Abstract
Abstract. Although experimental data clearly confirm the existence of self‐renewing mammary stem cells, the characteristics of such progenitor cells have never been satisfactorily defined. Using a double immunofluorescence technique for simultaneous detection of the basal cytokeratin 5, the glandular cytokeratins 8/18 and the myoepithelial differentiation marker smooth muscle actin (SMA), we were able to demonstrate the presence of CK5+ cells in human adult breast epithelium. These cells have the potential to differentiate to either glandular (CK8/18+) or myoepithelial cells (SMA+) through intermediary cells (CK5+ and CK8/18+ or SMA+). We therefore proceeded on the assumption that the CK5+ cells are phenotypically and behaviourally progenitor (committed adult stem) cells of human breast epithelium. Furthermore, we furnish evidence that most of these progenitor cells are located in the luminal epithelium of the ductal lobular tree. Based on data obtained in extensive analyses of proliferative breast disease lesions, we have come to regard usual ductal hyperplasia as a progenitor cell‐derived lesion, whereas most breast cancers seem to evolve from differentiated glandular cells. Double immunofluorescence experiments provide a new tool to characterize phenotypically progenitor (adult stem) cells and their progenies. This model has been shown to be of great value for a better understanding not only of normal tissue regeneration but also of proliferative breast disease. Furthermore, this model provides a new tool for unravelling further the regulatory mechanisms that govern normal and pathological cell growth.
INTRODUCTION
It is generally assumed that adult breast epithelium consists of luminal glandular and basal myoepithelial cells. The majority of the myoepithelial cells express the basal cytokeratins (CKs) 5/14 and SMA (smooth muscle actin), whereas luminal cells are thought to be characterized by expression of CKs 8/18/19 (Taylor‐Papadimitriou et al. 1991) and oestrogen/progesterone receptors (Anderson & Clarke 1999). The epithelial tissue of the resting breast has to be classified as a dynamic, immature epithelium that is constantly being renewed during menstrual cycles. In order to safeguard the integrity of this epithelium, any loss of parenchymal cells must be compensated by cells of identical phenotype. This may be achieved by means of mitosis within a population of differentiated cells, or by de novo replacement via selective differentiation following mitotic proliferation of progenitor cells or partially differentiated cells. Although there are some conclusive experimental data providing indirect evidence of the function precursor cells may take in this process both in cells of animal and human breast epithelium (Joshi et al. 1986; Sonnenberg et al. 1986; Smith & Medina 1988; Smith & Medina 1988; Purkis et al. 1990; Taylor‐Papadimitriou et al. 1991; O’Connell et al. 1994; Kordon & Smith 1998; Péchoux et al. 2000; Lakhani & O’Hare 2001; Boecker et al. 2002; Deugnier et al. 2002a; Gudjonsson et al. 2002), the lack of specific cell markers has prevented any real progress being made in this field (Williams & Daniel 1983; Smith & Medina 1988).
Transplantation studies have shown that portions of the mouse mammary tree or even single cells implanted into cleared fat pads develop an entire new gland (Williams & Daniel 1983; Smith & Medina 1988; Kordon & Smith 1998). Those experiments have confirmed that a single epithelial cell can give rise to 1012–1013 multipotent progenitor cells (Hayflick & Moorhead 1961; Daniel & Young 1971), and ultimately grow to a complete ductal lobular tree. However, the question of where such progenitor cells are localized within the breast tissue and how they can be characterized has never been satisfactorily resolved.
Drawing upon studies performed on human breast tissue using CK5/14 immunohistochemistry we assumed that CK5/14+ cells might represent progenitor cells of the breast epithelium (Böcker et al. 1992a; Böcker et al. 1992b). With the advent of new technologies for simultaneous detection of several antigens within the same cell, we were able to elucidate further the role of those CK5/14+ cells and their exact relation to the differentiated glandular and myoepithelial phenotype. More specifically, it was our aim to verify our hypothesis and find out whether this phenotypically distinct CK5/14+ subpopulation really acquires precursor cell functions in human breast epithelium.
By using specific monoclonal antibodies (mAbs) to the basal CK5, the glandular cytokeratin subgroups CK8/18 and the myoepithelial differentiation marker smooth muscle actin (SMA), we examined resting adult female breast epithelium and compared its main features with those of benign proliferative breast lesions such as ductal hyperplasia, and noninvasive and invasive breast cancer.
The data obtained in this way strongly suggest that in normal breast epithelium CK5+ cells do indeed display the phenotypic and behavioural characteristics of progenitor cells for both the glandular and myoepithelial cell lineages. On a broader view, this method enables phenotypic characterization of breast epithelium progenitor cells and their glandular and myoepithelial progenies, and furnishes information as to the spatial distribution of progenitor cells within the ductal lobular tree. With the advent of such double‐staining techniques and the use of the three molecular markers, the mechanisms that pertubate the survival of progenitor cells and their commitment to either of the two cell lines of normal resting breast epithelium could be investigated more extensively. In addition, we will be able to describe qualitative differences in the expression patterns between usual ductal hyperplasia and ductal carcinoma in situ. The variance in the expression of molecular markers cannot be easily reconciled with the prevailing concept of a gradual evolution of invasive breast cancer from usual ductal hyperplasia to ductal carcinoma in situ and, further, to definitive invasive cancers. The new tools will also deepen our understanding of the processes underlying the pathological proliferation of breast epithelium.
DOUBLE IMMUNOFLUORESCENCE STAINING EXPERIMENTS
The human resting breast epithelium contains CK5+/– progenitor cells that differentiate to either glandular and myoepithelial cells
By using double‐labelling experiments, we identified CK5+ progenitor cells with the potential to differentiate into lineage‐specific glandular (CK5+/CK8/18+) or myoepithelial precursor cells (CK5+/SMA+) and, finally, to differentiated end cells (either CK8/18+ or SMA+) (Fig. 1). During the lineage‐specific differentiation process there is a gradual decrease of CK5 and a simultaneous increase in either CK8/18 in the glandular or SMA in the myoepithelial cell line. Transitions from CK5+ progenitor cells to SMA+ myoepithelial cells were not found.
Figure 1.
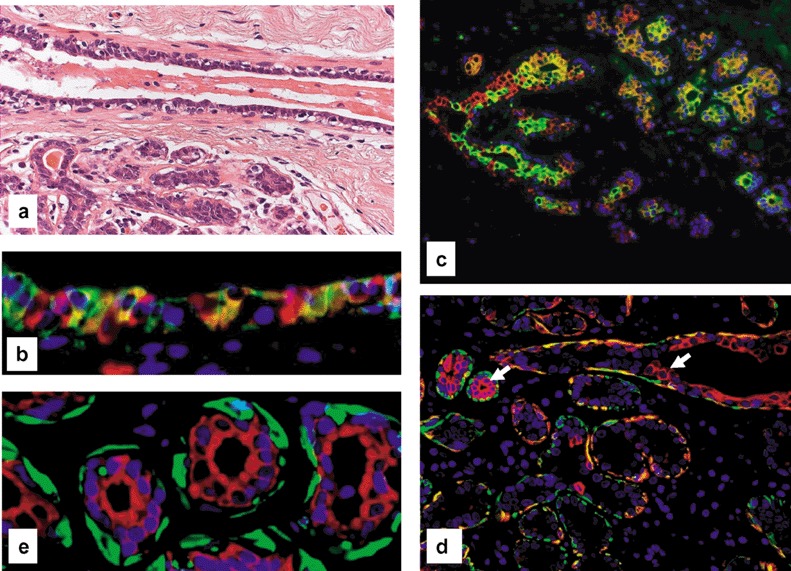
Normal breast epithelium. (a) Small duct and adjacent lobule. Note that the normal epithelium consists of a bilayer with luminal glandular and basal myoepithelial cells (haematoxylin and eosin). (b) Double‐fluorescence staining of the epithelium of a small duct for CK5 (FITC, green) and CK8/18/19 (Cy3, red) displaying few CK5+ progenitor cells (green signal), intermediary cells (hybrid signal) and CK8/18+ glandular cells (red signal). (c) Double‐fluorescence staining of the epithelium of two terminal ducts and adjacent lobules of a resting breast for CK5 (green signal for FITC) and CK8/18 (Cy3, red) revealing the same cellular components as seen in the small duct in (b), indicating a relatively immature glandular epithelium. (d) Double‐fluorescence staining of the epithelium of a small duct and adjacent lobule of a resting breast for CK5 (FITC, green) and SMA (Cy3, red). The arrow marks a progenitor cell expressing CK5 alone. Note that most cells in the outer layer represent intermediary myoepithelial cells expressing both CK5 and SMA (hybrid colour). The differentiated myoepithelial express only SMA (green signal). Note also that the inner epithelium of both duct and of two acini for CK5 (FITC, green) and SMA (Cy3, red) contain precursor cells. (e) Double‐fluorescence staining of the epithelium of lobular acini (red signal) Panels for SMA (FITC, green) and CK8/18 (Cy3, red) clearly showing a glandular and myoepithelial cell lineage. Note the absence of any transitional cells, indicating that there is no transdifferentiation between those two lineages.
Thus the following phenotypical subsets of cells of breast epithelium can be distinguished: (1) progenitor cells (only CK5+); (2) intermediary glandular cells (CK5+; CK8/18+); (1) glandular cells (only CK8/18+); (3) intermediary myoepithelial cells (CK5+; SMA+); and (4) myoepithelial end cells (only SMA+).
The CK5 epithelial progenitor cell resides mainly within the luminal cell population
CK5+ cells were consistently observed throughout the luminal layer of the breast epithelium of the complete ductal lobular tree of the resting breast. Most of the luminal cells represent intermediary glandular cells (CK5+/CK8/18+), and only a few are differentiated glandular cells (CK8/18+) (Fig. 1). In the resting breast epithelium, the number of cells in basal position that only express CK5 seems to be extremely small. The vast majority of basal cells are in transition to myoepithelial end cells, which implies that they immunostain for both SMA and CK5. The number of myoepithelial end cells (SMA+) varies from breast to breast and even within different lobules of the same breast, but is usually smaller than the number of intermediary myoepithelial cells.
The luminal compartment displays considerable and fundamental spatial differences between the ductal and lobular system
In the resting breast, the lobules usually display a wide spectrum of cells, from progenitor cells (CK5+), intermediary glandular (CK5+, CK8/18+) and glandular end cells (CK8/18+), which in many lobules appears to be similar to the cellular composition of the luminal cell lining of the ductal tree. On the other hand, there may be more ‘mature’ lobules with mainly differentiated CK8/18+ glandular cells (Fig. 2). Only under the effects of hormones of pregnancy/lactation does the lobular luminal epithelium become fully differentiated to lactating secretory end cells, whereas the luminal epithelium of the ductal tree, including the terminal ducts, remains a regenerative immature epithelium with progenitor cells and their progenies (Fig. 3).
Figure Figur 2.
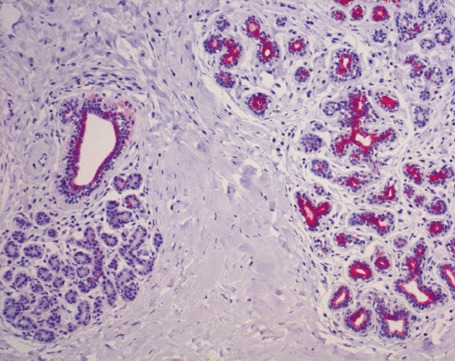
HRP immunohistochemical labelling of CK5 immunohistochemistry of a duct and several adjacent lobules of normal resting breast. Note that the inner epithelium of the duct and of the lobules on the right‐hand side stain intensively, indicating that this epithelium is immature and contains many putative CK5+ (precursor?) cells. In contrast, the lobule on the left is not stained at all. This epithelium has lost CK5.
Figure 3.
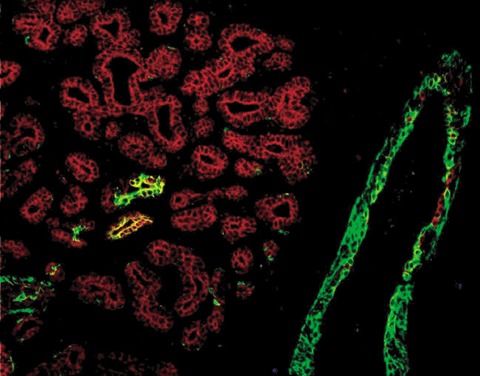
Lactating mammary‐gland epithelium compared with epithelium of normal resting breast. Double‐labelling for CK5 and CK8/18 of terminal ducts (ID) and adjacent lobules. The lobules contain differentiated CK8/18+ cells only, in contrast to the normal breast, as shown in Fig. 1(c).
Usual ductal hyperplasia represents a proliferation of CK5+/– progenitor cells, intermediary glandular (CK5+/–; CK8/18+/–) and differentiated glandular cells (CK8/18+/–)
In double‐fluorescence studies of all cases of usual ductal hyperplasia (UDH) (Fig. 4), we found a typical mosaic pattern of glandular cells in different stages of glandular differentiation. Some of the proliferating epithelial cells turned out to represent CK5+ progenitor cells that differentiate through intermediary transitional (CK5+, CK8/18+) to glandular end cells (only CK8/18+). Myoepithelial cells (SMA+) were not among the intraductally proliferating cells. The outer cell layer seems to contain the same cellular phenotypic elements of the myoepithelial cell lineage as seen in normal breast epithelium.
Figure 4.
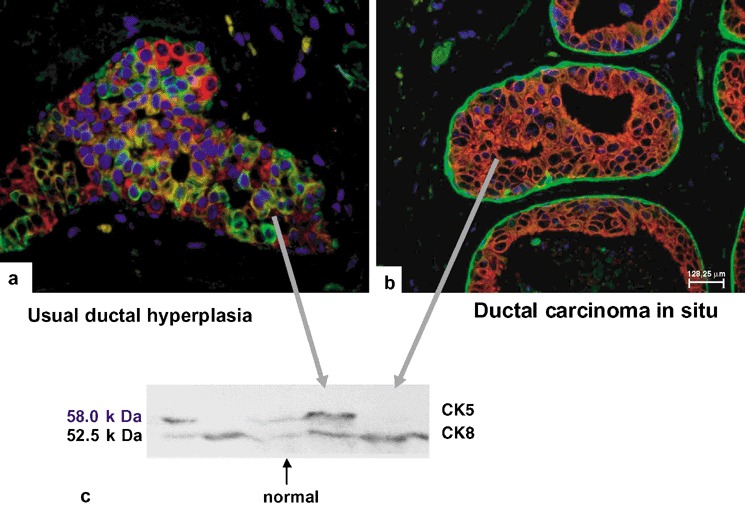
Usual ductal hyperplasia and ductal carcinoma in situ. Double‐fluorescence staining for CK5 (greenl) and CK8/18 (red). Note that UDH (a) contains the whole spectrum of cells, including CK5+ progenitor (green signal), intermediary glandular cell (hybrid signal) and differentiated glandular cells (red signal), whereas DCIS (b) displays a purely glandular phenotype (red). (c) Western blotting of breast tissue preparations and cultured cells. Lane 1, A‐431 cells (total cellular proteins); lane 2, A‐549 cells (total cellular proteins); lane 3, normal breast tissue (microdissected terminal ducts and lobular units); lane 4 and 5, usual ductal hyperplasia and DCIS (microdissected ductal lesions). Western blotting immunoreaction with anti‐CK5 and anti‐CK8. Note the appearance of CK5 and CK8 bands in preparations of normal breast epithelium and of UDH (lanes 3 and 4). The CK8 band occurs in preparations from DCIS only.
Breast cancer cells usually display a differentiated glandular epithelial phenotype
Sixteen of 17 IBCs displayed the same characteristic CK8/18+ and CK5− immunoprofile of the tumour cells. An identical pattern was observed in 15 of 16 cases of ductal carcinoma in situ (DCIS) of varying grades, and in three lobular neoplasias. In contrast to the findings in neoplastic cells, the outer cell layer displayed the same phenotypic composition as found in the normal breast epithelium. We observed only two cases in which the tumour cells coexpressed CK5 and CK8/18.
WESTERN‐BLOTTING EXPERIMENTS
To corroborate the double‐labelling immunofluorescence data, microdissected breast tissue samples were subjected to Western‐blotting analysis. Using mABs against CK5 and CK8, distinct signals were observed in normal breast tissue, such as a 58‐kDa band corresponding to the basal keratin CK5 (Fig. 4c, lane 3) and a similar signal on a 52.5‐kDa band corresponding to the glandular keratin CK8. In UDH the same two bands were identified. In DCIS, a strong signal was detected only for the CK8 band, whereas a CK5‐specifc band was visible as a faint signal, most probably corresponding to the non‐neoplastic basal layer (Fig. 4, lane 4). In control cells with established keratin composition, i.e. presence of CK5 and of CK8 in A‐431 cells and the presence of CK8 in A‐549 cells (Fig. 4c, lanes 1 and 2), we found the expected corresponding bands.
DISCUSSION
According to current theories on hierarchies of cell growth in different organs, it seems likely that several levels must be distinguished: a slowly cycling, self‐renewal stem‐cell pool, a larger pool of more mature transit‐amplifying cells capable of proliferation, and differentiated end cells that have lost their proliferation potential. Our current data suggest that the two differentiated cell types of breast epithelium are linked in a progenitor–progeny relationship to the CK5+ cells, and provide evidence that CK5+ cells of the human resting breast epithelium give rise to both the glandular and myoepithelial cell lineages. The results of double‐immunofluorescence experiments performed on specimens of human resting breast provide morphological evidence for the presence of a CK5+ cell population fulfilling the morphological requirements to be referred to as a common progenitor (adult stem) cell for the normal breast epithelium. This common precursor cell is able to differentiate to either glandular (CK8/18+) or myoepithelial cells (SMA+), respectively (Fig. 5) through intermediary cells. Over the course of the lineage‐specific differentiation process there is a gradual decrease in CK5, along with an increase in either CK8/18 in the glandular or SMA in the myoepithelial cell lineages. Differentiated cells were characterized by expression of either CK8/18 or SMA alone. Thus the acquisition of the differentiated phenotype of resting breast epithelium is inextricably linked to changes in the levels of basal cytokeratin 5. These data indicate that commitment to one lineage involves suppression of the alternative choice. When microdissected tissues were subjected to Western blotting analysis, the data were in accordance with previous results obtained by two‐dimensional gel electrophoresis, which showed that the keratin pattern of normal breast tissue comprises CK7, CK8, CK18 and CK19 in addition to CK5, CK14, CK15 and CK17, but not CK6 (Moll 1998). In view of the results obtained and the existing literature, we have come to the conclusion that CK14 and CK17 represent the major type I partners of CK5 (Cooper et al. 1985; Nagle et al. 1986). Recently, published data on gene expression in normal breast tissue and in breast cancer have also revealed patterns that are characteristic of the ‘basal’ and ‘luminal’ phenotypes (Perou et al. 2000). Such similarities further enhance the viability of our stem‐cell concept. With reference to these results, a new cell biological concept as depicted in the schematic drawing in Fig. 5 is proposed.
Figure 5.
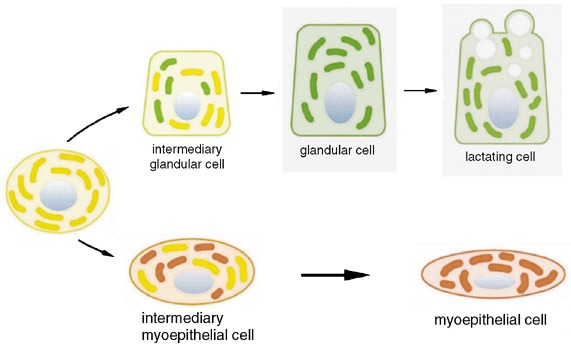
A new progenitor cell concept based on our fluorescence immunolabelling and Western blotting findings. CK5+ progenitor cells (yellow) give rise to both glandular cells (CK8/18+ green) and myoepithelial cells (SMA+ red) via intermediary cells that coexpress CK5 with the lineage‐specific marker (either CK8/18+ or SMA+).
While the existence of progenitor cells can be more or less taken for granted, questions as to their exact localization within the breast parenchyma have not yet been resolved. Apparently, most of the CK5+ cells reside in the luminal epithelium of the double‐layered breast epithelium (Fig. 6). Relatively small in number, they do not amount to more than 4% of all epithelial cells. Most of the luminal cells are intermediary glandular cells (CK5+/CK8/18+), interspersed with only a few differentiated glandular ones (CK8/18+). In view of these findings, we consider the luminal epithelium of the resting breast to consist of rather immature regenerative epithelium. The myoepithelial layer is usually composed of intermediary and differentiated myoepithelial end cells. In the resting breast epithelium, the number of cells in basal position that only express CK5 seems to be extremely small. The vast majority of basal cells are in transition to myoepithelial end cells, which is reflected by the fact that they immunostain for both SMA and CK5 (Fig. 1c).
Figure 6.
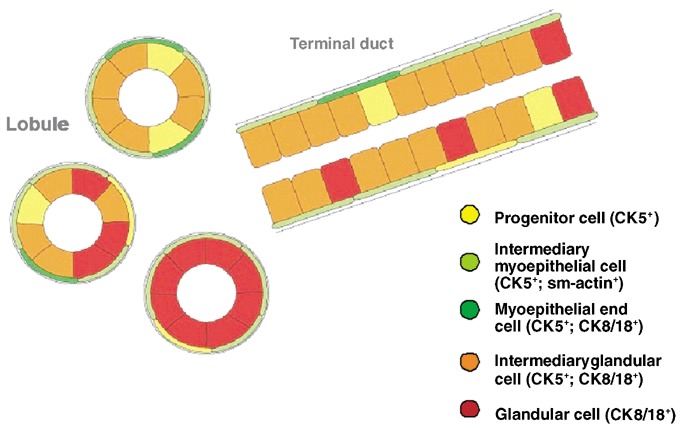
Architectural distribution of the cells of the new progenitor cell model in the normal resting breast epithelium.
Lineage marking can be used to show the functional differences of the luminal epithelium in lobules and ducts. The luminal compartment not only displays considerable cellular heterogeneity, but also fundamental differences in the spatial arrangement of the ductal and lobular systems, which can best be exemplified by comparing the resting with the lactating breast. In the former, the lobules usually display a wide spectrum of cells from progenitor cells (CK5+) to intermediary glandular (CK5+, CK8/18+) and glandular end cells (CK8/18+). In terms of cellular composition, the latter seem to be similar to the luminal cell lining of the ductal tree. In some cases apparently more ‘mature’ lobules mainly display differentiated CK8/18+ glandular cells. Under the effects of hormones of pregnancy/lactation, nearly all luminal cells differentiate into CK8/18+ secretory end cells that produce milk, while the progenitor cells with their progenies prevail in the terminal ducts. Our hypothesis is that the specific spatial organization of the glandular precursor cells in terminal ducts may play a decisive role in the replacement of the lactating cells in the lobules after weaning.
Thus the master plan of the elaborate terminal duct lobular units is phenotypically a two‐tiered organization of: (1) a regenerative area of ducts and especially terminal ducts, and (2) a functional area of lobules that only fulfil the tissue‐specific function during lactation. The concept of a progenitor (adult stem) cell therefore seems essential not only to the understanding of normal physiological regeneration but also of the cellular mechanisms of lactation and involution after weaning.
There are several other lines of evidence in favour of the notion that the CK5+ cell compartment contains the breast epithelial stem cell. Recently, Deugnier et al. demonstrated that a CK5+ and P‐cadherin+ mammary cell line that had been transplanted to a cleared mammary fat pad produced alveoli‐like structures containing CK5+ and CK8+ cells and other signs of differentiation (Deugnier et al. 2002b). At least in an experimental model, CK5+ cells thus harbour the capacity for self‐renewal. As, in our current understanding of stem cells, this capacity for self‐renewal is virtually inexhaustible, it is most likely that they will also express telomerase. In this context, Di Renzo and colleagues demonstrated such telomerase activity in an immortalized mammary epithelial cell line positive for CK5 and p63 (DiRenzo et al. 2002). The latter has been characterized as a ‘stem‐cell’ marker in keratinocytes, pointing again to a possible pre‐cursor or stem‐cell role of CK5+ cells. In both models, it could be shown that these cells seem to require a specific repertoire of growth factors and their respective receptors. The expression of the epidermal growth factor receptor (EGFR) in this cellular compartment in both studies was essential for their basic growth properties.
Although the number of CK5+ cells in breast epithelium is small, it seems rather unlikely that there is such a number of first‐order stem cells, because these are thought to reside within a niche or group of cells and an extracellular matrix capable of providing an optimal microenvironment. It is possible, and has to be proven, that such a stem‐cell niche of breast epithelium might consist of surrounding transit‐amplifying, differentiating glandular cells, myoepithelial cells and their surrounding environment, including the basement membrane and stromal cells with their usual humoral and physical activities. Such a microenvironment may regulate the stem cells and their proliferating and differentiating progenies via perturbating factors and epithelial–mesenchymal crosstalk (Brittan & Wright 2002). Given such a scenario, what might be the exact number, functional characteristics and localization of the first‐order stem cells? In view of the data of Diallo et al. (2001) and Tsai et al. (1996) on the clonal cellular content of terminal duct‐lobular units of the human breast epithelium and the terminal differentiation of pregnant/lactating breast epithelium, it is tempting to speculate that different TDLUs may have their own stem cells, and that such stem cells are located in the terminal ducts of TDLUs rather than in the lobules itself.
WHAT IS THE CONSEQUENCE OF THESE RESULTS FOR OUR UNDERSTANDING OF BREAST CARCAINOGENESIS?
UDH on the one and DCIS on the other hand represent different types of intraductal proliferations that appear to be caused by alterations of proliferative and differentiation capacities at different cellular levels, and are associated with an increased risk for invasive breast cancer. UDH as the paradigm for a benign intraductal proliferative lesion appears to be a CK5+ progenitor (adult stem) cell lesion with the same differentiation potential as seen in the glandular lineage of the normal breast. As the lesions clearly contain CK5+ progenitor cells, it seems likely that these cells are already committed to glandular differentiation and that the primary defect is at the glandular precursor level, in contrast to DCIS, which arise from differentiated CK8/18+ cells.
On the level of invasive breast, a large majority of cases (>95%) shows a purely glandular phenotype (CK5−/CK8/18+), with only a small proportion displaying a glandular precursor phenotype (CK5+/CK8/18+). Carcinomas with a pure stem‐cell phenotype (CK5+/CK8/18−/SMA−) and malignancies of myoepithelial type are exceptionally rare (Lakhani & O’Hare 2001). These data are in full agreement with cell‐culture studies showing that CK5 mRNA and protein is expressed in normal mammary epithelial cells, while it is absent from tumour‐derived cell lines (Trask et al. 1990). Furthermore, many breast cancers are oestrogen receptor‐positive, as are the differentiated Ck8/18+ glandular cells (Kelsey et al. 1993; Clarke et al. 1997; Elston & Ellis 1998; Shoker et al. 2000; Allred et al. 2001; Willett et al. 2001). Furthermore, several recent publications have demonstrated that invasive carcinomas with a CK5+ ‘basal’ phenotype can be distinguished by their RNA expression pattern (Perou et al. 2000) and are associated with a worse prognosis (Sorlie et al. 2001; van De Rijn et al. 2002). Interestingly, the vast majority of these tumours was associated with p53 mutations (Sorlie et al. 2001). Apart from the clinical implications, these tumours are also characterized by a clearly distinguishable ER‐negative, EGFR‐positive, highly proliferating immunophenotype and a distinct genotype (Korsching et al. 2002). According to these data, CK5+ breast cancers are a unique breast cancer subgroup, representing a distinct feature of poorly differentiated breast cancers in a previously proposedprogression model (2001a, 1999a, 1999b, 2001b). The different subgroups of invasive breast cancer in the context of the progenitor cell model therefore seem to arise from different cell populations within the normal female breast. Is is obvious that breast cancer cells therefore partially maintain and reflect the physiological state of their precursors and are not necessarily the final stage of a continuing de‐differentiation (Olsson 2001).
In conclusion, we present direct evidence that CK5‐positive cells display stem‐cell properties and are capable of forming glandular epithelial and myoepithelial end cells. This cell model opens a new field for investigation in that distinct cell subtypes can now be evaluated in terms of their potential to generate lineage‐specific end cells. We have begun to analyse the differential expression of factors that might perturb the different subsets of cells. If we were to understand the regulatory mechanisms that govern growth and differentiation (Siziopikou & Schnitt 2000) or even elimination of ‘superfluous’ cells in normal breast regeneration, we might be able to design strategies of chemo‐prevention to eradicate transformed cells at an early stage (Dooley et al. 2001) or even cells of noninvasive breast cancer.
ACKNOWLEDGEMENT
Grants were received from Deutsche Krebshilfe; 10–1681‐Bü‐I.
REFERENCES
- Anderson E, Clarke RB (1999) Epithelial stem cells in the mammary gland: casting light into dark corners. Breast Cancer Res. 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böcker WJ, Bier B, Freytag G, Brömmelkamp B, Jarasch E‐D, Edel G, Dockhorn‐Dworniczak B, Schmid KW (1992a) An immunohistochemical study of the breast using antibodies to basal and luminal keratins, alpha‐smooth muscle actin, vimentin, collagen IV and laminin. Part I: normal breast and benign proliferative lesions. Virchows Arch. A 421, 315. [DOI] [PubMed] [Google Scholar]
- Böcker WJ, Bier B, Freytag G, Brömmelkamp B, Jarasch E‐D, Edel G, Dockhorn‐Dworniczak B, Schmid KW (1992b) An immunohistochemical study of the breast using antibodies to basal and luminal keratins, alpha‐smooth muscle actin, vimentin, collagen IV and laminin. Part II: Epitheliosis and ductal carcinoma in situ . Virchows Arch. A 421, 323. [DOI] [PubMed] [Google Scholar]
- Boecker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, Buerger H, Wai D, Diallo RI, Brandt B, Herbst H, Schmidt A, Lerch MM, Buchwallow IB (2002) Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab. Invest. 82, 737. [DOI] [PubMed] [Google Scholar]
- Brittan M, Wright NA (2002) Gastrointestinal stem cells. J. Path. 197, 492. [DOI] [PubMed] [Google Scholar]
- Buerger H, Mommers EC, Littmann R, Simon R, Diallo R, Poremba C, Dockhorn‐Dworniczak B, Van Diest PJ, Boecker W (2001a) Ductal invasive G2 and G3 carcinomas of the breast are the end stages of at least two different lines of genetic evolution. J. Pathol. 194, 165. [DOI] [PubMed] [Google Scholar]
- Buerger H, Otterbach F, Simon R, Poremba C, Diallo R, Decker T, Riethdorf L, Brinkschmidt C, Dockhorn DB, Boecker W (1999a) Comparative genomic hybridization of ductal carcinoma in situ of the breast‐evidence of multiple genetic pathways. J. Pathol. 187, 396. [DOI] [PubMed] [Google Scholar]
- Buerger H, Otterbach F, Simon R, Schafer KL, Poremba C, Diallo R, Brinkschmidt C, Dockhorn DB, Boecker W (1999b) Different genetic pathways in the evolution of invasive breast cancer are associated with distinct morphological subtypes. J. Pathol. 189, 521. [DOI] [PubMed] [Google Scholar]
- Buerger H, Schmidt H, Beckmann A, Zanker KS, Boecker W, Brandt B (2001b) Genetic characterisation of invasive breast cancer: a comparison of CGH and PCR based multiplex microsatellite analysis. J. Clin. Pathol. 54, 836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 57, 4987. [PubMed] [Google Scholar]
- Cooper D, Schermer A, Sun T‐T (1985) Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications and limitations. Lab. Invest. 52, 243. [PubMed] [Google Scholar]
- Daniel CW, Young LJ (1971) Influence of cell division on an aging process. Life span of mouse mammary epithelium during serial propagation in vivo . Exp. Cell Res. 65, 27. [DOI] [PubMed] [Google Scholar]
- Deugnier MA, Faraldo MM, Janji B, Rousselle P, Thiery JP, Glukhova MA (2002b) EGF controls the in vivo developmental potential of a mammary epithelial cell line possessing progenitor properties. J. Cell Biol. 159, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deugnier MA, Teuliere J, Faraldo MM, Thiery JP, Glukhova MA (2002a) The importance of being a myoepithelial cell. Breast Cancer Res. 4, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo R, Schaefer KL, Poremba C, Shivazi N, Willmann V, Buerger H, Dockhorn‐Dworniczak B, Boecker W (2001) Monoclonality in normal epithelium and in hyperplastic and neoplastic lesions of the breast. J. Pathol. 193, 27. [DOI] [PubMed] [Google Scholar]
- DiRenzo J, Signoretti S, Nakamura N, Rivera‐Gonzalez R, Sellers W, Loda M, Brown M (2002) Growth factor requirements and basal phenotype of an immortalized mammary epithelial cell line. Cancer Res. 62, 89. [PubMed] [Google Scholar]
- Dooley WC, Ljung BM, Veronesi U, Cazzaniga M, Elledge RM, O'Shaughnessy JA, Kuerer HM, Hung DT, Khan SA, Phillips RF, Ganz PA, Euhus DM, Esserman LJ, Haffty BG, King BL, Kelley MC, Anderson MM, Schmit PJ, Clark RR, Kass FC, Anderson BO, Troyan SL, Arias RD, Quiring JN, Love SM, Page DL, King EB (2001) Ductal lavage for detection of cellular atypia in women at high risk for breast cancer. J. Natl. Cancer Inst. 93, 1624. [DOI] [PubMed] [Google Scholar]
- Elston CW, Ellis IO (1998) The Breast. Edinburgh: Harcourt Brace. [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov‐Jessen L, Bissell MJ (2002) Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 16, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585. [DOI] [PubMed] [Google Scholar]
- Joshi K, Smith JA, Perusinghe N, Monoghan P (1986) Cell proliferation in the human mammary epithelium. Differential contribution by epithelial and myoepithelial cells. Am. J. Pathol. 124, 199. [PMC free article] [PubMed] [Google Scholar]
- Kelsey JL, Gammon MD, John EM (1993) Reproductive factors and breast cancer. Epidemiol. Rev. 15, 36. [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921. [DOI] [PubMed] [Google Scholar]
- Korsching E, Packeisen J, Agelopoulos K, Eisenacher M, Voss R, Isola J, Van Diest PJ, Brandt B, Boecker W, Buerger H (2002) Cytogenetic alterations and cytokeratin expression patterns in breast cancer: integrating a new model of breast differentiation into cytogenetic pathways of breast carcinogenesis. Lab. Invest. 82, 1525. [DOI] [PubMed] [Google Scholar]
- Lakhani SR, O'Hare MJ (2001) The mammary myoepithelial cell ‐ Cinderella or ugly sister. Breast Cancer Res. 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R (1998) Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell. Biochem. 31, 205. [PubMed] [Google Scholar]
- Nagle RB, Bocker W, Davis JR, Heid HW, Kaufmann M, Lucas DO, Jarasch ED (1986) Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J. Histochem. Cytochem. 34, 869. [DOI] [PubMed] [Google Scholar]
- O'Connell P, Pekkel V, Fuqua S, Osborne CK, Allred DC (1994) Molecular genetic studies of early breast cancer evolution. Breast Cancer Res. Treat. 32, 5. [DOI] [PubMed] [Google Scholar]
- Olsson H (2001) A hypothesis about tumour development and the clinical features of hereditary breast cancers. Eur. J. Cancer 37, 2023. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, Van De Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen‐Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406, 747. [DOI] [PubMed] [Google Scholar]
- Purkis PE, Steel JB, MacKenzie IC, Nathrath WB, Leigh IM, Lane EB (1990) Antibody markers of basal cells in complex epithelia. J. Cell Sci. 97, 39. [DOI] [PubMed] [Google Scholar]
- Shoker BS, Jarvis C, Clarke RB, Anderson E, Munro C, Davies MP, Sibson DR, Sloane JP (2000) Abnormal regulation of the oestrogen receptor in benign breast lesions. J. Clin. Pathol. 53, 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siziopikou KP, Schnitt SJ (2000) MIB‐1 proliferation index in ductal carcinoma in situ of the breast: relationship to the expression of the apoptosis‐regulating proteins bcl‐2 and p53. Breast J. 6, 400. [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D (1988) A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J. Cell Sci. 90, 173. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Daamis H, Calafat J, Hilgers J (1986) In vitro differentiation and progression of mouse mammary tumor cells. Cancer Res. 46, 5913. [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, Van De RM, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein LP, Borresen‐Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor‐Papadimitriou J, Wetzels RHW, Ramaekers FC (1991) Intermediate filament protein expression in normal and malignant human mammary epithelial cells In: Dickson RB, Lippmann ME, eds. Genes, Oncogenes and Hormones: Advances in Cellular and Molecular Biology of Breast Cancer. Boston, MA: Kluwer, 355. [DOI] [PubMed] [Google Scholar]
- Trask DK, Band V, Zajchowski DA, Yaswen P, Suh T, Sager R (1990) Keratins as markers that distinguish normal and tumor‐derived mammary epithelial cells. Proc. Natl. Acad. Sci. USA 87, 2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Lu Y, Nichols PW, Zlotnikov G, Jones PA, Smith HS (1996) Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res. 56, 402. [PubMed] [Google Scholar]
- Van De Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Kochli OR, Mross F, Dieterich H, Seitz R, Ross D, Botstein D, Brown P (2002) Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am. J. Pathol. 161, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Rockhill B, Hankinson SE, Hunter DJ, Colditz GA (2001) Epidemiology and nongenetic causes of breast cancer In: Harris JR, Lippman ME, Morrow M, Kent Osborne C, eds. Diseases of the Breast. Philadelphia, PA: Lippincott Williams & Wilkins, 175. [Google Scholar]
- Williams JM, Daniel CW (1983) Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev. Biol. 97, 274. [DOI] [PubMed] [Google Scholar]


