Abstract
Background and Aims:
Optimal pain control can be a challenge in cirrhotic patients. The aim was to compare the analgesic efficacy and side effects of intravenous fentanyl patient-controlled analgesia (PCA) with and without bupivacaine boluses in transversus abdominis plane (TAP) and rectus sheath space (RSB) in cirrhotics undergoing liver surgery.
Material and Methods:
A double-blinded randomized controlled trial (n = 55, child's A) was conducted. Catheters were inserted surgically in TAP and rectal sheath space during surgical closure. Fentanyl PCA + TAP + RSB group (gp) (n = 30): (0.2 ml/kg of 0.25% bupivacaine, 8 hourly) was compared with fentanyl PCA gp (n = 25): [0.2 ml/kg of saline (placebo) injected in catheters 8 hourly] for 48 h postoperatively. Plasma bupivacaine was measured with an enzyme-linked immunosorbent assay at 10 min, 30 min, 1 h, 2 h, and 4 h after each injection and 30 min before next injection.
Results:
Fentanyl consumption was reduced in (PCA + TAP + RSB) gp compared to PCA gp (Day 1: 325.4 ± 169.1 vs. 1034 ± 231.7, Day 2: 204.44 ± 62.9 vs. 481.6 ± 158.3 μg, P < 0.05). Both groups demonstrated effective pain control at rest [Visual Analog Scales (VAS) <3), but on movement pain control with bupivacaine was better (P < 0.05). Increased demand for rescue opioids was observed prior to next scheduled bupivacaine injection in 10/30 patients on Day 1 and 2/30 on Day 2, in association with a reduced bupivacaine serum levels compared to 10 min after injection (47.6 ± 22.7 vs. 93.6 ± 61.0 ng/ml, respectively, P < 0.05). Bupivacaine did not exceed referred toxic levels.
Conclusion:
Repeated bupivacaine TAP and RSB with PCA fentanyl improved pain control, reduced opioids demand with no toxicity. Time interval between injections needs to be reduced to avoid breakthrough pain.
Keywords: Cirrhotic, liver resection, postoperative pain, rectal sheath block, transversus abdominis block
Introduction
In Egypt, the main indication for hepatic resection is malignancy as a consequence of hepatitis C (genotype 4).[1] An increase in bioavailability of opioids is expected particularly with the temporary reduction in function or volume of the liver after resection.[2] Epidural analgesia in view of expected coagulopathy can increase risk of epidural hematoma.[3,4,5]
Recently, Melloul et al. published guidelines to enhanced recovery after liver surgery and concluded that no evidence exists to prefer epidural, and wound infiltration and intrathecal opioids are good alternatives when combined with multimodal analgesia.[6] Transversus abdominis plane (TAP) block can help provide analgesia,[7,8] but the safety of injecting local anesthetics in this plane was questioned.[9]
Primary goal of the study was to assess the analgesic efficacy of intravenous fentanyl patient-controlled analgesia (PCA) with and without combined TAP and rectal sheath local anesthetic blocks through catheters surgically inserted during closure of abdominal wall muscles. Secondary goal was to study changes in plasma bupivacaine levels with an enzyme-linked immunoassay (ELISA) technique, and correlated to any toxicity.
Material and Methods
The trial was approved by the local Ethics and Research Committee of the Institutional Research Board (IRB, 00105/2015) and was registered with the Pan African Clinical Trials registry of South African Cochrane Registry as a randomized controlled trial (RCT) with blinding (PACTR 201407000849363), (www.pactr.org). A written informed consent was taken for each patient.
Adult hepatitis C patients (Child classification A) with cirrhosis confirmed by ultrasonography, scheduled for an elective liver resection were included in the study. Patients were excluded from the study if they had any previous history of an allergic reaction to a local anesthesia drug, an objection to the suggested local anesthesia techniques, and if unable to use the PCA machine. A previous abdominal surgical scar, the need for post-operative ventilation (intraoperative complications), and a history of opioid addiction were also among the other exclusion criteria.
Patients were randomly allocated into one of two groups with opaque closed envelopes and drugs were prepared by the pharmacy department. Contents were kept blind to the attending staff. Catheters were inserted in both TAP and rectal sheath spaces surgically under direct vision during the closure of the anterior abdominal muscle layers (right inverted L-shaped incision; a combination of midline and subcostal incisions on one side).
PCA group included intravenous fentanyl PCA regime and placebo saline 0.9% injection through the surgically inserted catheters in the transversus abdominis and posterior rectus sheath spaces.
The second group (PCA + TAP + RSB) included combined regional anesthesia blocks of the TAP and posterior rectus sheath with local anesthetic injected in the related catheters, together with an intravenous fentanyl PCA regime. Nature of the procedure was explained to the patients, who were also taught to assess the intensity of pain by the Visual Analog Scale (VAS) and how to use the syringe pump for PCA.
General anesthesia was induced with propofol 1.5–2 mg/kg and fentanyl 2 μg/kg. Depth of anesthesia monitoring was done (Entropy, USA, GE) Rocuronium 0.6 mg/kg (Esmeron, Organon, USA) was used to facilitate endotracheal intubation guided by nerve stimulation. Anesthesia was maintained with a mixture of air, oxygen, sevoflurane, fentanyl 1 μg/kg/h, and rocuronium. Fentanyl infusion was continued till full recovery.
Before final closure of the abdomen two multihole catheters (an epidural catheter, 20G) were placed under direct vision in both groups in the plane between transversus abdominis and internal oblique muscles and another between rectus abdominis muscle and the posterior wall of the rectus sheath with special attention to blood vessels in this space. First injection was performed after closure of the skin and before extubation. Following emergence from anesthesia, all the patients were transferred to post-anesthesia care unit and later to the intermediate care unit.
Repeated local anesthetic drug injection protocol
Injection of 0.2 ml/kg of saline was given in both catheters at the conclusion of surgery and every 8 h in the (PCA) group. Injection of 0.2 ml/kg of 0.25% bupivacaine was given at the conclusion of surgery and every 8 h in the (PCA + TAP + RSB) group (maximum of 1 mg/kg dosage of bupivacaine in each catheter at any time of injection).
Intravenous fentanyl were administered immediately after surgery once the patient was fully awake in the post-anesthesia care unit (PACU) through a PCA pump (Fresenius -Le Grand Chemin – F38590 BREZINS, Germany) programmed for a demand-only mode with no basal rate; the program delivered a bolus of 15 μg fentanyl, with a 10 min lockout interval and a maximum fentanyl dose per hour of 90 μg. All settings were saved and the key locked.
Plasma bupivacaine was measured by an ELISA assay at 30 min, 1 h, 2 h, 4 h, 8 h, 24 h, and 48 h after admission to the PACU, (IDELISA T Minc Biotechnology Company London Canada). The kit is for in vitro research screening purposes and can be used for blood and urine as prescribed by the manufacturers. All patients were monitored clinically for any possible signs of toxicity from bupivacaine with all required safety measures.
The presence and severity of pain was assessed by an investigator blinded to the trial using a VAS[10](score from 0 to 10, with 0 indicating no pain and 10 indicating worst pain). All patients were asked to score their pain at rest and on movement (knee flexion). Postoperative nausea and vomiting (PONV) were scored from 0 to 3,[11]0: none, 1: yes, does not require treatment, 2: yes, requires and relieved by treatment, and 3: yes, but not relieved by treatment. Sedation was assessed by sedative score (Ramsay score).[12] The following were reported; total fentanyl consumptions for 48 h, signs and symptoms of local anesthetic drug toxicity and plasma bupivacaine levels, complications and PONV.
Statistical analysis and design
Primary outcome of this RCT was VAS. A mean difference of 10% and standard deviation of 13.2 and 11.0 was used for calculation. Sample size using (IBM SPSS Sample power) software and was also confirmed using the Lenth Java Applets for Power and Sample Size (Computer software).[13,14] SPSS (Statistical Package for Social Science 18) program was used for statistical analysis. Kolmogorov–Smirnova test revealed that variables are normally distributed and parametric statistics were carried out. Exploration of data yielded descriptive statistics including the minimum and maximum, range, mean, standard deviation, median, and inter-quartile range for each variable. Comparisons were carried out between the two studied groups using independent t-test (t-test). Within each group comparisons was carried out using repeated measures analysis of variance (ANOVA). Box-and-whiskers graph was made. Pearson Chi-square test (χ2) and Fisher exact test were used to measure association between qualitative variables. Correction of P value for multiple testing was set P to 0.01 to detect significance (Bonforroni correction of multiple comparisons). In the present study an alpha level was set to 1% with a significance level of 99%, and a beta error accepted up to 20% with a power of study of 80%.
Results
Fifty-five patients were included in the study [Figure 1], 25 in the PCA gp and 30 in the PCA + TAP + RSB gp. Patient characteristics and demographics were comparable [Table 1].
Figure 1.
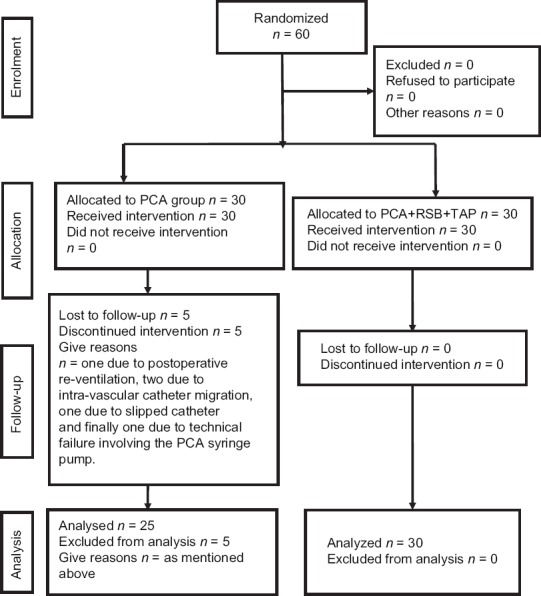
Consort flow diagram showing patients’ allocation at different stages of the study
Table 1.
Patients’ characteristics for (PCA) group versus (PCA + RSB + TAP) group
| Variables | PCA group (n=25) | PCA + RSB + TAP (n=30) | p value |
|---|---|---|---|
| Age (years) | |||
| 54.3±7.7 | 54.4±6.6 | P=0.954 | |
| Gender | |||
| Males | 14 (56.0) | 17 (56.7) | |
| Females | 11 (44.0) | 13 (43.3) | P=0.960 |
| Weight (kg) | |||
| 81.6±15.7 | 82.7±8.7 | P> 0.05 | |
| ASA (II, III) | 14/11 | 13/17 | |
| Liver surgery | |||
| Focal lesion right lobe | 10 (40) | 12 (40) | |
| Focal lesion in left lobe | 13 (52) | 12 (40) | |
| Hemangioma in right lobe | 0 | 2 (6.7) | |
| Hemangioma in left lobe | 1 (4) | 0 | |
| Cyst in right lobe | 1 (4) | 3 (10) | |
| Hydatid cyst in right lobe | 0 | 1 (3.3) | |
Data are presented as mean±standard deviation (SD), number, %, and Student’s t-test were used comparing age, weight, and sex. P: Probability of error (significant if<0.05). χ2: Pearson Chi-square. NS=P > 0.05 which considered not significant. ASA=American Society of Anesthesiologist physical status
Heart rate (HR) during first 48 h postoperative (beat/min) were significantly less in PCA + TAP + RSB gp than PCA gp. (Day 1, 84.2 ± 7.5 vs. 91.8 ± 5.5, Day 2, 78.4 ± 7.7 vs. 86.3 ± 5, respectively, (P < 0.001), but with no significant difference in mean blood pressure.
During rest pain control was equally effective for both groups (VAS ≤3), but on movement and early ambulation the VAS for pain was reduced during the first two postoperative days [Figures 2 and 3], when TAP and rectal sheath block (RSB) were combined with intravenous opioids.
Figure 2.
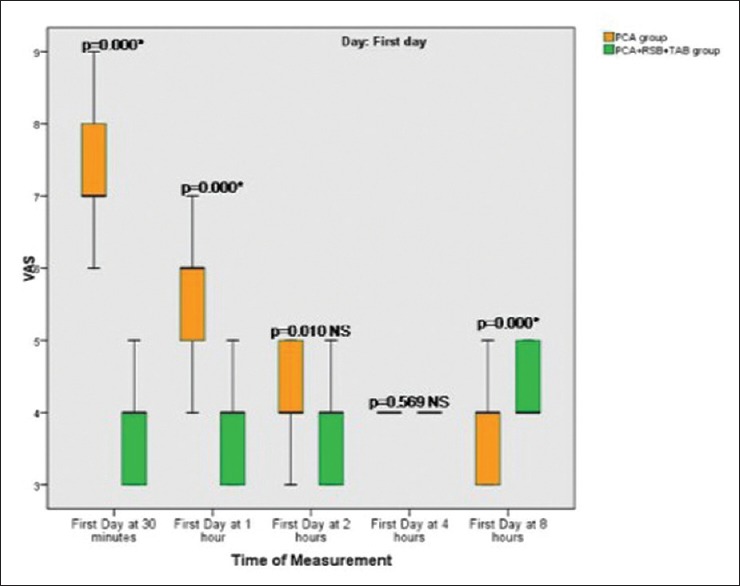
Box plot graph of Visual Analogue Scale (VAS) on movement on postoperative Day 1 showing; median values (line within the box) and inter-quartile range for 25 patients in intravenous fentanyl patient controlled analgesia group (PCA) + placebo saline injection in RSB and TAP group, and 30 patients in multimodal analgesia group (PCA + 0.25% bupivacaine in RSB and TAP). P < 0.01, changes considered significant. *Significant. NS = P >0.01 which considered not significant
Figure 3.
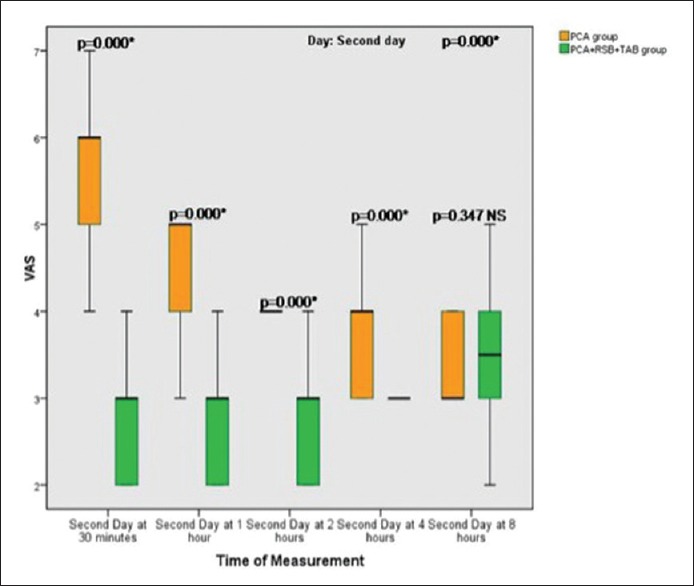
Box plot graph of Visual Analogue Scale (VAS) showing median values (line within the box) and inter-quartile range in postoperative Day 2 in 25 patients in intravenous fentanyl patient controlled analgesia group (PCA) + placebo saline injection in RSB and TAP versus 30 patients in multimodal analgesia group (PCA + 0.25% bupivacaine in RSB and TAP). P <0.01, changes considered significant. NS = P >0.01 which considered not significant
This was also associated with significant reduction in total intravenous fentanyl consumption during the first and second postoperative days in (TAP + RSB + PCA) group compared to PCA group (352.4 ± 169.1 vs. 1034.0 ± 231.8 μg, P < 0.001) and (204.4 ± 62.9 vs. 481.6 ± 158.3 μg, P < 0.001 respectively).
There was no difference in need for rescue analgesics in the two groups to maintain a VAS ≤3 at rest during the first postoperative day and during the second day [Table 2]. There was no difference in the duration of intensive care stay between both groups median (P = 0.359).
Table 2.
Number of patients during rest in need for rescue analgesic drugs in intravenous fentanyl patient controlled analgesia group (PCA) + placebo saline injection in RSB and TAP and the multimodal analgesia group (PCA + 0.25% bupivacaine in RSB and TAP)
| Variables | PCA (n=25) | PCA + RSB + TAP (n=30) | Significance |
|---|---|---|---|
| First day | |||
| 11 (44.0) | 10 (34.5) | P=0.474 | |
| Second day | |||
| 5 (20.0) | 2 (6.9) | P=0.153 | |
Data are presented as number and (%) in both groups. Pearson Chi-square. P: Probability of error (significant if <0.05)
Bupivacaine consumption in the study group during the first 48 h was 438.4 ± 96 mg. Bupivacaine plasma levels peaked within 10 min after injection of the first doses with a gradual decrease before next top up dose. No toxic blood levels were observed with ELISA or reported clinically in any case [Figure 4].
Figure 4.
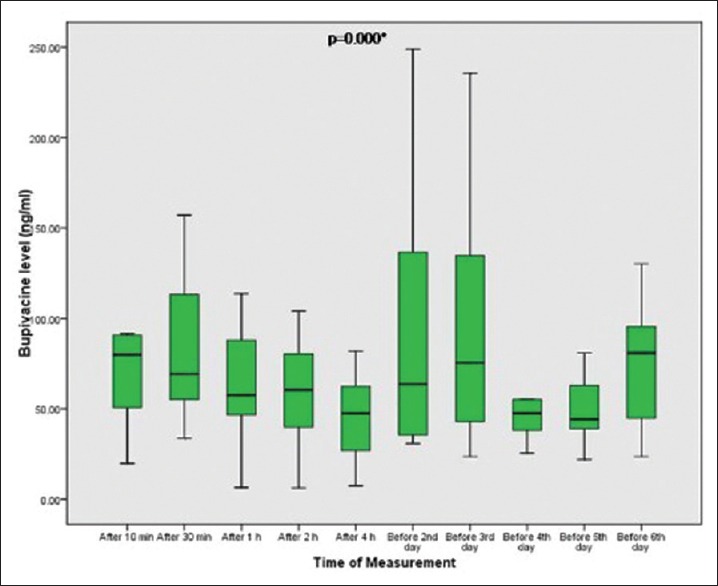
Plasma levels of bupivacaine in the multimodal analgesia group (PCA + RSB + TAP) after injection of first dose at different times and just before the following repeated doses in a duration of 48 h. Data are presented as median and interquartile. ANOVA: Repeated measures analysis of variance. P: probability of error (significant if <0.01). **Significant compared to baseline levels (after 10 min)
PONV was frequently encountered in the PCA group during the first and second days [Table 3], while PCA + TAP + RSB group demonstrated early intestinal motility, this could be due to the lower consumption of fentanyl. No respiratory depression was reported in any patient and Ramsay Sedation Score was comparable despite difference in fentanyl consumption [Table 4].
Table 3.
Significant postoperative nausea and vomiting (PONV) during first and second days in (PCA) group and (PCA + RSB + TAP) group. Scores: 0: none. 1: yes, does not require treatment. 2: yes, requires and relieved by treatment. 3: yes, but not relieved by treatment
| PONV Score | PCA (n=25) | PCA + RSB + TAP (n=30) | |
|---|---|---|---|
| PONV score (First day) | 0 | 10.0 (40.0) | 26 (86) |
| 1 | 3 (12.0) | 2 (6) | |
| 2 | 11 (44.0) | 2 (6) | |
| 3 | 1 (4.0) | 0 (0.0%) | |
| PONV Score (Second day) | 0 | 15 (60.0) | 27 (90.0) |
| 1 | 1 (4.0) | 2 (6) | |
| 2 | 8 (32.0) | 1 (3.3) | |
| 3 | 1 (4.0) | 0 (0.0) |
Data are presented as number and (%) in both groups. For first day: χ2=14.205, P(MC)=0.001 *[MC: Monte Carlo Sig. (2-sided)] and for second day χ2=9.833, P(MC)=0.009 * [MC: Monte Carlo Sig. (2-sided)]
Table 4.
Ramsay Sedation score in first and second postoperative day among intravenous fentanyl patient-controlled analgesia group (PCA) versus (PCA + RSB + TAP) group
| Sedation Score | PCA (n=25) | PCA + RSB + TAP (n=30) | |
|---|---|---|---|
| Sedation score (First day) | 1 | 2 (8.0) | 0 (0.0) |
| 2 | 19 (76.0) | 29 (96) | |
| 3 | 4 (16.0) | 1 (3.3) | |
| Sedation score (Second day) | 1 | 0 (0.0) | 1 (3.3) |
| 2 | 20 (80.0) | 28 (93.3) | |
| 3 | 5 (20.0) | 1 (3.3) |
Data are presented as number and (%). For first day: χ2=5.474, P(MC)=0.057 NS [MC: Monte Carlo Sig. (2-sided)] and, for second day: χ2=4.583, P(MC)=0.081 NS [MC: Monte Carlo Sig. (2-sided)]
Discussion
The opioid sparing effect of TAP and RSB was observed in this current RCT, which is of importance for patients with hepatic cirrhosis undergoing liver surgery. Previous trials by Sharma et al.,[15] McDonnell et al., Allcock et al., Elkassabany et al., and Nash et al.[16,17,18,19] in their studies came to a similar conclusion when TAP block was adopted as part of a multimodal pain control regime.
Siddiqui et al. performed a metaanalysis and also found that these blocks helped to reduce the requirement of opioids and was comparable to morphine effect.[20] The use of an open surgical technique to identify the TAP accurately during the closure of the abdominal wall to perform regional anesthesia blocks and insert catheters for continuous analgesia was used by some investigators (Serag Eldin et al.[7] Brady et al., Milan et al., and Mrunalini et al). They stated that open TAP blocks are safe and reduce postoperative opioid requirements and sedation after liver resection and other surgeries as hemi-colectomies, liver transplant, and emergency laparotomy, respectively.[21,22,23]
Serag Eldin et al. found that the consumption of fentanyl was reduced by 20% with TAP block, but when RSB was combined with TAP in an attempt to block more sensory fibers and dermatomes, as in our current trial, the consumption of fentanyl was reduced by >60%. Multimodal analgesia can improve pain relief and quality of recovery as reported by De Oliveira Jr et al. and Carney et al.[24,25]
The use of anterior abdominal wall blocks as an alternative to the thoracic epidurals widely used in healthy liver patients was mainly suggested due to the expected coagulation changes (prolonged INR) which can be of special concern for patients with cirrhotic livers undergoing liver surgery.[3]
Monitoring for signs of overdose toxicity was given specific attention in this current trial due to Griffiths et al.[26] demonstrating that ropivacaine after TAP block can reach neurotoxic plasma levels. Results of our RCT demonstrated that plasma bupivacaine concentrations were lower than the potential toxic levels at all measuring points, with no patient demonstrating any clinical signs of toxicity.
Ganapathy et al.[27] reported no associating toxicity when ropivacaine was infused in the TAP block continuously for 24–72 h in patients undergoing laparotomy. They also observed that the level of analgesia was effective and comparable to the thoracic epidural analgesia.
Suresh et al.[28] also studied the plasma levels of bupivacaine after TAP block but in a different population in neonates and reported similar findings of low risk to local anesthetic toxicity at this group age.
Several incidents of toxicity in association with TAP block were reported by Naidu and Richebe and Scherrer et al.[29,30] and others.[31,32] Monitoring every patient with TAP blocks is essential for at least 45 min post-injection. Intermittent TAP block injection was preferred in this current study rather than continuous infusion to reduce the incidence of systemic toxicity in this study population of cirrhotic patients, with a possible altered protein binding and delayed metabolism which can lead to a high free bupivacaine plasma levels.
Reported breakthrough pain prior to the next anticipated injection was the only disadvantage for adopting bolus injections rather than continuous infusion. Reducing the 8 h interval could be studied in future to improve pain management.
The TAP block affects the somatic sensory nerves and not the visceral, this was unfortunately reflected in our study in a percentage of patients in need for rescue opioids. Gadsden et al.[31] described the TAP block as an evolving regional anesthetic technique for postsurgical pain management after abdominal surgery.
Another limitation for open TAP and RSB was the performance of an one-sided block due to the unilateral L shape by surgical incision adopted by most of the surgeons performing liver resection, limiting the ability to perform bilateral open TAP and rectal sheath blocks performed which could have helped to increase the efficacy of these blocks.
The inability to alter the settings of intravenous fentanyl PCA pumps during the study to meet with the demands of pain relief, lead to the use of additional rescue opioids with the increase in frequency of movement and physiotherapy to enhance recovery during the early postoperative period. Future studies are required to investigate the optimal concentrations and settings of PCA pumps for this group of hepatic patients.
One of the other limitations of the study was not using the more specific technique of high-performance liquid chromatography (HPLC) for plasma level of bupivacaine due to higer cost. The ELISA was able to demonstrate that bupivacaine did not exceed referred toxic plasma levels and provided trend of changes in plasma concentration at a reduced cost.[32]
Most of cases managed with TAP and RSB and consuming less opioids had a lower incidence of PONV, while patients managed solely on PCA fentanyl suffered significantly more, with about 50% in need for medical symptomatic relief. Petersen and his colleagues in their systematic search of the literature involving surgical procedures found significant reductions in postoperative opioid requirements with a reduction in PONV when TAP blocks were used.[33] In contrast to the above findings, Mrunalini et al. found no difference.[23]
In conclusion, a multimodal approach combining TAP and RSB with PCA fentanyl improved pain control and reduced opioid demand. Time intervals between local anesthetic drug injections need to be studied to reduce outbreaks of pain. Repeated bupivacaine injections in cirrhotic patients were not associated with any clinical signs of toxicity in this study. Bupivacaine serum levels measured with an ELISA technique were lower than referred toxic serum levels.
Financial support and sponsorship
Funding by Departmental Resources of Anaesthesia and Intensive Care Department, Liver Institute, Menoufiya University, Egypt.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Abdel-Wahab M, El-Husseiny T, El-Hanafy E, El Shobary M, Hamdy E. Prognostic factors affecting survival and recurrence after hepatic resection for hepatocellular carcinoma in cirrhotic liver. Langenbecks Arch Surg. 2010;395:625–32. doi: 10.1007/s00423-010-0643-0. [DOI] [PubMed] [Google Scholar]
- 2.Rudin A, Lundberg JF, Hammarlund-Udenaes M, Flisberg P, Werner MU. Morphine metabolism after major liver surgery. Anesth Analg. 2007;104:1409–14. doi: 10.1213/01.ane.0000261847.26044.1d. [DOI] [PubMed] [Google Scholar]
- 3.Bedawy A, Fayed N, Morad W, Madany S, Nematalah F, Yassen K. Rotational thrombo elastometry and standard coagulation tests for hepatic patients undergoing major liver resection. J Anesth Clin Res. 2012;3:243–7. [Google Scholar]
- 4.Page A, Rostad B, Staley C, Levy J, Park J, Goodman M, et al. Epidural analgesia in hepatic resection. J Am Coll Surg. 2008;206:1184–92. doi: 10.1016/j.jamcollsurg.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Fayed N, Abo E-WH, Gab-Alla N, Yassen, K, Lotfy, M Comparison between intravenous patient controlled analgesia and patient controlled epidural analgesia in cirrhotic patients after hepatic resection. Middle East J Anaesthesiol. 2014;22:467–76. [PubMed] [Google Scholar]
- 6.Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CH, et al. Guidelines for perioperative csre for liver surgery: Enhanced Recovery after surgery (ERAS) society recommendations. World J Surg. 2016;40:2425–40. doi: 10.1007/s00268-016-3700-1. [DOI] [PubMed] [Google Scholar]
- 7.Niraj G, Kelkar A, Jeyapalan I. Comparison of analgesic efficacy of subcostal transversus abdominis plane blocks with epidural analgesia following upper abdominal surgery. Anaesthesia. 2011;66:465–71. doi: 10.1111/j.1365-2044.2011.06700.x. [DOI] [PubMed] [Google Scholar]
- 8.Serag Eldin M, Mahmoud F, El Hassan R, Raouf M, Afifi M, Yassen K, et al. Intravenous patient-controlled fentanyl with and without transversus abdominis plane block in cirrhotic patients post liver resection. Local Reg Anesth. 2014;7:27–37. doi: 10.2147/LRA.S60966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths J, Barron F, Grant S, Bjorksten A, Hebbard P, Royse C. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105:853–6. doi: 10.1093/bja/aeq255. [DOI] [PubMed] [Google Scholar]
- 10.Maintyre P, Schug S, Scott D, Visser E, Walker S. Assessment and management of pain and its treatment. In: Maintyre P, Schug S, Scott D, Visser E, Walker S, editors. Acute Pain Management: Scientific Evidence. 3rd ed. Melbourne: ANZCA & FPM; 2010. pp. 35–46. [Google Scholar]
- 11.Maddali M, Mathew J, Fahr J, Zarroug A. A prospective study of incidence of postoperative nausea and vomiting in a tertiary care hospital in Oman. Middle East J Anesthesiol. 2003;17:131–41. [PubMed] [Google Scholar]
- 12.Ramsay M, Newman K, Jacobson R, Richardson C, Rogers L, Brown B, et al. Sedation levels during propofol administration for outpatient colonoscopies. Proc (Bayl Univ Med Cent) 2014;27:12–5. doi: 10.1080/08998280.2014.11929037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh B, Waghmare V, Shah V, Mehta T, Butala B, Parikh, G, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane block for retroperitoneoscopic donor nephrectomy: A randomized controlled study. Saudi J Anesth. 2013;7:43. doi: 10.4103/1658-354X.109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field A. Sample size calculation. In: Field A, editor. Discovering Statistics Using SPSS. 2 ed. London, California, New Delhi: SAGE Publications Ltd; 2006. pp. 143–217. [Google Scholar]
- 15.Sharma P, Saxena A, Bansal R, Mittal A, Shrivastava U. Evaluation of postoperative analgesic efficacy of transversus abdominis plane block after abdominal surgery: A comparative study. J Nat Sci Biol Med. 2013;4:177–80. doi: 10.4103/0976-9668.107286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonnell J, O’Donnell B, Curley G, Heffernan A, Power C, Laffey J. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: A prospective randomized controlled trial. Anesth Analg. 2007;104:193–7. doi: 10.1213/01.ane.0000250223.49963.0f. [DOI] [PubMed] [Google Scholar]
- 17.Elkassabany N, Ahmed M, Malkowicz S, Heitjan D, Isserman J, Ochroch E. Comparison between the analgesic efficacy of transversus abdominis plane (TAP) block and placebo in open retropubic radical prostatectomy: A prospective, randomized, double-blinded study. J Clin Anesth. 2013;25:459–65. doi: 10.1016/j.jclinane.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Allcock E, Spencer E, Frazer R, Applegate G, Buckenmaier III C. Continuous transversus abdominis plane (TAP) block catheters in a combat surgical environment. Pain Med. 2010;11:1426–9. doi: 10.1111/j.1526-4637.2010.00894.x. [DOI] [PubMed] [Google Scholar]
- 19.Nash H, Khoda B, Heppell S, Turner M. TAP blocks in breast reconstructions using abdominal wall tissue. Anesthesia. 2011;66:750–1. doi: 10.1111/j.1365-2044.2011.06798.x. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui M, Sajid M, Uncles D, Cheek L, Baig, M A meta-analysis on the clinical effectiveness of transversus abdominis plane block. J Clin Anesth. 2011;23:7–14. doi: 10.1016/j.jclinane.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Brady R, Ventham N, Roberts D, Graham C, Daniel T. Open transversus abdominis plane block and analgesic requirements in patients following right hemi colectomy. Annals R Coll Surg Engl. 2012;94:327–30. doi: 10.1308/003588412X13171221589856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milan Z, Duncan B, Rewari V, Kocarev M, Collin R. Subcostal transversus abdominis plane block for postoperative analgesia in liver transplant recipients. Transplant Proc. 2011;43:2687–90. doi: 10.1016/j.transproceed.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 23.Mrunalini P, Raju N, Nath V, Saheb S. Efficacy of transversus abdominis plane block in patients undergoing emergency laparotomies. Anesth Essays Res. 2014;8:377–82. doi: 10.4103/0259-1162.143153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Oliveira G, Jr, Fitzgerald P, Marcus R-J, Ahmad S, McCarthy R. A dose-ranging study of the effect of transversus abdominis block on postoperative quality of recovery and analgesia after outpatient laparoscopy. Anesth Analg. 2011;113:1218–25. doi: 10.1213/ANE.0b013e3182303a1a. [DOI] [PubMed] [Google Scholar]
- 25.Carney J, McDonnell J, Ochana A, Bhinder R, Laffey J. The transversus abdominis plane block provides effective postoperative analgesia in patients undergoing total abdominal hysterectomy. Anesth Analg. 2008;107:2056–60. doi: 10.1213/ane.0b013e3181871313. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths JD, Le NV, Grant S, Bjorksten A, Hebbard P, Royse C. Symptomatic local an aesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br J Anaesth. 2013;110:996–1000. doi: 10.1093/bja/aet015. [DOI] [PubMed] [Google Scholar]
- 27.Ganapathy S, Sondekoppam R, Terlecki M, Brookes J, Adhikary S, Subramanian L. Comparison of efficacy and safety of lateral-to-medial continuous transversus abdominis plane block with thoracic epidural analgesia in patients undergoing abdominal surgery: A randomised, open-label feasibility study. Eur J Anaesthesiol. 2015;32:797–804. doi: 10.1097/EJA.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 28.Suresh S, De Oliveira G., Jr Blood bupivacaine concentrations after transversus abdominis plane block in neonates: A prospective observational study. Anesth Analg. 2016;122:814–7. doi: 10.1213/ANE.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 29.Naidu RK, Richebe P. Probably local anesthetic systemic toxicity in a postpartum patient with acute fatty liver of pregnancy after a transversus abdominis plane block. A A Case Rep. 2013;1:72–4. doi: 10.1097/ACC.0b013e3182973a2f. [DOI] [PubMed] [Google Scholar]
- 30.Scherrer V, Compère V, Loisel C, Dureuil B. Cardiac arrest from local anesthetic toxicity after a field block and transversus abdominis plane block: A consequence of miscommunication between the anesthesiologist and surgeon. A A Case Rep. 2013;1:75–6. doi: 10.1097/ACC.0b013e3182973a3f. [DOI] [PubMed] [Google Scholar]
- 31.Gadsden J, Ayad S, Gonzales J, Mehta J, Boublik J, Hutchins J. Evolution of transversus abdominis plane infiltration techniques for postsurgical analgesia following abdominal surgeries. Local Reg Anesth. 2015;8:113–7. doi: 10.2147/LRA.S96253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato N, Fujiwara Y, Harato M, Kurokawa S, Shibata Y, Harada J, et al. Serum concentration of lidocaine after transversus abdominis plane block. J Anesth. 2009;23:298–300. doi: 10.1007/s00540-008-0721-4. [DOI] [PubMed] [Google Scholar]
- 33.Petersen P, Mathiesen O, Torup H, Dahl J. The transversus abdominis plane block: A valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol Scand. 2010;54:529–35. doi: 10.1111/j.1399-6576.2010.02215.x. [DOI] [PubMed] [Google Scholar]


