA1 or ASA2 status, requiring either knee or hip replacement. The patients were assessed in preoperative period, and buprenorphine patch of 10 mg (sustained release of 10 μg/h) was applied either on the chest or on outer side of the arm 12 h before surgery. Total knee arthroplasty/total hip arthroplasty was done under combined spinal epidural blockade. Epidural infusion with 0.125% bupivacaine at a rate of 4–5 mL/h was continued in postoperative period. Intravenous opioid analgesics were avoided in postoperative period, and whenever required only iv paracetamol 1g was given. Outcome in terms of requirement of iv analgesic, visual analog pain score, any associated nausea vomiting, itching, and level of somnolence was noted in postoperative period at 1,2,3,4,8,12,16,20,24,48, and 72 h, respectively.
Results:
None of the patient required rescue analgesia in the first 2 h. During 72 h postoperative period of observation 32% of patients demanded rescue analgesics at 8th hour, followed by 20% at 4th hour and 16% at 12th hour.
Conclusion:
Preoperative application of transdermal patch significantly reduces the requirement of postoperative intravenous opioid and nonopioid analgesic drugs.
Keywords: Buprenorphine, hip replacement, knee replacement, transdermal patch
Introduction
Buprenorphine is a highly lipophilic, semi-synthetic derivative of thebaine, a morphine alkaloid.[1] The molecular structure of buprenorphine confers suitability for use in a variety of preparations, including the transdermal route. Transdermal drug delivery systems (TDDSs) are non-invasive, simple, compliant, and sustained methods of delivery. For usage as transdermal patch, buprenorphine is incorporated into an adhesive polymer matrix (acrylate vinyl acetate), from which it is continuously released into the systemic circulation over a period of 7 days.[2] Buprenorphine transdermal patches are available in three strengths 5, 10, and 20 mg with rate of drug release of 5, 10, and 20 μg/h, respectively.
No literature is available about the use of transdermal buprenorphine patch for postoperative analgesia after total knee arthroplasty and total hip arthroplasty. But literature supports use of transdermal buprenorphine patch for postoperative analgesia after abdominal surgical procedure.[3] We conducted this study to evaluate the effect of preoperative application of buprenorphine transdermal patch on analgesic requirement in perioperative period after knee and hip replacement surgeries.
Material and Methods
Following institutional ethical committee approval and written informed consent, a prospective study was conducted in 50 patients of either gender belonging to ASA1 or ASA2 status, requiring either knee or hip replacement. Out of total 50 patients, 26 underwent total knee replacement (TKR) for primary osteoarthritis knee and 24 underwent total hip replacement (THR) for secondary osteoarthritis hip. Patients with skin allergies, refusal to consent, and severe hepatic dysfunction were excluded from the study. The patients included in study were assessed in preoperative period, and buprenorphine patch of 10 mg (sustained release of 10 μg/h) was applied either on chest or on outer side of arm 12 h before surgery. The transdermal buprenorphine patch should be applied to intact skin on the flat surfaces of the upper outer arm, upper chest, upper back, or the side of the chest. The skin should be dry, clean, non-irritated, non-hairy (hair should be trimmed with scissors, not shaved), and without large scars. The transdermal patch was applied 12 h preoperatively, as plasma concentration of buprenorphine increases only slowly within 12–24 h, to reach the analgesic threshold level (100 pg/mL). Total knee arthroplasty/total hip arthroplasty was performed under combined spinal epidural blockade. Epidural infusion with 0.125% bupivacaine at the rate of 4–5 mL/h with elastometric infusion pump was continued in postoperative period. Intravenous opioid analgesics and opioid even as additive to epidural infusion were avoided in postoperative period. Rescue analgesia was given with only iv paracetamol 1g whenever required according to patient's request irrespective of the Visual Analog Scale (VAS) score [Figure 1]. Outcome in terms of requirement of iv analgesic, visual analog pain score, any associated nausea vomiting, itching, and level of somnolence was noted in postoperative period at 1,2,3,4,8,12,16,20,24,48, and 72 h, respectively. Nausea vomiting and pruritis were noted in terms of yes or no. Postoperative pain was noted as per the visual analog score.
Figure 1.
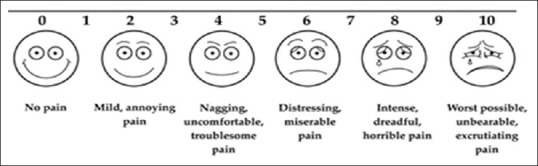
Visual analog pain score
Somnolence was categorized as follows:
Fully awake
Somnolent and responds to call
Somnolent and no response to verbal stimulation
Asleep and responds to only painful stimulation.[4]
Statistical analysis
Data collected were subjected to statistical analysis. Data analysis was carried out using IBM SPSS ver. 21 (International buisness machines corporation, IBM India Private Limited, Bangalore, Karnataka, India) installed on Windows 7 operating system. MSExcel 2007 and MSWord 2007 software were also used in this study. We used Chi-Square test to test the significance of the independence of attributes. The significance of a test is validated at 5% level of significance and power of 80% having the effect size of 0.5, the desired samples size is 38. However, to improve the efficacy of the test and keeping other factors in view, we collected the sample size of 50. P Value <0.05 was considered significant.
Results
The study was carried out on 50 patients; 54% of the patients were males and 46% were females [Table 1]. The average age of the patients was 55 years with a standard deviation of 14.82 [Table 2]. The average weight of the patients was 72.22 kg with a standard deviation of 11.36 [Table 3]. Of the total 50 patients, 52% underwent TKR for primary osteoarthritis knee and 48% underwent THR for secondary osteoarthritis hip [Table 1]. For a total of 50 patients, 550 VAS responses (11 observations for each patient) were collected at various time intervals as mentioned above over a period of 72h postoperatively [Table 4]. The majority of the responses were mild, annoying pain (26.7%), followed by nagging, uncomfortable, troublesome pain (22.7%), and no pain (22.0%). Not even a single patient complained of worst possible, unbearable, and excruciating pain. The overall trend showed that there was gradual increase in pain among all the 50 patients from the first hour (no pain) to fourth hour (nagging, uncomfortable, troublesome pain) and gradually decreased to mild, annoying pain by 72 h.
Table 1.
Demographic data
| No. of patients | % of patients | |
|---|---|---|
| Gender of the patients | ||
| Female | 23 | 46.0 |
| Male | 27 | 54.0 |
| Diagnosis of the patients | ||
| Primary osteoarthritis knee | 26 | 52.0 |
| Secondary osteoarthritis hip | 24 | 48.0 |
| Type of surgery | ||
| Total hip replacement | 24 | 48.0 |
| Total knee replacement | 26 | 52.0 |
Table 2.
Descriptive statistics of age of patients
| No. of patients | Minimum | Maximum | Mean | Std. deviation | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| 50 | 21 | 75 | 55.00 | 14.827 | −0.926 | 0.090 |
Table 3.
Descriptive statistics of weight of patients
| No. of patients | Minimum | Maximum | Mean | Std. deviation | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| 50 | 53 | 92 | 72.22 | 11.316 | 0.119 | −1.197 |
Table 4.
Distribution of patients’ response to VAS
| No. of patients/time | VAS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total | |
| 1 h | 21 | 4 | 18 | 1 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 50 |
| 2 h | 16 | 4 | 9 | 3 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 50 |
| 3 h | 12 | 4 | 10 | 4 | 12 | 1 | 7 | 0 | 0 | 0 | 0 | 50 |
| 4 h | 9 | 3 | 11 | 3 | 10 | 1 | 8 | 1 | 4 | 0 | 0 | 50 |
| 8 h | 4 | 5 | 10 | 4 | 16 | 2 | 5 | 0 | 4 | 0 | 0 | 50 |
| 12 h | 5 | 5 | 17 | 2 | 13 | 2 | 5 | 0 | 1 | 0 | 0 | 50 |
| 16 h | 8 | 3 | 13 | 3 | 14 | 1 | 8 | 0 | 0 | 0 | 0 | 50 |
| 20 h | 7 | 6 | 15 | 1 | 15 | 0 | 6 | 0 | 0 | 0 | 0 | 50 |
| 24 h | 11 | 7 | 9 | 4 | 9 | 0 | 10 | 0 | 0 | 0 | 0 | 50 |
| 48 h | 11 | 9 | 18 | 2 | 5 | 0 | 5 | 0 | 0 | 0 | 0 | 50 |
| 72 h | 17 | 9 | 17 | 1 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 50 |
| Total no. of observations | 121 | 59 | 147 | 28 | 122 | 7 | 56 | 1 | 9 | 0 | 0 | 550 |
VAS, Visual analog score
None of the patients complained of pruritis at any point of time during the observation period of 72 h postoperatively [Table 5]. Of the total 550 observations for 50 patients during an observation period of 72h, only 6 were noted as yes for nausea and vomiting [Table 6].
Table 5.
Observation for pruritis
| Time of observation | Pruritis (yes/no) | |
|---|---|---|
| No. | Total | |
| 1 h | 50 | 50 |
| 2 h | 50 | 50 |
| 3 h | 50 | 50 |
| 4 h | 50 | 50 |
| 8 h | 50 | 50 |
| 12 h | 50 | 50 |
| 16 h | 50 | 50 |
| 20 h | 50 | 50 |
| 24 h | 50 | 50 |
| 48 h | 50 | 50 |
| 72 h | 50 | 50 |
| Total no. of observations | 550 | 550 |
Table 6.
Observation for Nausea and Vomiting
| Time of observation | Nausea and vomiting (yes/no) | ||
|---|---|---|---|
| No | Yes | Total | |
| 1 h | 49 | 1 | 50 |
| 2 h | 50 | 0 | 50 |
| 3 h | 50 | 0 | 50 |
| 4 h | 50 | 0 | 50 |
| 8 h | 48 | 2 | 50 |
| 12 h | 49 | 1 | 50 |
| 16 h | 49 | 1 | 50 |
| 20 h | 50 | 0 | 50 |
| 24 h | 50 | 0 | 50 |
| 48 h | 49 | 1 | 50 |
| 72 h | 50 | 0 | 50 |
| Total no. of observations | 544 | 6 | 550 |
Of the total 550 observations for somnolence [Table 7] in 50 patients during period of observation of 72h, it was observed that 74% were fully awake irrespective of time except during the night time, followed by somnolent and responded to call (25.3%) and somnolent and no response to verbal stimulation (0.7%). It was observed that none of the patient required rescue analgesia in the first 2 h. During 72 h period of observation postoperatively, the maximum demand for rescue analgesia by patients (32%) was observed at 8th hour followed by at 4th hour (20%) and 12th hour (16%) subsequently [Table 8].
Table 7.
Somnolence categories
| No. of patients/time | ||||
|---|---|---|---|---|
| Fully awake | Somnolent and responds to call | Somnolent and no response to verbal stimulation | Total | |
| 1 h | 35 | 14 | 1 | 50 |
| 2 h | 34 | 16 | 0 | 50 |
| 3 h | 39 | 11 | 0 | 50 |
| 4 h | 42 | 8 | 0 | 50 |
| 8 h | 43 | 6 | 1 | 50 |
| 12 h | 30 | 20 | 0 | 50 |
| 16 h | 34 | 15 | 1 | 50 |
| 20 h | 41 | 9 | 0 | 50 |
| 24 h | 41 | 8 | 1 | 50 |
| 48 h | 34 | 16 | 0 | 50 |
| 72 h | 34 | 16 | 0 | 50 |
| Total no. of observations | 407 | 139 | 4 | 550 |
| % of responses | 74% | 24.3% | 0.7% | 100 |
Table 8.
Rescue analgesia/VAS
| No. of patients/time | VAS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 8 | 9 | 10 | Total | |
| @1 h | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 |
| @2 h | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 |
| @3 h | 46 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 50 |
| @4 h | 40 | 0 | 0 | 0 | 3 | 1 | 4 | 2 | 0 | 0 | 50 |
| @8 h | 34 | 1 | 3 | 1 | 2 | 2 | 4 | 3 | 0 | 0 | 50 |
| @12 h | 42 | 0 | 1 | 0 | 5 | 0 | 1 | 1 | 0 | 0 | 50 |
| @16 h | 48 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 50 |
| @20 h | 44 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 50 |
| @24 h | 41 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 50 |
| @ 48 h | 44 | 0 | 1 | 0 | 2 | 0 | 3 | 0 | 0 | 0 | 50 |
| @72 h | 47 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 50 |
| Total no. of patients | 486 | 2 | 8 | 1 | 13 | 4 | 29 | 7 | 0 | 0 | 550 |
VAS, Visual analog score
Discussion
Inadequate postoperative pain control may cause delay in recovery, prolonged hospital stay, delay in start of physiotherapy, and may even adversely affect the outcome of surgery.[5] TDDS is a easy to use, non-invasive, safe, and reliable method of drug delivery. It allows continuous drug delivery and maintains sustained plasma levels of the drug. It also decreases the requirement of rescue analgesics by providing sustained pain relief and reducing the incidence of breakthrough pain. Due to slow and sustained release of drug, sudden increases in plasma drug levels are avoided, reducing the incidence of adverse effects associated with drugs. Buprenorphine patches have been used effectively for chronic low back pain and for pain associated with osteoarthritis.[6,7,8,9]
In this study, we determined the effect of preoperative application of buprenorphine transdermal patch on analgesic requirement in postoperative period in hip and knee replacement surgeries. The use of continuous epidural analgesia during post-operative period may preclude early mobilization due to motor effects of local anesthetic drug. Use of transdermal buprinorphine patch in addition to continuous epidural infusion can even promote early tapering of epidural analgesia and reduce the requirement of opioid or non-opioid analgesics during mobilization and physiotherapy. During postoperative period after 8 h, patients were instructed to do toe movements and active quadriceps exercises. After 24 h, patients were shifted from postoperative recovery unit to their rooms or wards, and straight leg raising and gluteal muscle exercises were started forTKR and THR patients, respectively, thereafter. At 48 h, drain was removed and check X-ray was done in radiology department. After 72 h, physiotherapy was started for TKR and THR.
We could manage postoperative analgesia in our study cases with preoperative application of buprenorphine transdermal patch of 10 mg (sustained release of 10 μg/h) and postoperative continuous epidural infusion of 0.125% bupivacaine at a rate of 4–5 mL/h without fentanyl or any other additives. Only inj. paracetamol 1g iv was given whenever required according to demand from patient irrespective of VAS score at that time. As the pain is a subjective feeling and tolerance to pain varies from patient to patient, we did not consider VAS score as criteria for providing rescue analgesia. Some patients required analgesic supplementation with even lower VAS score. We completely avoided postoperative use of opioid or any other analgesics through any route except iv paracetamol. Maximum demand (32%) of rescue analgesia in postoperative period among our study cases was observed at 8th hour, which corresponds to the time when patients are instructed to do toe movements and starting of active quadriceps exercises. Rescue analgesia demand by 20% patients at 4th hour may correspond to segment regression of spinal anaesthesia. Although it is drawback of our study that we did not monitor segment regression of spinal anaesthesia in postoperative period. No literature is available about use of transdermal buprenorphine patch in patients undergoing joint replacement surgery. Vittorio Emenuele et al. studied the efficacy and safety of preoperative application of transdermal buprinorphine patch for postoperative pain management of elderly patients with proximal femoral fractures.[10] They also found it effective with high patient satisfaction score. In another study by Conaghan et al., transdermal buprenorphine patch and paracetamol were compared with codeine and paracetamol in elderly patients with osteoarthritis of the hip or knee joints and there was significantly less requirement of escape medication in buprenorphine patch and paracetamol group when compared with other group.[9]
Conclusion
Preoperative application of transdermal buprenorphine patch (10 mg) is effective for postoperative analgesia after hip and knee replacement surgeries. It reduces the requirement of postoperative rescue analgesics too. Further studies with greater sample size are required to prove its efficacy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Budd K. Buprenorphine. In: Bullingham RES, editor. Clinics in anaesthesiology. London: WB Saunders Company Ltd; 1983. pp. 147–52. [Google Scholar]
- 2.Evans HC, Easthope SE. Transdermal buprenorphine. Drugs. 2003;63:1999–2010. doi: 10.2165/00003495-200363190-00003. [DOI] [PubMed] [Google Scholar]
- 3.Arshad Z, Prakash R, Gautam S, Kumar S. Comparison between transdermal buprenorphine and transdermal fentanyl for postoperative pain relief after major abdominal surgeries. J Clin Diagn Res. 2015;9:UC01–4. doi: 10.7860/JCDR/2015/16327.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav M, Kumar P B, Singh M, Gopinath R. Intrathecal magnesium sulfate as a spinal adjuvant in two different doses, combined with 0.5% heavy bupivacaine for infraumbilical surgeries. Anesth Essays Res. 2015;9:364–8. doi: 10.4103/0259-1162.159764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Likar R. Transdermal buprenorphine in the management of persistent pain – Safety aspects. Ther Clin Risk Manag. 2006;2:115–25. [PMC free article] [PubMed] [Google Scholar]
- 6.Landau CJ, Carr WD, Razzetti AJ, Sessler NE, Munera C, Ripa SR. Buprenorphine transdermal delivery system in adults with persistent noncancer-related pain syndromes who require opioid therapy. Clin Ther. 2007;29:2179–93. doi: 10.1016/j.clinthera.2007.10.010. Erratum in Clin Ther 2009;31:677. [DOI] [PubMed] [Google Scholar]
- 7.Gordon A, Rashiq S, Moulin DE, Clark AJ, Beaulieu AD, Eisenhoffer J. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15:169–78. doi: 10.1155/2010/216725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munera C, Drehobl M, Sessler NE, Landau C. A randomized, placebo-controlled, double-blinded, parallel-group, 5-week study of buprenorphine transdermal system in adults with osteoarthritis. J Opioid Manag. 2010;6:193–202. doi: 10.5055/jom.2010.0017. [DOI] [PubMed] [Google Scholar]
- 9.Conaghan PG, O’Brien CM, Wilson M, Schofield JP. Transdermal buprenorphine plus oral paracetamol vs an oral codeine-paracetamol combination for osteoarthritis of hip and/or knee: A randomised trial. Osteoarthritis Cartilage. 2011;19:930–8. doi: 10.1016/j.joca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Privitera C, Guzzetta G. Transdermal (TDS) buprenorphine patches for postoperative pain management in orthopaedic surgery in the elderly. Reg Anesth Pain Med. 2008;33:e187. [Google Scholar]


