Abstract
Patients with peritoneal carcinomatosis were considered incurable with dismal survival rates till hyperthermic intraperitoneal chemotherapy after optimal cytoreductive surgery evolved. Perioperative management for these procedures is complex and involves an optimal cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy. In this article we highlight the perioperative concerns in these patients including anesthetic challenges, such as optimal fluid management, maintaining blood pressure, control of body temperature, coagulation and electrolyte derangement and renal toxicity of chemotherapeutic drugs. We have also discussed the postoperative problems and their management.
Keywords: Anesthesia, fluid management, hyperthermic intraperitoneal chemotherapy, postoperative care
Introduction
Primary peritoneal neoplasm and metastasis to peritoneum from gynecologic or gastrointestinal cancer have a very poor prognosis. They were considered an incurable palliative condition for long until, Dr Paul Sugarbaker showed that surgical removal of visible tumour for peritoneal mesothelioma combined with loco-regional heated chemotherapeutic drugs improved quality of life and survival of patients.[1] Nowadays, this hyperthermic intraperitoneal chemotherapy (HIPEC) is a well-established treatment for pseudomyxoma peritonei, and colorectal, ovarian and gastric cancers with isolated peritoneal metastases. Perioperative management is complex and is associated with massive fluid shift, blood loss, temperature imbalance and hemodynamic alterations. The anesthesiologist plays an important role during HIPEC and cytoreductive procedure. A team approach with the surgical colleagues for the care of these patients improves patient outcomes.
History
Cytoreductive surgery for ovarian cancer was first described by Joe Vincent Meigs in New York to reduce macroscopic disease.[2,3] Subsequently effect of hyperthermic intraperitoneal chemotherapy on killing cancer cells was established. This led to development of modern HIPEC procedure. A short history is described in the Table 1.
Table 1.
History of development of modern hyperthermic intraperitoneal chemotherapy procedure
| Year | Development |
|---|---|
| 1979-1980 | TIFS developed to deliver heated chemotherapy into the peritoneum and tested in humans for administration of HIPEC for locally advanced abdominal malignancy[4] |
| Mid to late 1980s | Dr. Sugarbaker reported survival benefits in patients with peritoneal dissemination[1] |
| 1995 | Dr. Sugarbaker standardized the procedure and described stepwise approach to cytoreduction[5] |
| 2016 | LE-HIPEC technique described where the HIPEC given after the closure of the abdominal wound and spread with the help of laparoscopic approach[6] |
HIPEC=Hyperthermic intraperitoneal chemotherapy, TIFS=Thermal transfusion infiltration system, LE=Laparoscopy-enhanced
Rationale
Intraperitoneal delivery ensures high concentrations of the cytotoxic drug in the local tumor-bearing peritoneum uniformly and keeps the systemic drug levels low. A “Three Compartment Model” is suggested wherein a high concentration gradient of chemotherapeutic drug is maintained between the peritoneal cavity and the plasma by this ‘peritoneal-plasma barrier’ (third compartment).[7] Portal vein carries cytotoxic drug from peritoneal surface to liver as first pass metabolism and ensures exposure of hepatic micrometastasis to cytotoxic chemotherapy.[8]
Tissue penetration
The intraperitoneal chemotherapy penetrates tissues upto a depth of 5mm only. So before intraperitoneal instillation of drug, adequate cytoreductive surgery should be performed to remove visible tumour mass to ensure penetration of the chemotherapy into the remaining tumor cells.[9]
Hyperthermia
Hyperthermia in the range of 41 to 43°C causes selective destruction of malignant cells by reversible nonselective inhibition of RNA synthesis and mitosis arrest. It increases the tissue penetration for the drug, leads to vascular stasis in the microcirculation of malignant tumours and results in anaerobic glycolysis and acidosis. This acidic microenvironment in malignant cells induces lysosomal enzymes and causes accelerated death of fragile malignant cells.[10]
Enhanced cytotoxicity of heated chemotherapeutic drugs
Heat and cytotoxic drugs act synergistically to increase the drug uptake in malignant cells by improving membrane permeability and transport. Heat causes changes in pharmacokinetics and pharmacodynamics of chemotherapy drugs and increases drug penetration and its action in tissues.[11]
Indications of hyperthermic intraperitoneal chemotherapy
Appropriate patient selection is the most challenging step in the success of the HIPEC procedure. The benefits may be minimal in patients with advanced intra-abdominal disease. The important parameters that need to be assessed during the screening of the patients for the surgery include:
Preoperative cancer index (PCI) describes the volume and extent of disease radiologically. It integrates peritoneal implant size and distribution of peritoneal nodules. The abdomen and pelvis are divided into 9 regions and intestines are divided into 4 regions. Each lesion is measured and given a score on basis of size (LS-0 denotes absence of cancer; LS-1 tumor size <0.5 cm in diameter; LS-2 tumor deposit 0.5-5 cm and LS-3 tumor deposit >5 cm). The PCI score is calculated by adding scores for each region to get a score between 1-39.[12,13] It is an important, commonly used and validated prognostic index. A PCI >17.4 doesn’t decrease the chances of any benefit with HIPEC and if PCI is >20 HIPEC should not be done.
Histopathology of the tumor for invasiveness of malignancy is an important parameter to be considered before HIPEC in patients with low-grade disseminated peritoneal adenomucinosis and high-grade peritoneal mucinous carcinomatosis.[14] HIPEC may be beneficial in patients with PCI >20 also and decision should be individualized based on other patient factors. Patients with histopathologically proven extra-abdominal disease, extraperitoneal disease like >3 liver metastases, retroperitoneal lymph nodes or unknown primary tumour are contraindications for HIPEC surgery.[15]
Preoperative CT scan to ascertain the extent of extra abdominal spread in the thorax, and pelvis.
Completeness of the cytoreduction (CC) score is an important prognostic indicator on basis of the visible tumor left after cytoreduction.(CC-0 no visible tumor; CC-1 persisting tumor nodule <2.5 mm; CC-2 tumor nodule between 2.5mm-2.5cm and CC-3 tumor nodule >2.5 cm).[12] A score of CC-0 or CC-1 are ideal candidates for HIPEC after cytoreduction.
Technique
There are two methods for intraperitoneal administration of hyperthermic chemotherapy:
-
The Open abdomen technique is performed by the “Coliseum technique”, as described by Sugarbaker.[16] The infusate is maintained at a temperature of 43-45°C to ensure a temperature of 41-43°C of the intraperitoneal fluid [Figures 1 and 2]. The drug in the perfusate is recirculated within the peritoneal cavity to maintain a minimum intraperitoneal temperature of 41.5°C. This ensures even distribution of temperature or chemotherapeutic drug throughout the abdominal cavity. An open approach allows direct access of the cavity during administration of chemotherapeutic drugs and allows for manipulation of fluid and bowel for optimal distribution of drug within the abdomen. But it is difficult to achieve and maintain hyperthermic state due to the heat dissipation by the exposed abdomen. Moreover, heated chemotherapy may lead to aerosolisation and cause inhalational exposure of operating room personnel.
Peritoneal cavity expander (PCE) is a modified open technique. It consists of an acrylic cylinder with in-flow and out-flow tubes that are secured over the wound. The small bowel floats freely and manually manipulated in the PCE filled with heated perfusate. This ensures more uniform distribution of the drug as compared to a closed technique. It may lead to oozing around the wound and tumor recurrence inside parietal wound
In closed technique, after cytoreduction the temperature probes and catheters are placed to instill chemotherapy and abdomen wall is sutured prior to infusion of chemotherapy. Due to closed abdomen, intraabdominal pressure rises which helps in deeper tissue penetration of drug, this reduces the heat loss to minimal and ensures that hyperthermia is easy to achieve and maintain. The advantage of closed technique is that there is minimal exposure of the operating theatre personnel to aerosolized chemotherapy. However, the disadvantage of closed technique is that it may lead to uneven distribution of the hyperthermic chemotherapy, the drug may pool and accumulate in dependent parts of the abdomen and lead to postoperative ileus, bowel perforation, and fistula.
Figure 1.
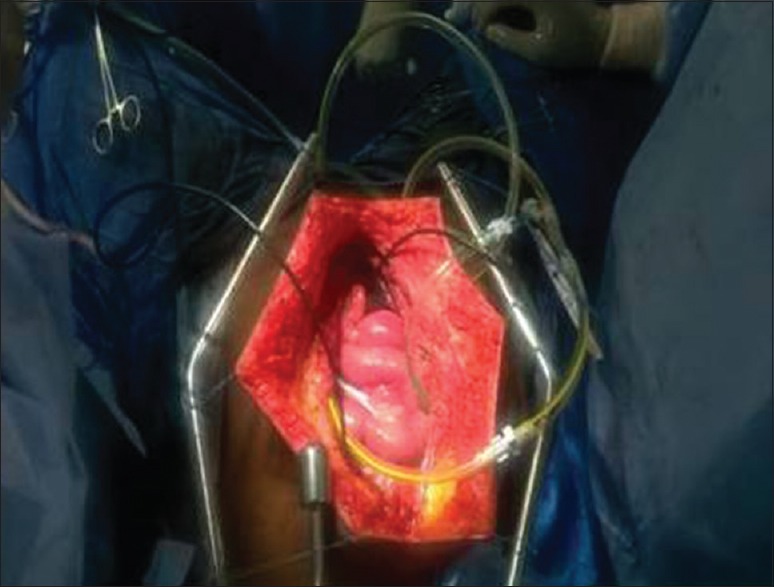
Coliseum technique of HIPEC: After cytoreduction, Tenckhoff catheter and closed suction drains are inserted in abdominal wall for administration of HIPEC
Figure 2.

Hyperthermic intraperitoneal chemotherapy machine
Anesthetic Implications
The anesthesiologist has a crucial role during HIPEC and cytoreductive procedure. A team approach for care of these patients improves patient outcomes.[17] Unique aspects of HIPEC surgery are hemodynamic fluctuations, hypothermia and induced hyperthermia, the potential for chemotherapeutic drug induced nephrotoxicity. Anesthesiologists need to manage intravenous fluid therapy, blood transfusion and electrolyte balance to maintain optimal end-organ perfusion, and prevent renal injury.
Preoperative Concerns
Operative risk assessment for comorbidities like diabetes, hypertension and heart disease and their implications and additional risk to previous radiotherapy and chemotherapy. Traditionally ASA classification is done to assess risk of anesthesia.[18] In addition Eastern Cooperative Oncology Group (ECOG) performance status of patients should be assessed.[19] It describes a patient's functional status in terms of their ability to care for themself, daily activity, and physical ability. The surgery is extensive and may require prolonged hospital stay. The patients and their caregivers should be informed regarding therapy, expected outcome, cost involved, risk involved, quality of life and need for systemic chemotherapy
-
Prehabilitation is an important part of preparation for a good surgical outcome. It should include:
- Patient's preoperative nutritional status and albumin levels strongly predicts length of hospital stay and overall survival in these patients.[20,21] Malnutrition may be present in >50% patients with ovarian cancer and advanced colorectal cancer with peritoneal metastasis. Preoperative malnutrition may lead to prolonged hospital stay, increased complications and cost of care. Some of the clinical indicators of malnutrition include weight loss >5% in past three months, reduced intake and a Body Mass Index (BMI) <18.5 kg/m2.[20,21] There is limited literature on preoperative nutrition building in HIPEC patients but well accepted guidelines for surgical patients can be used. The nutritional support preferably enteral should be instituted to improve anemia and albumin
- Patients coming for HIPEC surgery often have ascites and pleural effusion. This may lead to basal atelactasis and predispose the patients to increased risk of postoperative respiratory complications. Intensive spirometry should be prescribed in the preoperative period to reduce the incidence of pulmonary complications[22]
- Patients undergoing HIPEC surgeries are at increased risk of venous thrombo-embolism (VTE) and a decision to start VTE prophylaxis (preoperatively and 4-6 weeks postoperatively) using heparin (low molecular weight/unfractionated) and/or mechanical pneumatic compression stockings should be taken
Pulmonary function assessment and management: These patients may have ascites and pleural effusion which leads to diaphragmatic splinting and basal atelectasis and predispose them to hypoxia in preoperative period. A detailed medical history and investigations (CXR/CT, arterial blood gas (ABG) analysis, pulmonary function tests, cardiopulmonary exercise testing (CPET) if feasible should be done to determine pulmonary function.[23]
Cardiovascular assessment: Large amount of fluid shift occurs during cytoreductive phase due to large raw surface produced by peritonectomy, ascitic drainage and significant blood loss during this procedure. In addition hyperthermia during HIPEC leads to peripheral vasodilatation and reduction in peripheral vascular resistance and mean arterial pressure. This leads to hyperdynamic circulation with increase in heart rate, central venous pressure (CVP), cardiac index.[21,23]
Intraoperative Concerns
-
Induction and maintenance of anesthesia
- These patients usually have abdominal distension due to ascites which decreases functional residual capacity. This may predispose the patient to increased desaturation and aspiration. So, rapid sequence induction should be considered for induction of anesthesia in these patients[15]
- Patients undergoing HIPEC are prone to coagulation abnormalities in the perioperative period due to preoperative chemotherapy, nutritional deficiencies and hypoalbuminemia due to ascitic fluid drainage. The long duration of surgery, female gender, old age and malignancy predispose these patients to risk for thrombosis. Various studies have reported deranged prothrombin time (PT) with international ratio (INR), decreased AT III and fibrinogen values, a prolonged actived partial thromboplastine time (aPTT) and a thrombocytopenia.[24] Additionally, patients may have platelet dysfunction due to extreme variation in the temperature. Advanced point of care coagulation monitoring such as thromboelastography (TEG) may help to detect complex coagulation disorders such as hyperfibrinolysis, thrombocytopathia, or factor XIII deficiency[25]
- These patients have a large laparotomy incision and should be provided with good perioperative analgesia.[24] Primary opioid based analgesia (Morphine) should be used with caution as it may be associated with increased respiratory complications and need for ventilator support. Epidural provides good analgesia but is associated with an increased risk of hemodynamic disturbance due to extensive surgery and these patients are prone to develop spinal hematoma due to coagulation abnormalities and thrombocytopenia. So, the coagulation abnormalities should be assessed and documented to be normal before the insertion and removal of epidural catheter and ensure that the procedure is atraumatic and done by experienced anesthesiologists to avoid complications.[26] Also, this should be timed according to the effect of the anticoagulant drug being used. Other alternative techniques for analgesia like paravertebral blocks (single and continuous) and sub costal TAP block provide good alternatives with less side effects.
-
Invasive monitoring
During the heated chemotherapy phase hyperthermia leads to vasodilatation and hyperdynamic circulation with tachycardia and increased cardiac output. This normalizes after completion of the heated therapy. In addition increased intra-abdominal pressure in the closed abdomen technique, may further decrease venous return and aggravate hemodynamic instability.[27] Functional hemodynamic monitoring in the form of CO, SVV and DELTA SV particularly when PCI is recommended >15 to guide the goal directed fluid therapy to maintain normovolemia and prevent acute renal failure.[28] Extravascular lung water (EVLW) is the amount of water that is present outside the pulmonary vasculature. It includes interstitial, intracellular, alveolar and lymphatic fluid and does not include pleural effusions. Measurement of EVWL using volume view of Vigileo (EV1000) measuring lung water is particularly helpful in predicting fluid overload and overall morbidity during and after HIPEC[29]
- Decision to put central venous lines (CVP)/pulmonary artery (PA) catheter should be individualized as their usefulness in predicting fluid responsiveness is limited. CVP and PA pressures are static parameters and maynot reflect volume status or volume responsiveness accurately, but a change in parameters over a period may help us to guide the fluids.[30] Also these patients will require vasopressors so a CVP line can be used for giving the vasopressors to maintain the blood pressure
- An arterial line is a must to monitor beat to beat blood pressure to guide inotropes and ABG sampling
- Positive pressure ventilation-induced changes in stroke volume on a rhythmic basis can be useful to predict fluid responsive subgroups
- In high risk patients cardiac output monitoring using Flotrac/Vigileo may be considered
- The addition of dynamic measures of cardiac preload and fluid responsiveness, such as CO, SV, and SVR, may help us to implement goal (or flow) directed fluid therapy.
Drugs given in HIPEC are hydrophilic, have high molecular weight and a slow peritoneal clearance. Patient's height, weight and body surface area should be documented to calculate doses of anesthesia drug and chemotherapy and the dose modifications in renal, hepatic and cardiac dysfunction. The specific side effects of the chemotherapy drugs used and drug-specific potential toxicities should be known, preempted and managed by the anesthesiologist[31] [Table 2].
-
Perioperative thermoregulation
- Extreme temperature fluctuation is very crucial in these patients. So; body temperature is monitored by two probes. One temperature probe is placed in the oesophagus for core temperature monitoring and the temperature of the abdominal cavity is measured by thermistors present in the inlet and outlet drains of the HIPEC machine.[18,32] Usually at least 3 to 4 probes are placed in the abdominal cavity during HIPEC 2 in sub diaphragm and 2 on both sides of pelvis. It is essential to maintain temp >41 at least in all areas
- During debulking phase patients are prone to hypothermia due to extensive surgical resection and exposure, excessive fluid loss and prolonged duration of surgery. An intraoperative core hypothermia should be prevented to prevent coagulation and metabolic derrangements.[23] Hypothermia can be prevented by convective warming devices like forced air warming blankets (Bair Hugger ®), warm intravenous fluids and increased ambient operating room temperature
- During HIPEC phase the patients usually develop raised core body temperature (nasopharyngeal) of up to 40.5°C due to the hyperthermic perfusate. This leads to hypermetabolic phase and hyperdynamic circulation. Due to systemic hyperthermia peripheral vasodilatation occurs which causes heat loss from the core to the periphery and environment. Thus the anesthesiologist must maintain normothermia by setting the warming device to ambient or off mode and using the underbody mattress to cool the patient. Cold intravenous fluids and placement of ice packs in the axillae of the patient may be required to normalize the temperature. If despite all these measures, core body temperature rises to ≥39°C then the perfusionist should be advised to reduce the instillate temperature.
-
Fluid management
- During cytoreduction of the tumor the intraoperative fluid loses may be as high as 8-12 ml/kg and may be associated with significant blood loss depending upon the extent of resection.[18] During the HIPEC phase the saline enriched chemotherapeutic drug may increase the intra abdominal pressure, decrease venous return and decrease the cardiac output.[33] Since this procedure leads to massive fluid shift, intraoperative crystalloids and colloids are administered to ensure adequate perfusion pressure and urine output without causing fluid overload.[34] Patients with poor cardiac reserve maynot tolerate a high volume of intravenous fluid, and may require vasopressors and inotropes to be used judiciously
- These patients may have a blood loss from few hundred ml to up to 9000 ml due to surgical reasons and coagulation abnormalities.[18] The patients with higher disease load, high PCI and with extensive resections may require blood transfusions.[18] If available salvage of lost blood with subsequent irradiation (50 Gy) to eliminate cancer cells can be considered as an alternative to transfusing large volumes of banked blood as this blood is more physiological and may be associated will less transfusion related problems.[35] Various studies have suggested a lower transfusion trigger from 6-8 g/dl to reduce 30 day morbidity and mortality.[36] A recent study has suggested that a liberal transfusion trigger of 9 g/dl reduces major postoperative complications in patients having major cancer surgery.[37] It may be difficult to access the accurate blood loss in these patients and traditional methods of measurement of hemoglobin are done in laboratory and take time which may be unacceptable in such surgeries.[38] in complex surgeries like HIPEC continuous measurement of hemoglobin (SpHb) values may facilitate our decision regarding transfusions.[39] Different criteria exist for determining the need to perform a blood transfusion, among which hemoglobin concentration plays a fundamental role. To obtain accurate levels of hemoglobin, analytical methods are traditionally used that are based on blood samples, which are analyzed in a laboratory at intermittent intervals. On occasion, the results from the analyses can take some time and do not show the evolution of the patient between the moment of extraction and when the analytical results are received. In a stressful environment such as that of an operating theatre, these delays and uncertainties mean that some blood transfusions are unnecessary, especially in cases of stable anemia or perceptible but not very significant blood losses, and can represent up to 10% of all transfusions performed
- Various strategies for fluid management have been described like “liberal”, “restrictive” or “goal directed”. Goal-directed fluid therapy instead of liberal fluid administration provides better perioperative results and lesser postoperative complications[33]
- The appropriate choice of fluids is debatable. Recent metaanalysis that showed colloids increase the adverse events and are not routinely recommended for resuscitation.[40] Colloids replenish intravascular volume in a ratio of 1:1 in a fluid responsive patient and may be used along with crystalloids to maintain ‘optovolemia’. In patients with massive ascitic drainage the perioperative protein loss may be in range of upto 700 g per day and may necessitate albumin supplementation[41]
- Urine output: Haemodynamics, hyperthermia and use of cytotoxic chemotherapy during HIPEC increases the risk of renal injury.[42] Intraoperative measurement of urine output is a reliable, non-invasive and a surrogate marker of renal perfusion. One should aim at a minimum urine output of 0.5ml/kg/hr during cytoreduction; 2-4ml/kg/hr during HIPEC phase and 1-2ml/kg post-HIPEC.[42] The diuretic should only be given after ensuring euvolemia and optimal renal perfusion.[43]
-
Intraoperative electrolyte disturbances[44]
- The type and volume of carrier fluid for the cytotoxic drugs affects their systemic absorption during HIPEC. The carrier solution is mostly normal saline and traditionally dextrose containing fluid is used with oxaliplatin because it was believed that the oxaliplatin is degraded by chloride containing solutions.[45] This view is recently being challenged and it has been found to be stable in chloride based solutions. Moreover, these solutions are readily absorbed from the peritoneal cavity and result in fluid overload. The patient may develop hyperglycemia due to absorption of dextrose containing peritoneal instillate carrier solution (especially with oxaliplatin). This hyperglycemia may cause intravascular fluid shift and dilutional hyponatremia which is rapidly correctable with glycemic correction. The patient may develop lactic acidosis mainly due to increased glucose metabolism and anaerobic metabolism
- Chemotherapy agents may also lead to dyselectrolytemia. Cisplatin causes hypomagnesaemia which leads to cardiac arrhythmias while oxaliplatin causes lactic acidosis, hyperglycemia and hyponatremia. Electrolyte disturbances like calcium, potassium and magnesium should be replaced if required
- Frequent ABG, and electrolytes (sodium, potassium, calcium and magnesium) are desired to be measured to detect and manage the abnormalities early.
Table 2.
Common end organ toxicities of various chemotherapy drugs during Hyperthermic intraperitoneal chemotherapy
| Chemotherapy drug | Adverse effects |
|---|---|
| Cisplatin | Nephrotoxicity, peripheral neuropathy, myelotoxicity |
| Oxyplatin | Neurotoxicity (laryngeal/pharyngeal dysthesia), gastrointestinal bleeding |
| Doxorubicin | Cardiotoxicity (arrhythmia, cardiomyopathy), myelotoxicity |
| Mitomycin C | Nephrotoxicity, Pulmotoxicity, Myelosupression |
| Irinotecan | Myelotoxicity |
Postoperative/Critical Care Management
Management of Hemodynamic: Fluid loss during initial 72 hours after surgery may be as high as 4.1 litres per day due to oozing of protein-rich fluid from the raw surface area due to peritonectomy.[46] Most of the patients require vasopressors support in the early postoperative period to counteract the vasodilatation due to HIPEC. Postoperative intravenous fluid therapy should be guided by hemodynamic changes, urine output and losses from drains and nasogastric tube. Blood and blood products like fresh frozen plasma should be transfused depending on the drain output, hemoglobin and hematocrit value, and coagulation profile. The patient may continue to lose protein rich fluid in the exudates and it may lead to decrease in albumin levels in the postoperative period. So, one should supplement Albumin if it falls below 3.0 g/dl to maintain adequate intra vascular volume[47]
Coagulation profile should be monitored during the postoperative period. The patient may continue to have thrombocytopenia due to the cytotoxic drugs used and dilutional coagulopathy due to massive fluid shift and blood loss.[48] This coagulation dysfunction peaks at 24 to 48 h post surgery with normalization coagulation profile in 72 hr.[49] Fresh frozen plasma and platelet should be transfused to correct any documented coagulation abnormality only and not as routine prophylaxis. TEG is an important point of care monitor to identify the coagulation abnormalities and manage them
Electrolyte imbalance due to significant fluid shift during the surgery and immediate postoperative period is expected. Therefore they should be measured and replaced periodically
Analgesia: A multimodal analgesia with a combination of local anesthetics and opioids in thoracic epidural and intravenous NSAIDs and opioids provides excellent analgesia. This plays an important role in early ambulation, early extubation and decrease postoperative ileus[50]
Stress Ulcer Prophylaxis: These patients are at risk of stress ulcer due to need for mechanical ventilation, hypotension necessitating vasopressors, non-steroidal anti-inflammatory drugs and coagulopathy.[51] So, all patients should receive prophylactic H2 receptor antagonists or proton pump inhibitors after HIPEC
Thromboprophylaxis: Since patients are at risk of thrombosis, thromoboprophylaxis with mechanical devices like intermittent pneumatic compression and graduated compression stockings should be provided to prevent DVT.[52] Pharmacological agents like heparin/LMWH should be started when coagulation profile is normalized and bleeding risk is minimal
Feeding/Nutrition: Enteral nutrition is always better than parenteral nutrition. Early enteral feeding helps in bowel movement and reduces translocation of bacteria, thereby decreases infective complications in surgical patients. Parenteral nutrition need to be given if the patient is unable to take orally due postoperative ileus, stress ulcer or anastomotic leak
Respiratory support: These patients are hypoxic due to ascites, pleural effusion and atelectasis. Although respiratory parameters improve after CRS due to ascitic drainage, some patients require postoperative ventilation due to hemodynamic instability, diaphragmatic injury or multiple comorbidities preventing safe extubation
Infection control: The infective complications due to immunosuppression (neutopenia and leucopenia after chemotherapy) may be present and the intensivist should have a a low threshold to escalate to higher antibiotics if required.
Safety Concerns
At high temperature, aerosols and vapours are produced from a chemotherapy drug. The staff involved in HIPEC surgery is at risk of inhalation of these aerosols or may get direct contact with chemotherapy due to spillage. This contact and inhalation of chemotherapy may have a deleterious effect on the body and the staff should be educated about the handling of chemotherapy. Moreover high risk groups like pregnant women, nursing mother, history of abortions, those planning pregnancy, history of teratogenicity, those with prior chemotherapy or radiotherapy or immunosuppressive therapy, people working with radiotherapy and those with allergic reactions to latex/cytotoxic drugs or skin diseases should be excluded from the HIPEC team. A mechanism should be in place for proper disposal of chemotherapy drug containers and tubings of HIPEC equipment. The national institute of occupational safety and health has recommended that the containers for disposal of chemotherapeutic waste should be leak proof and the labels should clearly mention “cytotoxic agent”. They should be emptied when half full.[53]
Conclusion
Perioperative care of patients undergoing cytoreductive surgery and HIPEC procedure is complex and involves management of excessive fluid and protein losses, thermoregulation, coagulation disturbance and postoperative care. A higher PCI is associated with increased duration of surgery, increased blood loss, increased use of vasopressors, increased need of post operative ventilation and coagulation derangements. A successful outcome requires a team approach and continuity of care with a vigilant interaction of multiple disciplines. These patients need extensive prehabilitation and optimization to ensure uneventful smooth intraoperative course and early recovery and discharge.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sugarbaker PH. Surgical management of peritoneal carcinosis: Diagnosis, prevention and treatment. Langenbecks Arch Chir. 1988;373:189–96. doi: 10.1007/BF01274232. [DOI] [PubMed] [Google Scholar]
- 2.Neuwirth MG, Alexander HR, Karakousis GC. Then and now: Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. 2016;7:18–28. doi: 10.3978/j.issn.2078-6891.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González-Moreno S. Peritoneal surface oncology: A progress report. Eur J Surg Oncol. 2006;32:593–6. doi: 10.1016/j.ejso.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–60. [PubMed] [Google Scholar]
- 5.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lotti M. HIPEC and the necessary hyperthermia: Do we still need the open abdomen? Eur Rev Med Pharmacol Sci. 2017;21:4473–5. [PubMed] [Google Scholar]
- 7.Rubino MS, Abdel-Misih RZ, Bennett JJ, Petrelli NJ. Peritoneal surface malignancies and regional treatment: A review of the literature. Surg Oncol. 2012;21:87–94. doi: 10.1016/j.suronc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Flessner MF. The transport barrier in intraperitoneal therapy. Am J Physiol Renal Physiol. 2005;288:F433–42. doi: 10.1152/ajprenal.00313.2004. [DOI] [PubMed] [Google Scholar]
- 9.El-Kareh AW, Secomb TW. A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia. 2004;6:117–27. doi: 10.1593/neo.03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW, et al. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: Pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148–54. doi: 10.1016/s0959-8049(97)00370-5. [DOI] [PubMed] [Google Scholar]
- 11.Dudar TE, Jain RK. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 1984;44:605–12. [PubMed] [Google Scholar]
- 12.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–8. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 14.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390–408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Dubé P, Sideris L, Law C, Mack L, Haase E, Giacomantonio C, et al. Guidelines on the use of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal surface malignancy arising from colorectal or appendiceal neoplasms. Curr Oncol. 2015;22:e100–12. doi: 10.3747/co.22.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bree E, Tsiftsis DD. Principles of perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis. Recent Results Cancer Res. 2007;169:39–51. doi: 10.1007/978-3-540-30760-0_4. [DOI] [PubMed] [Google Scholar]
- 17.Sugarbaker PH. 4th ed. Grand Rapids, Michigan: Ludann Company; 2005. Technical Handbook for the Integration of Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy into the Surgical Management of Gastrointestinal and Gynecologic Malignancy. [Google Scholar]
- 18.Raspe C, Piso P, Wiesenack C, Bucher M. Anesthetic management in patients undergoing hyperthermic chemotherapy. Curr Opin Anaesthesiol. 2012;25:348–55. doi: 10.1097/ACO.0b013e32835347b2. [DOI] [PubMed] [Google Scholar]
- 19.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 20.Vashi PG, Gupta D, Lammersfeld CA, Braun DP, Popiel B, Misra S, et al. The relationship between baseline nutritional status with subsequent parenteral nutrition and clinical outcomes in cancer patients undergoing hyperthermic intraperitoneal chemotherapy. Nutr J. 2013;12:118. doi: 10.1186/1475-2891-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Shim SH, Oh IK, Yoon SH, Lee SJ, Kim SN, et al. Preoperative hypoalbuminemia is a risk factor for 30-day morbidity after gynecological malignancy surgery. Obstet Gynecol Sci. 2015;58:359–67. doi: 10.5468/ogs.2015.58.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheshadri DB, Chakravarthy MR. Anaesthetic considerations in the perioperative management of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Indian J Surg Oncol. 2016;7:236–43. doi: 10.1007/s13193-016-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esquivel J, Angulo F, Bland RK, Stephens AD, Sugarbaker PH. Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open “coliseum technique”. Ann Surg Oncol. 2000;7:296–300. doi: 10.1007/s10434-000-0296-2. [DOI] [PubMed] [Google Scholar]
- 24.Thong SY, Chia CS, Ng O, Tan G, Ong ET, Soo KC, et al. A review of 111 anaesthetic patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Singapore Med J. 2017;58:488–96. doi: 10.11622/smedj.2016078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt C, Creutzenberg M, Piso P, Hobbhahn J, Bucher M. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia. 2008;63:389–95. doi: 10.1111/j.1365-2044.2007.05380.x. [DOI] [PubMed] [Google Scholar]
- 26.Desgranges FP, Steghens A, Rosay H, Méeus P, Stoian A, Daunizeau AL, et al. Epidural analgesia for surgical treatment of peritoneal carcinomatosis: A risky technique? Ann Fr Anesth Reanim. 2012;31:53–9. doi: 10.1016/j.annfar.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Shime N, Lee M, Hatanaka T. Cardiovascular changes during continuous hyperthermic peritoneal perfusion. Anesth Analg. 1994;78:938–42. doi: 10.1213/00000539-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Mavroudis C, Alevizos L, Stamou KM, Vogiatzaki T, Eleftheriadis S, Korakianitis O, et al. Hemodynamic monitoring during heated intraoperative intraperitoneal chemotherapy using the FloTrac/Vigileo system. Int Surg. 2015;100:1033–9. doi: 10.9738/INTSURG-D-14-00138.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jozwiak M, Teboul JL, Monnet X. Extravascular lung water in critical care: Recent advances and clinical applications. Ann Intensive Care. 2015;5:38. doi: 10.1186/s13613-015-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–8. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 31.Raspé C, Flöther L, Schneider R, Bucher M, Piso P. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2017;43:1013–27. doi: 10.1016/j.ejso.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: A consensus statement. Society of surgical oncology. Ann Surg Oncol. 2007;14:128–33. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 33.Raue W, Tsilimparis N, Bloch A, Menenakos C, Hartmann J. Volume therapy and cardiocircular function during hyperthermic intraperitoneal chemotherapy. Eur Surg Res. 2009;43:365–72. doi: 10.1159/000248164. [DOI] [PubMed] [Google Scholar]
- 34.Colantonio L, Claroni C, Fabrizi L, Marcelli ME, Sofra M, Giannarelli D, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg. 2015;19:722–9. doi: 10.1007/s11605-015-2743-1. [DOI] [PubMed] [Google Scholar]
- 35.Hansen E, Knuechel R, Altmeppen J, Taeger K. Blood irradiation for intraoperative autotransfusion in cancer surgery: Demonstration of efficient elimination of contaminating tumor cells. Transfusion. 1999;39:608–15. doi: 10.1046/j.1537-2995.1999.39060608.x. [DOI] [PubMed] [Google Scholar]
- 36.Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: Systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ. 2015;350:h1354. doi: 10.1136/bmj.h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Almeida JP, Vincent JL, Galas FR, de Almeida EP, Fukushima JT, Osawa EA, et al. Transfusion requirements in surgical oncology patients: A prospective, randomized controlled trial. Anesthesiology. 2015;122:29–38. doi: 10.1097/ALN.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 38.Shander A. Financial and clinical outcomes associated with surgical bleeding complications. Surgery. 2007;142:S20–5. doi: 10.1016/j.surg.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Ribed-Sánchez B, González-Gaya C, Varea-Díaz S, Corbacho-Fabregat C, Pérez-Oteyza J, Belda-Iniesta C, et al. Economic analysis of the reduction of blood transfusions during surgical procedures while continuous hemoglobin monitoring is used. Sensors (Basel) 2018;18 doi: 10.3390/s18051367. pii: E1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;28:CD000567. doi: 10.1002/14651858.CD000567.pub6. [DOI] [PubMed] [Google Scholar]
- 41.Vorgias G, Iavazzo C, Mavromatis J, Leontara J, Katsoulis M, Kalinoglou N, et al. Determination of the necessary total protein substitution requirements in patients with advanced stage ovarian cancer and ascites, undergoing debulking surgery. Correlation with plasma proteins. Ann Surg Oncol. 2007;14:1919–23. doi: 10.1245/s10434-007-9404-x. [DOI] [PubMed] [Google Scholar]
- 42.Miao N, Pingpank JF, Alexander HR, Royal R, Steinberg SM, Quezado MM, et al. Cytoreductive surgery and continuous hyperthermic peritoneal perfusion in patients with mesothelioma and peritoneal carcinomatosis: Hemodynamic, metabolic, and anesthetic considerations. Ann Surg Oncol. 2009;16:334–44. doi: 10.1245/s10434-008-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owusu-Agyemang P, Arunkumar R, Green H, Hurst D, Landoski K, Hayes-Jordan A, et al. Anesthetic management and renal function in pediatric patients undergoing cytoreductive surgery with continuous hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin. Ann Surg Oncol. 2012;19:2652–6. doi: 10.1245/s10434-012-2319-1. [DOI] [PubMed] [Google Scholar]
- 44.De Somer F, Ceelen W, Delanghe J, De Smet D, Vanackere M, Pattyn P, et al. Severe hyponatremia, hyperglycemia, and hyperlactatemia are associated with intraoperative hyperthermic intraperitoneal chemoperfusion with oxaliplatin. Perit Dial Int. 2008;28:61–6. [PubMed] [Google Scholar]
- 45.Mehta AM, Van den Hoven JM, Rosing H, Hillebrand MJ, Nuijen B, Huitema AD, et al. Stability of oxaliplatin in chloride-containing carrier solutions used in hyperthermic intraperitoneal chemotherapy. Int J Pharm. 2015;479:23–7. doi: 10.1016/j.ijpharm.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 46.Cooksley TJ, Haji-Michael P. Post-operative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC) World J Surg Oncol. 2011;9:169. doi: 10.1186/1477-7819-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanakoudis F, Petrou A, Michaloudis D, Chortaria G, Konstantinidou A. Anaesthesia for intra-peritoneal perfusion of hyperthermic chemotherapy. Haemodynamic changes, oxygen consumption and delivery. Anaesthesia. 1996;51:1033–6. doi: 10.1111/j.1365-2044.1996.tb14998.x. [DOI] [PubMed] [Google Scholar]
- 48.Schols SE, Lancé MD, Feijge MA, Damoiseaux J, Marcus MA, Hamulyák K, et al. Impaired thrombin generation and fibrin clot formation in patients with dilutional coagulopathy during major surgery. Thromb Haemost. 2010;103:318–28. doi: 10.1160/TH09-06-0396. [DOI] [PubMed] [Google Scholar]
- 49.Arakelian E, Gunningberg L, Larsson J, Norlén K, Mahteme H. Factors influencing early postoperative recovery after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2011;37:897–903. doi: 10.1016/j.ejso.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Ali M, Winter DC, Hanly AM, O’Hagan C, Keaveny J, Broe P, et al. Prospective, randomized, controlled trial of thoracic epidural or patient-controlled opiate analgesia on perioperative quality of life. Br J Anaesth. 2010;104:292–7. doi: 10.1093/bja/aeq006. [DOI] [PubMed] [Google Scholar]
- 51.Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian critical care trials group. N Engl J Med. 1994;330:377–81. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 52.Kucher N, Tapson VF, Goldhaber SZ DVT FREE Steering Committee. Risk factors associated with symptomatic pulmonary embolism in a large cohort of deep vein thrombosis patients. Thromb Haemost. 2005;93:494–8. doi: 10.1160/TH04-09-0587. [DOI] [PubMed] [Google Scholar]
- 53.Cass Y, Musgrave CF. Guidelines for the safe handling of excreta contaminated by cytotoxic agents. Am J Hosp Pharm. 1992;49:1957–8. [PubMed] [Google Scholar]


