Abstract
The progression of cells from G2 into mitosis is mainly controlled by formation of the cyclin B1/p34cdc2 complex. The behaviour of this complex in the irradiation‐induced G2 cell cycle delay is still unclear. A prior study demonstrated that the expression of the cyclin B1 protein is reduced by irradiation, and restored to control levels by the methylxanthine drug pentoxifylline, which is a potent G2 block abrogator. The present study shows that irradiation, and 2 mM pentoxifylline affect the expression of the cyclin‐dependent kinase p34cdc2 in HeLa cells. Irradiation induces p34cdc2 levels to increase and cyclin B1 levels to decrease. Addition of pentoxifylline at the G2 maximum reverses these trends. This is also evident from the cyclin B1/p34cdc2 ratios which decline after irradiation and are rapidly restored to control levels upon addition of pentoxifylline. It is concluded that cyclin B1 and p34cdc2 protein expression are important events and act in concert to control the irradiation induced G2 block. Analysis of cyclin B1 expression in whole cells and in isolated nuclei furthermore show that cyclin B1 is translocated from the nucleus into the cytoplasm when the G2 block is abrogated by pentoxifylline.
Introduction
G2 arrest is a response exibited by proliferating eukaryotic cells exposed to a variety of DNA damaging agents including X‐irradiation ( Weinert & Hartwell 1988), DNA alkylators ( Konopa 1988) and topoisomerase inhibitors ( Barlogie et al. 1976 ). It is presumed that G2 arrest facilitates DNA repair prior to mitosis. Exposure of irradiated cells to methylxanthines like caffeine and pentoxifylline induces mitosis before DNA repair is complete. This is thought to contribute to the enhanced cell killing observed when cells are irradiated in the presence of methylxanthines ( Lau & Pardee 1982). The biochemical mechanisms underlying the formation of the G2 block and its drug induced abrogation are not yet well understood.
Entry into mitosis is dependent on the activation of the maturation promoting factor (MPF) which contains a 34 kDa serine threonine cyclin‐dependent protein kinase, p34cdc2. Kinase activity is controlled by the formation of a complex between p34cdc2 and the mitotic cyclin B1 protein ( Murray 1998 ; Broek et al. 1991 ). Upon cyclin B1 binding, p34cdc2 becomes phosphorylated at threonine 161, which promotes affinity for cyclin B1, and at threonine 14 and tyrosine 15 by the Wee1 kinase, which inhibits kinase activity ( McGowan & Russel 1993). The subsequent dephosphorylation of threonine 14 and tyrosine 15 residues by the cdc25 phosphatases activates p34cdc2 kinase ( Murray 1992). The activation is also regulated by the subcellular location of the p34cdc2 complex ( Li, Meyer & Donoghue 1997). The cyclin B1/p34cdc2 complex, which is localized in the cytoplasm during interphase, is transported into the nucleus at the onset of mitosis ( Pines & Hunter 1991) and then phosphorylates nuclear substrates, e.g. the condensin complex ( Murray 1998)
It is clear that the formation and activation of the MPF complex is controlled at multiple levels. Recent investigations address the role of MPF in the formation of the G2 cell cycle arrest in greater detail. It is now generally thought that control of entry into mitosis involves the expression of cyclin B1 ( Muschel et al. 1991 ; Maity et al. 1996 ) and p34 cdc2 ( Lock & Ross 1990), the activation of the MPF complex by dephosphorylation ( McGowan & Russel 1993) and subcellular translocation ( Li et al. 1997 ).
Irradiation depresses cyclin B1 expression ( Bernhard et al. 1994a ; Kao et al. 1997 ; Hwang & Muschel 1998). Data from the authors' laboratory show that the addition of pentoxifylline to G2 blocked cells induces cyclin B1 expression ( Theron & Böhm 1998). Other authors have shown similar results in synchronized cells for caffeine and staurosporine ( Bernhard, McKenna & Muschel 1994b). The aim of this study was to evaluate the role of p34cdc2 protein expression in the radiation induced G2 arrest, and the influence of the G2 abrogator pentoxifylline. The importance of using asynchronous cell populations in this type of study has been previously emphasized ( Theron & Böhm 1998), in order to avoid the unscheduled expression of cyclins ( Gong, Traganos & Darzynkiewics 1995). p34cdc2 expression has previously been measured by flow cytometry in polyploid cells ( Baroja et al. 1996 ). To the authors' knowledge this aspect has not been investigated flow cytometrically in response to DNA damage and G2 block abrogation. Some authors suggest that the levels of p34cdc2 remain constant throughout the cell cycle and also after irradiation ( Lock & Ross 1990; Lock 1992; McGowan & Russel 1993). The present flow cytometric results in HeLa cells allow the correlation of p34cdc2 expression directly with cell cycle stage and showed a higher percentage of p34cdc2 expression in the G1 and G2 phases, with an increase in p34cdc2 expression in G2 during the radiation induced G2 block.
The measurement of cyclin B1 expression has previously lead to confusing reports on changes in protein expression. This can be attributed to the sharp rise in the numbers of G2 cells during block formation which obscures the change in the relative proportions of cyclin B1 to G2 cells. To avoid this pitfall, cyclin B1 expression was expressed as a ratio of cyclin B1 to the fraction of cells in G2 ( Theron & Böhm 1998). Accordingly, p34cdc2 expression was measured by calculating p34cdc2/G2 ratios.
The activity of the maturation promoting factor also strongly relies on the subcellular location after the binding of the cyclin B1 and p34cdc2 constituents ( Moore et al. 1999 ). It has been suggested that the cyclin B1/p34cdc2 complex is localized in the cytoplasm during interphase and transported into the nucleus at the onset of mitosis ( Pines & Hunter 1991; Tassan et al. 1994 ). This was confirmed by using a fusion protein between cyclin B1 and green fluorescent protein to trace the movement of MPF in subcellular components ( Hagting et al. 1998 ). Cyclin B1 has been reported to appear in the cytoplasm during late S phase, and then moves to the perinuclear region and during G2 enters the nucleus, where it is concentrated during mitosis ( Kakino et al. 1996 ). A unique cytoplasmic pool of cyclin B1 has also been documented for actively dividing cells, which rapidly increases during S phase and G2 phase and is then translocated to the nucleus during early prophase where it forms a complex with the nuclear subset of p34cdc2 ( David‐Pfeuty & Novain‐Dooghe 1996). It is now generally thought that the inactive cyclin B1/p34 complex indeed shuttles between the nucleus and cytoplasm in human cells ( Pines 1999).
This study used asynchronous HeLa cells and flow cytometry to examine the expression of p34cdc2 and the subcellular location of the MPF complex by analysing cyclin B1 expression in isolated nuclei and in whole cells after exposure to ionizing irradiation and after G2 block abrogation. The results clarify molecular events which contribute to the formation of the radiation induced G2 cell cycle delay and the mechanism of action of pentoxifylline as a G2 block abrogator.
MATERIALS and METHODS
Cell culture
Asynchronous populations of HeLa cervical carcinoma cells were plated in McCoy's medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml) and 10% fetal bovine serum in 75 cm2 culture flasks and cultured at 37°C in 95% O2/5% CO2. Samples in exponential phase were irradiated with 7 Gy of 60Co and allowed to grow for 10 h, which is the time needed for maximum G2 block formation in HeLa cells. Pentoxifylline was added to specific flasks to a final concentration of 2 mM. At different time intervals thereafter, ranging from 2 to 25 h, the pentoxifylline treated and control samples were trypsinized, fixed in 70% ethanol and stored at −20^C.
Determination of G2 block
Time of maximum cell cycle block in G2 was determined by irradiating HeLa cells with 7 Gy of 60Co, and sampling at 2‐h intervals for up to 25 h. The cells were fixed in 70% ethanol and stored overnight at ‐20^C. After washing in phosphate buffered saline (PBS), cells were resuspended in PBS containing 10 μg propidium iodide (PI; Sigma, St Louis, MI, USA) and 0.1% RNase A. Samples were incubated at 37^C for 20 min prior to flow cytometric analysis.
Immunocytochemistry
Fixed cells were prepared for multiparameter flow cytometry to simultaneously measure total DNA content and cyclin B1 ( Gong et al. 1994 ) or p34cdc2 expression ( Baroja et al. 1996 ) as previously described.
Briefly, the cells were washed in PBS and treated with 0.25% Triton X‐100 for 5 min on ice. After another wash in 5 ml PBS, the cell suspension of 5 × 105 cells/100 μl was incubated overnight at 4^C in a 1:400 dilution of mouse monoclonal anti‐cyclin B1 antibody (Pharmingen Clone GNS‐1, San Diego, CA, USA), or a purified monoclonal anti‐p34cdc2 antibody (clone HCDC1, ICN Biochemicals) in PBS containing 1% bovine serum albumin (BSA). The next morning, cells were washed in PBS and incubated for 30 min at room temperature in a 1:40 dilution of fluorescein isothiocyanate (FITC)‐conjugated goat anti‐mouse IgG antibody (Sigma) in PBS/1% BSA. The cells were washed again, resuspended in 10 μg/ml PI and 0.1% RNase A in PBS, and incubated for 20 min at room temperature prior to analysis. Negative controls were prepared in a similar way, except that an isotype‐specific antibody, mouse IgG (Sigma), was used instead of the cyclin B1 or anti‐p34cdc2 antibody.
Flow cytometry
To determine cyclin B1 or p34cdc2 levels, samples were analysed on a Becton‐Dickinson (San Jose, CA, USA) FACScan flow cytometer. Fluorescence data from 10 000 events were collected, stored and analysed using Lysis II software. To determine the time of maximum G2 block, samples were analysed for red (PI) fluorescence which was displayed as a DNA histogram. Markers placed at the G2 boundaries served to estimate the G2 content for each time point.
Cyclin B1 or p34cdc2 expression, and DNA data, were displayed in dot plots of red (PI) vs. green (FITC) fluorescence representing total cellular DNA content and cyclin B1 or p34cdc2 expression, respectively. Cell doublets were gated out by using the doublet discrimination module. Determination of the G2 content was as described above. The fraction of cells expressing either cyclin B1 or p34cdc2 was determined by gating only cells that displayed a positive green (FITC) fluorescence. The threshold for FITC positive cells was defined using the gate window set on the negative control sample which was prepared with the isotype‐specific antibody IgG1, on all the treated samples. All experiments were repeated at least twice and generated identical trends.
Definition of cyclin B1/G2, p34cdc2/G2 and cyclin B1/p34cdc2 ratios
The cyclin B1/G2 and p34cdc2/G2 ratios were calculated by comparing the fraction of cells expressing either cyclin B1 or p34cdc2 to the fraction of cells in the G2 phase of the cell cycle for each post‐irradiation time point. Cyclin B1/p34cdc2 ratios were obtained by dividing the B1/G2 ratios over the p34cdc2/G2 ratios.
Isolation of HeLa nuclei
Nuclei were obtained as described ( Heussen et al. 1987 ). Briefly, cells from exponentially growing cultures were washed once in ‘lysis’ buffer consisting of 10 mM Tris–HCl, 10 mM NaCl, 5 mM MgCl2, pH 7.4. Cells were then resuspended in ice‐cold lysis buffer at 1–3 × 106 cells/ml and allowed to swell for about 15–20 min on ice. Lysis of cells was completed by the dropwise addition of 10% (v/v) nonidet‐P40 (Shell Chemical Co.) in lysis buffer to a final concentration of 0.5% (v/v). During this step, the sample was mixed vigorously on a vortex. Released nuclei were sedimented at 300 g for 5 min in a swing bucket rotor and resuspended gently by stepwise addition of small volumes of PBS, pH 7.4, with gentle mixing on a vortex, essential to prevent clumping. The isolation of nuclei was confirmed by microscopy, and the sample was then subjected to the normal staining procedure for cyclin B1.
Results
In HeLa cells, the radiation‐induced G2 cell cycle block reaches a maximum at 10–12 h after a single dose of 7 Gy ( Fig. 1). The DNA histogram shows estimates of normal cell cycle fractions of 53% and 30% in G1 and G2, respectively. These values change to 30% and 62% for G1 and G2, respectively, at 10–12 h post‐irradiation. Pentoxifylline was added at this estimated time for maximum G2 block expression. From the dotplot of red (PI) vs. green (FITC) fluorescence it is evident that, unlike cyclin B1 ( Theron & Böhm 1998), p34cdc2 is not limited to the G2 phase of the cell cycle, but is also expressed in G1 phase cells ( Fig. 2a). However, it is predominantly expressed in the G2 phase during G2 arrest ( Fig. 2b).
Figure 1(R).
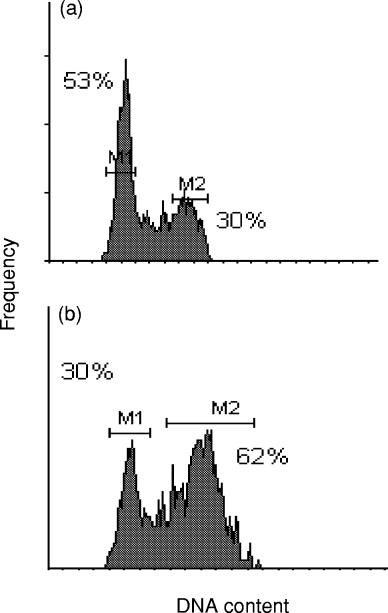
elationship between Ki‐67/MIB‐1 and FCM‐S‐values on a series of 330 breast cancers. Figure 2. Relationship between FCM‐S and [3H]dT LI values on a series of 330 breast cancers. Figure 3. Relationship between Ki‐67/MIB‐1 and [3H]dT LI values on a series of 330 breast cancers.
Figure 2(D).
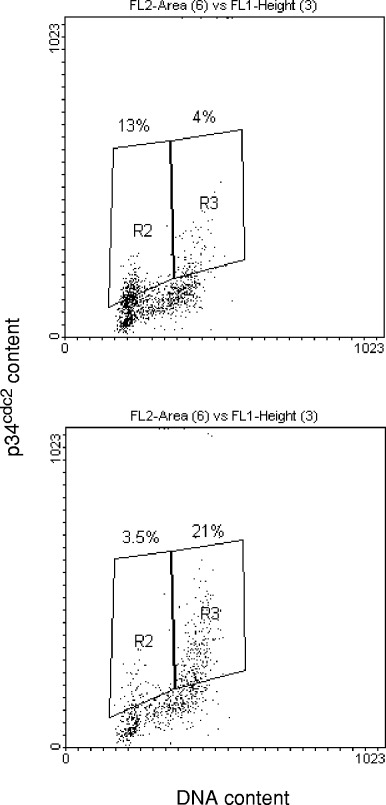
ot plots of green fluorescence (FITC) on the vertical scale vs. red fluorescence (PI) on the horizontal scale, representing p34cdc2 content and total DNA content, respectively. Percentages of cells expressing p34cdc2 are indicated for HeLa cells in the G1 and G2 phases, before (a) and 12 h after irradiation (b).
Figure 3(a) shows the increase in G2 fraction during radiation induced G2 arrest and the abrogation of the G2 block when pentoxifylline is added at the time of maximum G2 block. Similarly the expression of p34cdc2 increases after irradiation and drops after pentoxifylline treatment ( Fig. 3b). When the ratios of p34cdc2 to G2 fraction are plotted over time, an overexpression of p34cdc2 during G2 arrest becomes evident. Pentoxifylline treatment reduces the p34cdc2 expression to the control level of 0.1 ( Fig. 3c). Figure 4 shows the cyclin B1/G2 ratios for similar dose and time points in HeLa. Since cyclin B1 and p34cdc2 act in concert, the cyclin B1/p34cdc2 ratio was also plotted (see definition in Materials and methods section). Figure 5 shows that the G2 block maximum is associated with a very low cyclin B1/p34cdc2 ratio which gradually increases as the cells recover from the G2 block and enter mitosis after 40 h. Pentoxifylline added at the maximum G2 block rapidly restores the cyclin B1/p34cdc2 ratio over a very narrow time window, which reaches the control value after ∼7 h. Abrogation of the G2 block thus is associated with a rapid restoration of the critical cyclin B1/p34cdc2 ratio.
Figure 3(a).
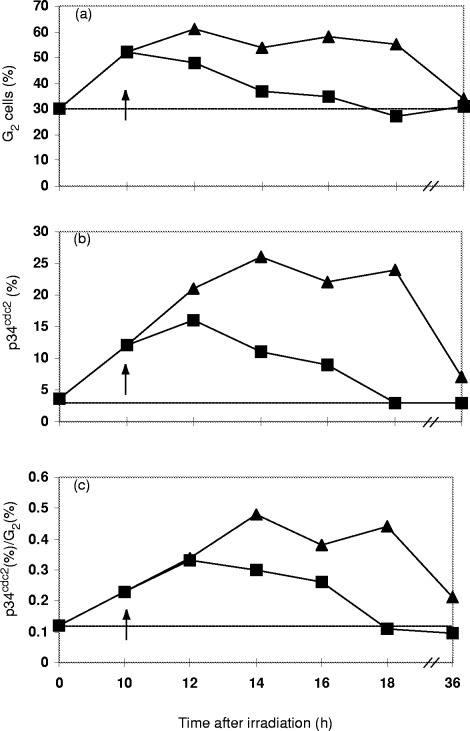
Increase of the G2 fraction during expression of the G2 block and decrease as the block resolves gradually over 36 h (∼). Decline of the G2 fraction over 6–8 h in response to addition of 2 mM pentoxifylline (&). (b) Increase of p34cdc2 expression during expression of G2 block (∼). Rapid decline of p34 after addition of 2 mM pentoxifylline within 8 h (&). (c) P34cdc2/G2 ratios plotted over 36 h after irradiation. Control cells (irradiation only) showing that restoration normally requires over 36 h (∼). Addition of 2 mM pentoxifylline and decline of ratio (&). Dotted lines show control levels of G2, p34cdc2 and G2/p34cdc2 in (a), (b) and (c), respectively.
Figure 4(C).
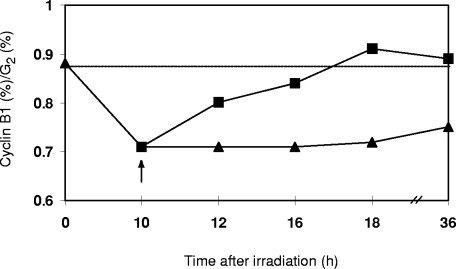
yclin B1/G2 ratios plotted over 36 h after irradiation. Decline and recovery of ratio as the block resolves (∼). Addition of 2 mM pentoxifylline at 10 h post‐irradiation results in rapid recovery of ratio within 6–8 h (&). Dotted line shows control ratio of cyclin B1/G2 in an unperturbed cell cycle.
Figure 5(C).
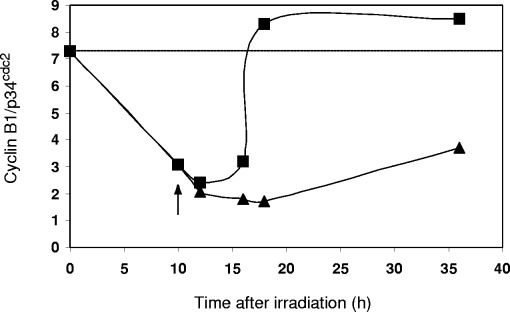
yclin B1/p34cdc2 ratios plotted over 36 h after irradiation. Decline and recovery of ratio after irradiation (∼). Abrogation of G2 block by 2 mM pentoxifylline and rapid restoration of ratio (&). Dotted line shows control ratio of cyclin B1: p34cdc2 in an unperturbed cell cycle.
In order to study the influence of pentoxifylline on subcellular translocation of the cyclin/cdk complex, cyclin B1 expression was compared in isolated nuclei and whole cells. Addition of pentoxifylline to whole cells at the time of maximum G2 block results in a rapid restoration of the control cyclin B1/G2 ratio within ∼7 h which then remains at control levels for up to 35 h. In the absence of pentoxifylline, these ratios were not fully restored within 35 h ( Fig. 6a). In nuclei ( Fig. 6b), the G2 block abrogation by pentoxifylline induces a sharp drop in cyclin B1 expression after 18 h. This suggests that cyclin B1 crosses the nuclear membrane after G2 block abrogation and re‐enters the cytoplasm where it is probably degraded by ubiquitin‐mediated proteolysis ( Pines 1999). Identical data trends were observed in A549 human lung carcinoma cells (not shown).
Figure 6(C).
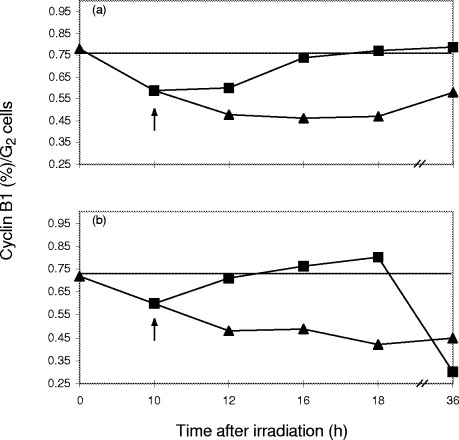
yclin B1/G2 ratios plotted over 36 h post‐irradiation for whole HeLa cells (a) and isolated HeLa nuclei (b). Recovery of G2 block in whole cells (a) and nuclei (b) after irradiation (∼). Effect of 2 mM pentoxifylline in whole cells (a) and nuclei (b) on cyclin B1/G2 ratio (&). The sharp drop in nuclear cyclin B1 between 18 and 36 h after irradiation suggests nuclear export. Dotted line shows ontrol ratio of cyclin B1: G2 in an unperturbed cell cycle.
Discussion
These results show that irradiation‐induced DNA damage has a profound influence on the expression of cyclin B1 and p34cdc2. These are constituents of the MPF complex and thus control the G2/M transition. A prior study demonstrated that cyclin B1 expression declines after exposure of HeLa cells to ionizing irradiation and that the cyclin levels are restored to control levels as a result of the G2 block abrogation by pentoxifylline ( Theron & Böhm 1998). In the present study, p34cdc2 and cyclin B1 expression were measured under identical conditions. The rise in p34cdc2 expression ( Fig. 3b) is in agreement with results from Smeets, Mooren & Begg (1994) who reported an increase of the hyperphosphorylated inactive form of p34cdc2 based on Western blots. Since G2 block formation is associated with an increase in the number of G2 cells and the level of p34cdc2 ( Fig. 3a,b), it was decided to use the p34/G2 ratios to assess changes of p34cdc2 expression relative to the number of cells in G2 ( Fig. 3c). G2, p34cdc2 and the ratio of p34cdc2/G2 show a maximum at 10–14 h. Addition of pentoxifylline rapidly restores the p34cdc2/G2 ratio to the control values within 8 h ( Fig. 3a,b,c). It therefore can be safely concluded that p34cdc2 is upregulated in response to DNA damage. It is possible that this upregulation results in the expression of a hyperphosphorylated, inactive form for the duration of the G2 block ( Smeets et al. 1994 ).
It appears that the existence of two MPF components enhances the sensitivity of the complex to small changes of the concentration of either constituent. This would result in an ‘all‐or‐nothing’ reaction resembling a switch. The required changes in substrate concentrations do not follow the normal Michaelis–Menten enzyme kinetics but are characterised by an ultrasensitive response ( Ferrel & Machleder 1998; Koshland 1998). In ultrasensitive kinetics, the maximum velocity is achieved by a 1–4‐fold change in substrate concentration, rather than the 80‐fold change observed in normal Michaelis–Menten kinetics ( Koshland 1998). The switch‐like response has previously been attributed to the translocation efficiency of the cyclin/cdk complex ( Ferrel 1998). Our data suggest that changes in the concentration of cyclin B1 and p34cdc2 indeed act in concert and like an ultrasensitive switch ( Fig. 5). Translocation of the complex accross the nuclear membrane would further ensure rapid control of mitotic entry. The change of the cyclin B1/p34cdc2 ratios closely resembles other ultrasensitive reactions, e.g. the cooperativity of O2 binding by haemoglobin ( Koshland 1998). Our experiments only assess the expression of the required amounts of cyclin B1 and p34cdc2 and do not address the additional activation expected from MPF phosphorylation and dephosphorylation. We suggest that the restoration of the control ratio of cyclin B1/p34cdc2 expression by pentoxifylline forms a crucial part of the mechanism of action of this G2 block abrogator.
When we assessed the influence of block abrogation on the subcellular location of cyclin B1 no significant differences were seen between cyclin B1 expression in whole cells and in nuclei, although overall levels seem consistently higher in whole cells. A sharp increase in cyclin B1 expression occurs in nuclei during G2 block abrogation by pentoxifylline. Subsequently (after ∼8 h), the B1 levels in nuclei falls sharply to even below the levels of irradiated control cells. This is most likely followed by ubiquitin‐mediated proteolysis of the cyclin B1 required for exit from mitosis ( King et al. 1996 ). It thus appears that pentoxifylline prompts mitotic entry and also exit from mitosis. The premature entry into mitosis could be the explanation for the enhanced cell kill observed when methylxanthines are combined with irradiation or chemotherapeutic drugs ( O'Connor 1996; Husain et al. 1998 ; Li et al. 1998 ). In p53 mutant cells, which cannot invoke a G1 block, a second challenge given at the G2 maximum results in even greater dose enhancement ( Wang et al. 1996 ; Binder, Serafin & Böhm 1999).
These data suggest that G2 block abrogation by pentoxifylline does not result in cell cycle perturbations and the uncoupling of mitosis, but in the resumption of normal cell cycle progression. These results are consistent with the hypothesis that the cell cycle is regulated in dimensions of both time (the timely synthesis and proteolysis of regulatory proteins) and space (localizing these regulators to the correct site) and that methylxanthines like pentoxifylline act at both levels to disrupt the G2 cell cycle delay. The manipulation of cell cycle checkpoints remain an important topic for strategies in anti‐cancer therapy.
Acknowledgements
Grants from the Federation of Research Development (FRD), the Cancer Association of South Africa (CANSA) and the Volkswagen Foundation to LB are gratefully acknowledged.
References
- Barlogie, B , Drewinko, B , Johnston, DA , Freireich, EJ. 1976. The effect of Adriamycin on the cell cycle traverse of a human lymphoid cell line. Cancer Res., 36, 1975. [PubMed] [Google Scholar]
- Baroja, A , De La Hoz, C , Alvarez, A , Ispizua, A , Bilbao, J , De Gandarias, JM. 1996. Genesis and evolution of high‐ploidy tumour cells evaluated by means of the proliferation markers p34cdc2, cyclin B1, PCNA and 3[H]‐thymidine . Cell Prolif., 29, 89. [PubMed] [Google Scholar]
- Bernhard, EJ , Maity, A , Muschel, RJ , McKenna, WG. 1994a. Increased expression of cyclin B1 mRNA coincides with diminished G2‐phase arrest in irradiated HeLa cells treated with staurosporine or caffeine . Rad. Res., 140, 393. [PubMed] [Google Scholar]
- Bernhard, EJ , McKenna, WG , Muschel, R. 1994b. Cyclin expression and G2‐phase delay after irradiation . Rad. Res., 138, S64. [PubMed] [Google Scholar]
- Binder, AB , Serafin, AM , Böhm, LJF. 1999. G2/M block abrogation drastically enhances the cytotoxicity of melphalan, daunorubicin and cisplatin in p53 mutant human tumour cells. Rad. Res. 2000; submitted for publication. [DOI] [PubMed]
- Broek, D , Bartlett, R , Crawford, K , Nurse, P. 1991. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature, 349, 388. [DOI] [PubMed] [Google Scholar]
- David‐Pfeuty, T & Novain‐Dooghe, Y. 1996. Human cyclin B1 is targeted to the nucleus in G1 phase prior to its accumulation in the cytoplasm. Oncogene, 13, 1447. [PubMed] [Google Scholar]
- Ferrel, J Jr 1998. How regulated protein translocation can produce switch‐like responses. TIBS, 23, 461. [DOI] [PubMed] [Google Scholar]
- Ferrel, JE Jr & Machleder, EM. 1998. The biochemical basis of an all‐or‐none cell fate switch in Xenopus oocytes. Science, 280, 985. [DOI] [PubMed] [Google Scholar]
- Gong, J , Li, X , Traganos, F , Darzynkiewics, Z. 1994. Expression of G1 and G2 cyclins measured in individual cells by multiparameter flow cytometry: a new tool in the analysis of the cell cycle. Cell Prolif., 27, 357. [Google Scholar]
- Gong, J , Traganos, F , Darzynkiewics, Z. 1995. Growth imbalance and altered expression of cyclins B1, A, E and D3 in MOLT‐4 cells synchronised in the cell cycle by inhibitors of DNA replication. Cell Growth Differentiation, 6, 1458. [PubMed] [Google Scholar]
- Hagting, A , Karlsson, C , Clute, P , Jackman, M , Pines, J. 1998. MPF localization is controlled by nuclear export. EMBO J., 17 (14), 4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussen, C , Nackerdien, Z , Smit, BJ , Böhm, L. 1987. Irradiation damage in chromatin isolated from V‐79 Chinese hamster lung fibroblasts. Rad. Res., 110, 84. [PubMed] [Google Scholar]
- Husain, A , Rosales, N , Schwartz, GK , Spriggs, DR. 1998. Lisofylline sensitizes p53 mutant human ovarian carcinoma cells to the cytotoxic effects of cis‐diamminedichloroplatinum. Gynec. Oncol., 70, 17. [DOI] [PubMed] [Google Scholar]
- Hwang, A & Muschel, R 1998. Radiation and the G2 phase of the cell cycle. Rad. Res., 150 (Suppl.), S52. [PubMed] [Google Scholar]
- Kakino, S , Sasaki, K , Kurose, A , Ito, H. 1996. Intracellular localization of cyclin B1 during the cell cycle in glioma cells. Cytometry, 24, 49. [DOI] [PubMed] [Google Scholar]
- Kao, GD , McKenna, WG , Maity, A , Blank, K , Muschel, RJ. 1997. Cyclin B1 availability is a rate limiting component of the radiation induced G2 delay in HeLa cells. Cancer Res., 57, 753. [PubMed] [Google Scholar]
- King, RW , Deshaies, RJ , Peters, JM , Kirschner, MW. 1996. How proteolysis drives the cell cycle. Science, 274, 1652. [DOI] [PubMed] [Google Scholar]
- Konopa, J. 1988. G2 block induced by DNA cross‐linking agents and its possible consequences. Biochem. Pharmacol., 37, 2303. [DOI] [PubMed] [Google Scholar]
- Koshland, DE. 1998. The era of pathway quantification. Science, 280, 852. [DOI] [PubMed] [Google Scholar]
- Lau, CC & Pardee, AB. 1982. Mechanism by which caffeine potentiates lethality of nitrogen mustard. Proc. Natl. Acad. Sci. USA , 79, 2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J , Meyer, AN , Donoghue, DJ. 1997. Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation. Proc. Natl. Acad. Sci. USA , 94, 502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y‐X , Weber‐Johnson, K , Sun, L‐Q , Paschoud, N , Mirimanoff, R‐O , Coucke, PA. 1998. Effect of pentoxifylline on radiation‐induced G2‐phase delay and radiosensitivity of human colon and cervical cancer cells . Rad. Res., 149, 338. [PubMed] [Google Scholar]
- Lock, RB. 1992. Inhibition of p34cdc2 kinase activation, p34cdc2 tyrosine dephosphorylation, and mitotic progression in chinese hamster ovary cells exposed to etoposide. Cancer Res., 52, 1817. [PubMed] [Google Scholar]
- Lock, RB & Ross, WE. 1990. Inhibition of p34cdc2 kinase activity by etoposide or irradiation as a mechanism of G2 arrest in chinese hamster ovary cells. Cancer Res., 50, 3761. [PubMed] [Google Scholar]
- Maity, A , Hwang, A , Janss, A , Phillips, P , McKenna, WG , Muschel, R. 1996. Delayed cyclin B1 expression during the G2 arrest following DNA damage. Oncogene, 13, 1647. [PubMed] [Google Scholar]
- McGowan, CH & Russel, P. 1993. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr 15. EMBO J., 12, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, JD , Yang, J , Truant, R , Kornbluth, S. 1999. Nuclear import of Cdk/cyclin complexes: identification of distinct mechanisms for import of Cdk2/Cyclin E and Cdc2/CyclinB1. J. Cell Biol., 144, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, A. 1992. Creative blocks: cell‐cycle checkpoints and feedback controls. Nature, 359, 599. [DOI] [PubMed] [Google Scholar]
- Murray, A. 1998. How to compact DNA. Science, 282, 425. [DOI] [PubMed] [Google Scholar]
- Murray, AW , Solomon, MJ , Kirchsner, MW. 1992. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature, 339, 280. [DOI] [PubMed] [Google Scholar]
- Muschel, R , Zhang, HB , Iliakis, G , McKenna, WG. 1991. Cyclin B1 expression in HeLa cells during the G2 block induced by ionising radiation. Cancer Res., 51, 5113. [PubMed] [Google Scholar]
- O'Connor, PM. 1996. Cell cycle checkpoints: targets for anti‐cancer therapy. Anti-Cancer Drugs 7 (Suppl.) , 3), 135. [Google Scholar]
- Pines, J. 1999. Checkpoint on the nuclear frontier. Nature, 397, 104. [DOI] [PubMed] [Google Scholar]
- Pines, J & Hunter, T. 1991. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle‐dependent nuclear transport. J. Cell. Biol., 115, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets, MFMA , Mooren, EHM , Begg, AC. 1994. The effect of radiation on G2 blocks, cyclin B1 expression and cdc2 expression in human squamous carcinoma cell lines with different radiosensitivities. Radiother. Oncol., 33, 217. [DOI] [PubMed] [Google Scholar]
- Tassan, JP , Schultz, SJ , Bartek, J , Nigg, EA. 1994. Cell cycle analysis of the acitivity, subcellular location and subunit composition of human CAK (CDK activating kinase). J. Cell. Biol., 127, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theron, T & Böhm, L. 1998. Cyclin B1 expression in response to abrogation of the G2/M block in HeLa cells . Cell Prolif., 31, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q , Fan, S , Eastman, A , Worland, PJ , Sausville, EA , O'Connor, PM. 1996. UCN‐01: a potent abrogator of G2 checkpoint function on cancer cells with disrupted p53. J. Natl. Cancer Inst., 88, 956. [DOI] [PubMed] [Google Scholar]
- Weinert, TA & Hartwell, LH. 1988. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cervisiae. Science, 241, 317. [DOI] [PubMed] [Google Scholar]


