Abstract
Notch signalling is a highly conserved intercellular signal transfer mechanism that includes canonical and non‐canonical pathways. It regulates differentiation and proliferation of stem/progenitor cells by means of para‐inducing effects. Expression and activation of Notch signalling factors (receptors and ligands) are critical not only for development of the dental germ but also for regeneration of injured tissue associated with mature teeth. Notch signalling plays key roles in differentiation of odontoblasts and osteoblasts, calcification of tooth hard tissue, formation of cusp patterns and generation of tooth roots. After tooth eruption, Notch signalling can also be triggered in dental stem cells of the pulp, where it induces them to differentiate into odontoblasts, thus generating fresh dentine tissue. Other signalling pathways, such as TGFβ, NF‐κB, Wnt, Fgf and Shh also interact with Notch signalling during tooth development.
| Contents: |
|---|
| Overview of the Notch signalling pathway |
| Analogous Notch signalling in mammals |
| Canonical Notch pathway |
| Non‐canonical Notch pathway |
| Expression and function of Notch signalling during tooth development |
| Dental development and cell population in the enamel producing organ |
| Expression pattern of Notch receptors |
| Expression pattern of Notch ligands |
| Feedback loop of Notch ligands and receptors |
| Activation of Notch signalling in adult teeth |
| Notch signalling activation in injured teeth |
| Notch signalling in dental stem cells |
| Interactions between Notch and other signalling in teeth |
| TGFβ and Notch signalling |
| NF‐κB and Notch signalling |
| Wnt and Notch signalling |
| Fgf and Notch signalling |
| Shh and Notch signalling |
| Conclusion and Perspectives |
Overview of Notch signalling
The Notch signalling pathway is an evolutionarily conserved mechanism for transmission of signals upon ligand‐receptor binding of adjacent cells, thereby enabling them to adopt different fates (1, 2). Homologues of Notch receptors and ligands, first studied in Drosophila, have been identified in nearly all metazoan phyla, from Caenorhabditis elegans to vertebrates. It is involved in cell differentiation, apoptosis and proliferation, thus controlling organ formation and morphogenesis (3, 4, 5, 6, 7, 8, 9, 10). Accumulated data on tooth development have demonstrated that proper expression of Notch signalling is critical for generation of dental epithelium and the enamel producing organ, differentiation of ameloblasts and odontoblasts, secretion of enamel and dentine matrix, proliferation and apoptosis of dental stem cells and further functions. Recent studies show that Notch signalling is involved in a highly complicated signalling network composed of a series of growth regulators, such as TGF‐β, NF‐κB, Wnt and Fgf.
Analogous Notch signalling in mammals
Notch receptors are type I transmembrane proteins with extracellular domains including EGF‐like repeats, Notch/Lin‐12 repeats and cytoplasmic RAM23 domains, as well as intracellular domains containing Ankyrin repeats and motifs required for signal transduction (11, 12, 13, 14, 15). There are four analogous receptors in mammals: Notch1, Notch2, Notch3 and Notch4. The primary translational product of a Notch receptor is synthesized in endoplasmic reticulum and then transported through secretory pathways to the trans‐Golgi network. After cleavage at the S1 site by a Furin‐like convertase, Notch gives rise to the mature heterodimeric receptor, which finally localizes to the plasmamembrane (16). Once bound with Notch ligand, the receptor may be cleaved at S2 and S3 sites by metalloprotease TNF‐α converting enzyme and γ‐Secretase complex, respectively, to generate the Notch intracellular domain (NICD) (17).
Notch receptors transduce signals from either typical ligands or atypical ligands in a context‐dependent manner. Delta homologues and Serrate homologues encoded by the Delta/Serrate/Lag2 (DSL) gene family are typical Notch ligands, which have higher affinity than atypical ligands. There are two types of typical Notch ligand in mammals, DLL‐type ligands (Delta‐like1, Delta‐like3 and Delta‐like4) and JAG‐type ligands (Jagged1, Jagged2). These ligands are type I transmembrane proteins, and generally contain both a conserved extracellular DSL domain and EGF‐like repeats (18, 19, 20, 21). Jagged1 and Jagged2 each also contains an extracellular cysteine rich domain and a von Willebrand factor type C domain (18, 21, 22, 23, 24). Interaction of a Notch receptor with a typical ligand requires direct binding of EGF‐like repeats of the receptor and the DSL domain of the ligand (25). At least three proteins with EGF‐like repeats have been identified as atypical Notch ligands, including DNER (26), F3/Contactin, and NB‐3, which are distinguished from typical ligands by absence of a conserved DSL domain, and lower affinity. DNER is a type I transmembrane protein, while F3/Contactin and NB‐3 are glycosyl phosphate‐dylinositol (GPI)‐anchored proteins (16, 26, 27). Atypical ligands are not yet well‐understood.
Canonical Notch pathway
The intracellular component of a Notch receptor (NICD) transduces signals to the cell nucleus and affects gene transcription levels by recruiting and binding to different sets of transcriptional regulators or cofactors. According to recent studies, Notch signalling pathway can be divided into two kinds of cascade, the canonical pathway and the non‐canonical pathway. The mode of activation of the canonical Notch signalling pathway was identified early as binding between typical Notch ligands and receptors. Briefly, canonical signalling pathway refers to a NICD‐CSL‐MAML cascade, in which NICD translocates into the nucleus and, with a sequence‐specific DNA (CGTGGGAA), activates binding to the transcription regulator CSL (CBF1 humans/Su (H) Drosophila/LAG1 C. elegans), also known as RBP‐Jκ in mammals (28). In the absence of NICD, CSL acts as a transcriptional repressor, recruiting a co‐repressor complex and inhibiting transcription of target genes that contain CSL binding sites (29, 30). Upon binding with NICD, CSL expels the co‐repressor complex and allows recruitment of a co‐activator complex. This NICD‐CSL complex also comprises Ski‐interacting protein (SKIP), Mastermind‐like proteins MAML1, MAML2, and MAML3, and co‐activator p300. SKIP and MAML both stabilize interaction of NICD and CSL (16, 31), while p300 has a key role in chromatin unwinding and transcription initiation (32, 33). Thus, CSL is converted to a key component of a transcription‐activator complex, driving transcription of specific target genes (2, 34, 35, 36, 37). Two families of basic helix‐loop‐helix transcription factors, Hes (in Drosophila ‘Hairy/Enhancer of Split’) and HERP (Hes‐related repressor protein) have been identified as immediate transcriptional targets of canonical Notch signalling (28, 38). Hes is a transcription repressor that suppresses expression of target genes as a primary Notch effector, while HERP is capable of forming heterodimers with Hes and cooperating for repression transcription. Hes1 can also auto‐regulate its own transcription, controlling Hes1 mRNA levels in a biological clock model of somitogenesis (39).
Non‐canonical Notch pathway
Evidence accumulated in recent years suggests the existence of a non‐canonical Notch signalling pathway, which is distinguished from the canonical signalling pathway by its target genes and mediators. As in most cases the molecular properties of the alternative cascade have not been clearly defined, the non‐canonical pathway is poorly understood. Essentially, the non‐canonical pathway can be divided into a CSL‐dependent pathway and a CSL‐independent pathway.
Over the past several years, CSL/RBP‐Jκ‐binding sites have been found in the promoters of many other genes which have been identified or postulated as direct targets of Notch signalling, such as cyclin D1 (40), p21 (41), GFAP (42), Nodal (43), IκBα (44), p50, p65, RelB, and c‐Rel (45). For instance, Oswald et al. demonstrated that RBP‐Jκ is capable of forming a higher‐order DNA binding complex with NICD on the promoter of NF‐κB2 (p100/p52), which is consistent with the canonical Notch pathway (46). Other reports demonstrated that the CSL‐dependent non‐canonical Notch pathway can be triggered by interaction of Notch1 or 2 with NB‐3 (an atypical receptor) (16, 27). According to these reports, the non‐canonical NICD/RBP‐Jκ signalling pathway can recruit Deltex1 proteins to form the NICD/RBP‐Jκ/Deltex1 complex, which activates the downstream gene MAG, a tissue‐specific transcription factor that induces terminal differentiation. Taken together, these reports indicate that CSL/RBP‐Jκ, as a co‐activator of transcription regulation complexes, may affect more downstream effectors with conserved sequences in spite of Hes/HERP family by DNA‐binding mechanism in a cell‐dependent or time‐dependent context.
There is little direct evidence for the existence of CSL‐independent non‐canonical Notch signalling. Lecourtois et al. have reported that sim is positively regulated when Notch is activated by a ligand distinct from Delta, and that its expression is little affected in Su(H) mutant embryos, which suggests that a Su(H)‐independent signalling pathway may exist from the Notch receptor to the sim promoter in Drosophila (47). A further group reports that uncleaved (S1, S2, or S3) Notch receptors can still block myogenesis, a finding consistent with the phenotype of wild‐type Notch (48, 49). However, the mechanism of most CSL‐independent Notch pathways had remained unclear until novel evidence was found of cross‐talk between Notch and the NF‐κB family. According to Shin et al., activated NICD can (i) directly interact with NF‐κB subunits and compete with IκBα, leading to retention of NF‐κB in the nucleus, or (ii) positively regulate IFN‐γ expression, a target gene of NF‐κB, through binding of NF‐κB‐NICD complex on the IFN‐γ promoter (50). In addition to its known transcriptional mechanism, NICD may exert rapid effects on specific target proteins in a CSL‐independent manner. Sriuranpong et al. describe that Notch signalling can negatively regulate protein levels of human MASH1 by direct degradation of bHLH transcription factor, most likely through activation of a specific E3 ubiquitin ligase (51).
Expression and function of Notch signalling during tooth development
Studies of the tooth development of rodents indicate that the Notch signalling pathway is deeply involved in the interactions between the oral/dental epithelium and cranial neural‐crest‐derived mesenchymal cells through which the tooth primordia is transformed into a complex mineralized organ (13, 52, 53, 54, 55, 56). The expression pattern of Notch 1, 2 and 3 and Notch receptors (Jagged1, Jagged2, Delta1) is space‐ and time‐dependent (Fig. 1).
Figure 1.
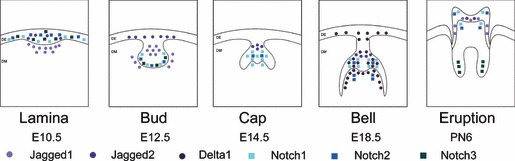
Expression pattern of Notch receptors. Expression patterns of Notch1, Notch2, Notch3, Jagged1, Jagged2 and Delta1 at different stages of developing tooth germ (DE, dental epithelium; DM, dental mesenchyma).
Dental development and cell populations in the enamel organ
Dental development is initiated with the formation of dental lamina arising from the oral epithelium, which, in mice, is triggered by an epithelial signal between E10 and E11 (57, 58). During E12, condensing epithelia cells in dental lamina invaginate into the underlying mesenchyme and give rise to a bud‐like structure, the precursor of the enamel organ. During the transitional bud‐to‐cap stage, several cell populations differentiate into three specific cell types: the inner enamel epithelium (IEE), the outer enamel epithelium (OEE) and, in the core, the stellate reticulum (SR). At E13.5, the cell population at the bottom of the bud suddenly stops proliferating and becomes a signalling centre of the dental epithelium, the primary enamel knot (EK), which secretes growth factors and other signalling molecules during E14, and which is responsible for regulation of tooth‐shape pattern (59, 60, 61). During subsequent morphogenesis of the molar teeth, more than two secondary EKs come up in the IEE above potential cusps and initiate the epithelial folding that results in the characteristic cusp patterns of teeth (62). During the bell stage, two to three layers of squamous epithelial cells, termed the stratum intermedium (SI), appear between the IEE and the SR. As development continues, the IEE differentiates into ameloblasts secreting the organic components of the enamel. The terminal division of the mesenchymal preodontoblasts gives rise to two cell layers: odontoblasts secreting dentin, and cells of the subodontoblastic layer (thought to be the progenitor pool).
Expression pattern of Notch receptors
Three of the Notch receptor family, Notch1, 2 and 3, are involved in developing rodent teeth during normal morphogenesis; each has a distinct temporospatial expression pattern (Table 1). The Notch receptor family is mainly expressed in germ during the early development stage, throughout the dental epithelium, and during the differentiation stage in the SI, gradually extending to the pulpal mesenchyme. Interestingly, in the mesenchymal cells, Notch transcription is detected in sub‐odontoblastic cells other than odontoblasts, suggesting that Notch signalling is responsible for maintenance of the precursor pool. Most notably, Notch is absent in epithelial cells in close contact with the mesenchyme, the basal cells, or IEE, which may be important for acquisition of the ameloblast fate.
Table 1.
Expression pattern of Notch receptors during tooth development in mouse
| Stage of development | Notch1 | Notch2 | Notch3 | References |
|---|---|---|---|---|
| Dental lamina | ||||
| (E10–E11) | Thickened dental epithelium | Thickened dental epithelium | Thickened dental epithelium | Mitsiadis et al. (13, 90, 91) |
| Bud stage | ||||
| (E12–E13) | Superficial dental epithelium | Superficial dental epithelium | Superficial dental epithelium | Mitsiadis et al. (13, 90, 91) |
| Cap stage | ||||
| (E14–E15) | EO, SR, EK, SI, PM | EO, SR | Perivascular structures | Mitsiadis et al. (13, 90, 91), Mucchielli and Mitsiadis (54), Harada et al. (64, 67) |
| Bell stage | ||||
| (E16–E19) | SI, cervical loop | SR, SI, OEE, cervical loop, PM | Basal layer | Mitsiadis et al. (13, 90, 91), Mitsiadis et al. (1), Harada et al. (64, 67) |
| Eruption stage | ||||
| (PN1–PN6) | EO, PM | EO, PM | EO, PM, FM | Mitsiadis et al. (13, 90, 91) |
EK, enamel knot; EO, enamel organ; FM, follicular mesenchyme; IEE, inner enamel epithelium; ; PM, papilla mesenchyme; SI, stratum intermedium; SR, stellate reticulum.
Molar teeth. During the development of molar tooth in mice, the Notch gene family is symmetrically expressed in the enamel organ. (i) During the lamina stage (E11–E12), all three Notch transcripts are intensely expressed in the mesial side of the thickened dental epithelium. (ii) In the bud stage (E13), Notch1, 2 and 3 mRNAs are expressed in superficial dental epithelium, but absent from the condensed mesenchyme (CM) (13). (iii) During the cap stage (E14–E15), Notch1 and 2 mRNAs are expressed in cells of the enamel organ, with the exception of its basal epithelium (13). Transcripts for Notch1 are found throughout the dental epithelium, including the SR and the epithelium overlapping the EK, with the strongest signal in cells forming the SI and overlying the basal layer (13, 54, 63). Notch2 transcripts are detected only in the oral half epithelium forming SR, and are weak or absent in the EK and cervical loop compartments (54, 63). Notch1 and 2 appear to be absent from the pulp mesenchyme, but Notch1 is expressed in dental papilla mesenchyme with a patchy pattern around the tooth germ (13, 63). Notch3 transcripts are absent in the enamel organ but presumably present in perivascular structures (13). (iv) During the bell stage (E16–E19), all Notch genes are expressed in the enamel organ, but become more and more restricted to specific subpopulations of cells. The expression of Notch1 occurs mainly in cells of the SI and cervical loop area (1, 13), while Notch2 transcripts are most abundant in SR, and Notch3 transcripts are detected in cells overlying the basal layer, such as SI and OEE (1, 13). Apart from the SR, Notch2 is also progressively expressed in the SI, OEE and cervical loop area (1, 64). Interestingly, transcripts of all three Notch genes are absent in preameloblasts. Notch2 and 3 are weakly expressed in the dental papilla mesenchyme of the cusp region, while Notch1 and Notch3 are also correlated with the endothelial cells of blood vessels (1, 13). At the late bell stage (E19), Notch1 and 3 transcripts are mainly expressed in SI, whereas Notch2 mRNA is also found in the SR and OEE. Weak Notch2 and Notch3 signals are observed in the dental papilla and follicular mesenchyme, but are absent from polarizing odontoblasts. (v) During the eruption stage (PN1–PN6), with its terminal differentiation of mesenchymal cells and preameloblasts, Notch1, 2 and 3 transcripts persist in the enamel organ. All Notch genes are transiently expressed in cells of the papilla mesenchyme of the cusp area just underlying differentiating odontoblasts, while Notch3 transcripts are also found in the dental follicular mesenchyme. In differentiated odontoblasts or ameloblasts, no expression of any Notch gene has been detected (13).
Incisor teeth. During the development of rodent incisors, the dental germ rotates 90° at the labial–lingual axis and demonstrates a Notch gene expression pattern that is distinct from that of molars.
In the early developing incisor (E11–E13), before the rotation, the expression pattern of Notch1 mRNAs is quite similar to that in molars (13), while Notch2 transcripts are restricted to the labial side of the dental epithelium. Notch2 mRNAs are also found in the lingual side of the dental furrow and are moderately expressed in the condensed dental mesenchyme of incisors (54).
During the bell stage (E18.5), Notch1, Notch2 and Notch3 are expressed in the enamel organ, showing asymmetrical expression patterns on the two sides. In the labial side, Notch1, 2 and 3 are expressed in SI, the sub‐odontoblast cell layer and the posterior IEE. However, none of the three Notch receptors is present in the preameloblasts, ameloblasts, anterior IEE or odontoblasts in the labial side (1). Furthermore, in the epithelial derivatives, Notch1 mRNAs are detected in the cell layer continuous to the SI, Notch2 expression is positive in the OEE and Notch3 transcripts are detected in the IEE (1). In contrast, on the lingual side, transcripts for Notch1 and Notch2 are only detected in the posterior region of the outer dental epithelium (ODE) (1). At the eruption stage, Notch1 transcripts are expressed in SI and the subodontoblast layer (64).
Expression pattern of Notch ligands
Three typical Notch ligands (Jagged1, Jagged2 and Delta1) are expressed in the developing tooth, showing distinct mRNAs transcripts expression in the IEE, ameloblasts, odontoblasts and sub‐odontoblastic layer cells (Table 2).
Table 2.
Expression pattern of Notch ligands during tooth development in mouse
| Stage of development | Jagged1 | Jagged2 | Delta1 | References |
|---|---|---|---|---|
| Dental lamina | ||||
| (E10–E11) | Condensed mesenchyme | DM | Mitsiadis et al. (25), Mustonen et al. (63) | |
| Bud stage | ||||
| (E12–E13) | SR, EK, FM | Oral epithelium | Mitsiadis et al. (25) | |
| Cap stage | ||||
| (E14–E15) | IEE | Mitsiadis et al. (25) | ||
| Bell stage | ||||
| (E16–E19) | IEE | EO, IEE, SI, odontoblast, ODE, IDE | Mitsiadis et al. (13, 90, 91), Mitsiadis et al. (25), Harada et al. (64, 67) | |
| Eruption stage | ||||
| (PN1–PN6) | IEE, ameloblast | IEE | Mitsiadis et al. (25), Mustonen et al. (63), Harada et al. (64, 67) | |
DE, dental epithelium; EK, enamel knot; FM, follicular mesenchyme; IDE, inner dental epithelium; IEE, inner enamel epithelium; ODE, outer dental epithelium; SI, stratum intermedium; SR, stellate reticulum.
Molar teeth. Jagged1 is absent in dental epithelium during the lamina and early bud stages (E11–E12), but unlike Notch genes, it is intensely expressed throughout the condensed mesenchyme at E12 (63). Throughout E13 to E13.5, Jagged1 is present in the SR and its expression region overlaps with that of Notch (63). Interestingly, at E13.5, Jagged1 mRNAs are transiently expressed in the enamel knot, but are absent in epithelial cells (63). At the late bud stage, Jagged1 is downregulated in the pulpal mesenchyme closest to the bud (63), but is maintained in cells of the peripheral dental mesenchyme forming the follicle that may contain the mesenchymal stem cells. At the cap stage of E14, Jagged1 is absent in dental papilla cells and the SR (63). During the PN1–PN8 stages, mRNAs for Jagged1 have been detected in the IEE and ameloblasts (63, 64).
Jagged2 mRNAs are expressed in the oral epithelium during E11–E13 (25), and in dental epithelium at the lamina stage of E11 (63). At E13–14, Jagged2 is weakly expressed in dental epithelium, including basal epithelial cells (63). At the cap stage, Jagged2 expression is restricted to IEE at E14 (25). During the bell stage and eruption stage (PN1 to PN8), Jagged2 expression is maintained in the IEE (25, 64).
Delta1 is weakly expressed in dental epithelium during tooth initiation and morphogenesis (E11–E14), but during cytodifferentiation (E16–E19), expression is upregulated in the epithelium‐derived ameloblasts and mesenchyme‐derived odontoblasts (1, 63). Delta1 is absent in dental mesenchyme from E12.5 to E15.5 (1). During the early bell stage (E16.5), Delta1 is restricted to the dental epithelium of the enamel organ, with strong expression in the IEE and SI and weak expression in the OEE and SR. At E18.5, a gradient of Delta1 expression is detected in differentiating odontoblasts, with the strongest signal at the tips of the cusps and progressively lower levels of expression in the developmentally less advanced odontoblasts farther down. Few transcripts of Delta1 are detected in the subodontoblastic layer (1).
Incisor teeth. During the development of mouse incisors, the expression of Delta1, but not those of Jagged1 or 2, is asymmetric at the labial‐lingual axis. During the bell stage, Jagged2 mRNAs are restricted to the IEE from E17 to E18.5 (25), while at E18.5, Delta1 is expressed in the posterior ODE and inner dental epithelium (1) on the lingual side. On the labial side, Delta1 is present in IEE‐derived cells such as preameloblasts and ameloblasts, and in mesenchyme‐derived cells such as odontoblasts, subodontoblast layer cells, and preodontoblasts (1). During the post‐natal stage, Jagged1 is positive in the IEE and ameloblasts at PN3 (64).
Feedback loop of Notch ligands and receptors
By preventing premature differentiation, Notch activation in mice may ensure a continuous supply of stem‐cell progenitors, which serve as the precursor pool both for odontoblasts and ameloblasts of the molars and incisors during development, and for the continuous replacement of incisors. A striking feature of Notch‐mediated cell communication is that at several sites, the Notch and Delta/Jagged expression patterns in the tooth germ are mainly complementary and confined to opposing cell layers (65), which were previously described as typical for Notch‐regulated cell‐type specification (66). For example:
-
1
During odontogenesis, Delta1 is expressed in differentiating ameloblasts and odontoblasts, whereas Notch1, Notch2 and Notch3 are confined to adjacent epithelial and mesenchymal cells (1, 13, 67, 68). In the cervical loop, Notch3 is restricted to cells of the stratum intermedium, while Notch1 is expressed only in the cells that will form the stratum intermedium. In contrast, Delta1 expression in the cervical loop is confined to the subpopulation of cells that is in continuity with the layer of cells that gives rise to ameloblasts (52),
-
2
Jagged2 mRNAs are found in the IEE and in the adjacent cell layer of the SI, whereas Notch1 mRNAs are expressed in the SI during the differentiation stage, suggesting that Jagged2‐Notch1 signalling is involved in regulating ameloblast differentiation (25).
-
3
An analogous situation exists along the dentin wall during early pulp repair in injured teeth, where Delta1 is upregulated in odontoblasts and Notch2 is expressed only in adjacent pulp cells other than odontoblasts (1, 53).
The specific complementary expression appears to be established by a Notch‐mediated feedback regulation mechanism between adjacent cells (1, 53). Similarly, the feedback regulation exerted by Delta‐Notch signalling, including positive regulation of Notch1 and Notch2 and negative regulation of Delta1 expression, may be responsible for the asymmetries and spatial segregation of Notch1, 2 and 3 and Delta1 in different cell layers (52). Furthermore, the Jagged2 ligand has also been shown to activate the Notch1 receptor in mammalian cells (25). Increasing evidence implies that feedback regulation may change the expression level of ligands and Notch receptors in opposing cells. On the epithelial side, the dental epithelial cells appear to constitute a developmentally equivalent group in which Delta‐Notch signalling between preameloblasts/ameloblasts and the adjacent SI may prevent immediately neighbouring cells from adopting an ameloblast fate through lateral inhibition (25). On the mesenchymal side, however, the expression of Delta1 in newborn odontoblasts may direct adjacent cells towards an alternative fate (i.e. cells of the subodontoblastic layer), or, alternatively, it may inhibit the adjacent cells from exiting the cell cycle, thus providing a feedback mechanism to control the proportion of cells that will differentiate into odontoblasts (52).
In the tooth germ of vertebrates, little is known about the ligand‐receptor pairs in the feedback regulation loops (69). What is known is that Delta1 can interact with any of the three Notch receptors, but the activation level differs among different receptors (52). Jagged1 is more effective than Delta1 in activating Notch2, while both Jagged1 and Delta1 can activate Notch1 efficiently (23, 69). Jagged1 transcripts disappear from the dental mesenchyme at the early bell stage and persist in the epithelial components only in the stratum intermedium at the stage at which Dll1 is upregulated in the dental epithelium (52). In contrast to the Notch family receptors and their ligand Jagged1, which are expressed during early tooth morphogenesis in both the epithelium and the mesenchyme (68), Delta1’s expression is not affected by epithelio‐mesenchymal interactions in dental explants, suggesting that signals intrinsic to both the epithelium and the mesenchyme are responsible for inducing Delta1 in dental tissues. Intrinsic properties of progenitors have been postulated to control the generation time of different cell types in other systems as well (52).
Activation of Notch signalling in adult teeth
Previous studies on intact rodent teeth have shown that both Notch and Delta proteins are absent from all adult dental tissues except the cervical loop of incisors (53, 67, 70). However, during repair processes of carious or injured adult teeth, Notch signalling is triggered in pulpal mesenchymal cells, particularly in those adjacent to the lesion area (that is, sub‐odontoblastic layer cells and odontoblasts) (70). This strongly suggests that Notch signalling is involved, not only in tooth development but also in homeostasis.
Notch signalling in injured teeth
In the pulp of injured teeth, both Notch receptor (Notch1, Notch2 and Notch3) and ligand (Delta1) staining have been detected but with different immunoreactivities. Notch staining is mainly restricted to mesenchymal cells adjacent to the lesion (that is, the tooth crown), while there is no immunoreactivity in odontoblasts (53). Notch2 protein, the most prominent Notch receptor reactivated in injured pulp, is strongly expressed in coronal pulp mesenchyme close to a lesion, while staining is less intense further from injured areas (53, 71). Faint Notch1 staining is found in some mesenchymal cells and vascular structures, both in the crown. Notch3 expression is mainly associated with vascular structures traversing roots of injured teeth, but is not expressed in those of coronal pulp (53). Interestingly, Notch2 expression is also activated in mesenchymal cells at a distance from the lesion (that is, in dental roots), suggesting that there is a potential progenitor pool at the root, which differentiates into odontoblasts or pulp fibroblasts under the influence of growth factors effusing from a lesion (53, 71). A complementary expression pattern of Delta1 has been found in odontoblasts, as well as in vascular structures adjacent to injured areas, suggesting an instrumental role for Notch‐Delta1 interaction in injured teeth (53). Notch upregulation (that is, of Notch and Delta1) may represent an early molecular event during regeneration processes of injured teeth, since expression is observed soon after injury (70). Upon binding with Delta1 ligands in adjacent cells, Notch2‐positive undifferentiated sub‐odontoblastic cells become engaged in a differentiation pathway leading to odontoblasts and/or pulp fibroblasts. These results highlight similarities between developmental and regenerative processes and add further weight to the hypothesis that activation of Notch is instrumental in tooth homeostasis (70). In periodontal lesions, immunoreactivity has been observed for Notch1 and 2 proteins, but not for Notch3 protein. Notch1 is weakly expressed in a proportion of osteocytes of the alveolar bone, whereas very strong Notch2 immunoreactivity was detected in cells of the periodontal ligament close to the site of injury, and in some cells of alveolar bone (53).
Notch signalling in dental stem cells
Dental pulp stem cells. Previous studies have shown that human dental pulp stem cells (DPSCs) can be seeded on to a scaffold to give rise to complex tooth structures, which suggests that DPSCs in adult teeth reserve the potential for dentinogenesis (72, 73, 74). Maintenance and/or differentiation of these stem cells is thought to be regulated by cell–cell interactions involving the Notch signalling pathway (75, 76, 77). Morsczeck et al. have reported that the Notch receptor in subcultured human dental follicle cells is a marker for undifferentiated cells (78). According to accumulated data, the Notch‐ligand signalling pathway is, unsurprisingly, responsible for specific differentiation and regulation of DPSC cell fate (79, 80).
Perivascular cells. Recent studies have shown that perivascular cells contain a population of DPSCs that can be stimulated by dental trauma and carious lesions via activation of Notch signalling (Notch3), and adopt the alternative cell fate of reparative dentine. Co‐expression of Notch3 and Rgs5, the latter as the marker of pericytes, has been detected in vascular structures during development, and in perivascular tissue and single capillary cells of injured teeth. These results point to the importance of vascular‐derived stem cells to pulp healing, and, furthermore, imply that Notch signalling plays a role in regulating stem‐cell fate specification (81).
Side population cells. Recently, side population (SP) cells have been identified in human periodontal ligament cells and porcine dental pulp tissues (82, 83). SP cells appear highly enriched for stem‐cell activity (80, 84), and have the capacity to differentiate into odontoblast‐like cells in vitro (85). It has been demonstrated that both Nestin and Notch1, acting as general markers of stem/progenitor cells and affecting self‐renewal and lineage‐specific differentiation (67, 86, 87), are expressed in SP cells of human adult dental pulp tissue (85).
Dental epithelial stem cells. Studies on mature rodent incisors suggest that a population of undifferentiated epithelial stem cells remains in the SR of the cervical loop (67). An epithelial stem cell expressing Notch1 may divide into two daughter cells, one that remains in the stem‐cell pool and the other that enters into the zone of rapidly dividing IEE cells – so‐called transit‐amplifying cells – which may differentiate into ameloblasts. In the cervical loop, Notch1 is restricted to the SR and is expressed most intensely in cells facing the IEE, while lunatic fringe, a regulator of Notch signalling, is expressed in the IEE starting from the cervical loop. Furthermore, localization of slowly dividing putative stem cells in peripheral SR cells correlates with the boundary between lunatic fringe and Notch1, suggesting that maintenance and fate of the stem cells is influenced by Notch signalling. In the ameloblast differentiation zone, the Notch signalling pathway regulates interactions between terminally differentiated ameloblasts expressing Jagged1, and SI cells expressing Notch1. These findings are consistent with recent data and support a role for Notch signalling in maintaining the differentiated state of ameloblasts.
Interactions between Notch and other signalling in teeth
TGFβ superfamily and Notch signalling
The TGFβ superfamily includes a large number of growth inducers, including actins/inhibins and bone morphogenic proteins (BMPs), which play a critical role in developmental processes. TGFβ growth factors, including BMPs and TGFβ1, bind to basement membranes of the germ, the dentine in mature teeth that is expressed in preodontoblasts/odontoblasts during the bell stage of molar development (88, 89, 90, 91, 92, 93, 94).
Regulation of Notch signalling by TGFβ. It has been shown that TGFβ signalling molecules are important for hard tissue formation after dental pulp injury (48, 49, 71) and during odontoblast differentiation (92). After occurrence of dentine lesions, TGFβ‐1, BMP‐2, BMP‐4 and BMP‐7 liberated from demineralized dentine of human pulp cells stimulate odontoblasts to elaborate reactionary dentine (70, 95, 96, 97). During tooth formation, odontoblast differentiation and dentine matrix synthesis is regulated by TGFβ‐1 and BMP‐2 (52, 92, 96, 98). TGFβ‐1 may also induce proliferation and migration of subodontoblastic cells and pulp fibroblasts (99). Several studies have demonstrated that the TGFβ superfamily may modulate differentiation by regulating expression of Notch receptors and ligands (1, 39, 67, 68, 100, 101). It has been shown that Delta‐1 expression correlates with ameloblast and odontoblast differentiation and is regulated by TGFβ‐1 and BMPs in E16.5 dental mesenchyme, in vitro (1, 102). TGFβ‐1’s regulation of Notch 2 expression is also involved in odontoblast differentiation and dentine formation after dental injury (98, 99). Furthermore, downregulation of Notch in early dental epithelium and mesenchyme is associated with expression of several growth factors and extracellular matrix molecules, including BMP‐2 and ‐4, at the epithelial/mesenchymal interface (94). Taken together, these results indicate that dental cell lineage restriction is under concomitant control of Notch and TGFβ‐1 pathways. However, the mechanism by which TGFβ/BMP signalling regulates Notch receptors and ligands requires further study.
Evidence for direct cross‐talk between Notch and TGFβ comes from three recent studies (103, 104, 105). Hes1 gene known to be a Notch target, can be activated by TGFβ signalling. Specifically, CSL recruits activated Smad3 (an intracellular transducer of TGFβ signalling) to the promoter via NICD (103). Similar cross‐talk also occurs between Notch and BMP. A further report has shown that the differentiation‐inhibiting effect of BMP4 on myogenic differentiation partly depends on functional Notch signalling, in which two Notch target genes, Hey1 and Hes1, are involved (104). Interaction between these two signalling pathways is supported by sequence analysis of Hey1 gene, which has revealed both Smad‐ and CSL‐binding sites in the Hey1 promoter. Finally, studies on endothelial cells (EC) indicate that Notch and BMP signals synergize and antagonize during regulation of EC migration (106). When EC are induced by long‐range effects of BMP ligands alone, activation of Smad1 leads to expression of Id1, an activator of EC migration. Once Notch signalling is activated, Herp2, a negative regulator of EC migration, can be upregulated through recruitment of a Smad1‐NICD complex (105). Taken together, the evidence indicates that BMP and TGFβ can feed into the Notch pathway by augmenting transcription of Notch target genes.
NF‐κB and Notch signalling
NF‐κB signalling is a cell fate regulatory network controlling expression of a multitude of genes involved in development, immunity, inflammation and cancer development (107). Observation of negative dominant mutant phenotype cIκBΔN mice has provided confirmation that NF‐κB is required, not only for cusp formation of molar tooth germs but for epithelial invagination of incisors as well. Moreover, Notch1 and Notch2 are downregulated in incisor tooth germ Ikkα mutant embryos, suggesting that Notch signalling may be involved in an NF‐κB‐independent Ikkα pathway (108).
Transcriptional interaction. Notch and NF‐κB pathways are integrated into cross‐talk, at least partly, by transcriptional cross‐regulation of various signalling members. Studies have shown that typical Notch ligands can transduce signals to the NF‐κB‐NICD complex, augmenting NF‐κB signalling. Consistent with this, a CSL‐responsive promoter element has been found in the DNA sequence of the NF‐κB protein p100/p52, which can be activated by Notch1 via RBP‐Jk (46). Furthermore, two groups have reported that NICD of Notch1 upregulates IκBα (44) and NF‐κB subunits (i.e. p50, p65, RelB and c‐Rel) (45) in a CSL‐dependent manner, thus reducing or stimulating NF‐κB activity. In addition, at least two reports have shown transcriptional upregulation of Jagged1 (109), Hes5, and Deltex1 (110) by NF‐κB.
Non‐transcriptional interaction. The effect of Notch1 on NF‐κB transcription regulation is dose‐dependent. Low levels of Notch1 stimulate NF‐κB transcriptional activity as mentioned above, while overexpressed Notch1 prevents NF‐κB‐dependent transactivation by a p50‐mediated physical interaction of Notch1 IC with p50‐p65 heterodimers (111, 112). The interaction domain, a 109 amino acid stretch, is mapped at the N terminus of Notch1 IC, overlapping with the RAM23 domain, which participates in Notch interaction with CSL (112). Other reports reveal that p50 physical interaction with Notch1 can also lead to NF‐κB activation. Shin et al. have shown that during the late stage of NF‐κB activation, Notch1 may compete with IκBα by physically interacting with p50/c‐Rel complexes, thus increasing nuclear retention of NF‐κB (50).
Wnt and Notch signalling
Wnt signalling plays key roles in tooth morphogenesis. Several Wnt genes are broadly expressed in dental epithelium, including EK, while others are upregulated in developing teeth (101, 113, 114). Wnt signals are context‐dependently transduced to the canonical and non‐canonical Wnt signalling pathways (115, 116, 117). So far, several interactions between Notch and Wingless/Wnt signalling have been established. First, Jagged1 gene is an evolutionarily conserved target of the canonical Wnt signalling pathway (26, 118). Second, a direct interaction between Dishevelled and Notch is important for the ability of Wingless to inhibit Notch (119). Third, NICD activity potentiates effects of LEF‐1 (a transcriptional activator in Wnt signalling) on a subset of promoters, and a weak physical interaction has been observed between NICD and LEF‐1 (120). Fourth, GSK‐3β has been shown to phosphorylate NICD (121) and finally, ablation of Notch1 in skin leads to enhanced β‐catenin signalling, the key mediator of Wnt signals (122).
Fgf and Notch signalling
Expression of Fgf3 and Fgf10 is restricted to mesenchyme underlying basal epithelial and IEE cells. Expression of lunatic fringe and Hes1 depends on mesenchymal signals, and both are positively regulated by Fgf10 (63). Harada et al., suggest that Fgf10 signalling from the mesenchyme may regulate the Notch pathway in dental epithelial stem cells via stimulation of lunatic fringe expression, thus stimulating division of both stem cells and IEE cells, while Fgf3 signalling only stimulates division of IEE cells (63, 67). BMP4 antagonizes the stimulatory effect of Fgf10 on lunatic fringe expression, but has a synergistic effect with Fgf10 on Hes1 expression (63).
Shh and Notch signalling
Sonic hedgehog (Shh) is a secreted signalling factor involved in growth and patterning of teeth, which develops from ectoderm and mesenchyme interactions (123, 124, 125, 126). During the lamina stage, Shh expression is detected in epithelium of the presumptive tooth domain. During bud to cap stage, Shh is upregulated in the EK, implicating Shh in patterning the tooth cap. During the cytodifferentiation stage, expression of Shh is extended to IEE and is maintained in differentiating ameloblasts. This suggests a key role for Shh in regulating cytodifferentiation of the IEE or the underlying odontoblast layer (127). One recent report presents novel evidence of cross‐talk between Shh and the Notch signalling pathway. It demonstrates that Shh signalling may be transduced to transcription factors other than RBP‐Jβ and thus stimulate Hes1, a principal effector of the Notch pathway in stem‐like cells such as C3H/10T1/2 mesodermal and MNS70 neural cells (128).
Conclusion
Notch signalling plays key roles in differentiation of odontoblasts and osteoblasts, calcification of tooth hard tissue, formation of cusp patterns and generation of tooth roots. After eruption, it can also be triggered in dental stem cells within pulp, where it induces them to differentiate into odontoblasts, thus generating fresh dentine. Other signalling pathways, such as TGFβ, NF‐κB, Wnt, Fgf and Shh also interact with Notch signalling during tooth development.
Acknowledgements
This work was funded by National Natural Science Foundation of China (30801304, 81071273, 31170929), Foundation for the Author of National Excellent Doctoral Dissertation of China (FANEDD 200977), Program for New Century Excellent Talents in University (NCET‐08‐0373) and Funding for Distinguished Young Scientists in Sichuan (2010JQ0066).
References
- 1. Mitsiadis TA, Hirsinger E, Lendahl U, Goridis C (1998) Delta‐notch signaling in odontogenesis: correlation with cytodifferentiation and evidence for feedback regulation. Dev. Biol. 204, 420–431. [DOI] [PubMed] [Google Scholar]
- 2. Souilhol C, Cormier S, Tanigaki K, Babinet C, Cohen‐Tannoudji M (2006) RBP‐Jkappa‐dependent notch signaling is dispensable for mouse early embryonic development. Mol. Cell. Biol. 26, 4769–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conlon RA, Reaume AG, Rossant J (1995) Notch1 is required for the coordinate segmentation of somites. Development 121, 1533–1545. [DOI] [PubMed] [Google Scholar]
- 4. Crowe R, Niswander L (1998) Disruption of scale development by Delta‐1 misexpression. Dev. Biol. 195, 70–74. [DOI] [PubMed] [Google Scholar]
- 5. de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ et al. (1997) Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124, 1139–1148. [DOI] [PubMed] [Google Scholar]
- 6. Dorsky RI, Chang WS, Rapaport DH, Harris WA (1997) Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature 385, 67–70. [DOI] [PubMed] [Google Scholar]
- 7. Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish‐Horowicz D (1995) Expression of a Delta homologue in prospective neurons in the chick. Nature 375, 787–790. [DOI] [PubMed] [Google Scholar]
- 8. Henrique D, Hirsinger E, Adam J, Le Roux I, Pourquie O, Ish‐Horowicz D et al. (1997) Maintenance of neuroepithelial progenitor cells by Delta‐Notch signalling in the embryonic chick retina. Curr. Biol. 7, 661–670. [DOI] [PubMed] [Google Scholar]
- 9. Kopan R, Nye JS, Weintraub H (1994) The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix‐loop‐helix region of MyoD. Development 120, 2385–2396. [DOI] [PubMed] [Google Scholar]
- 10. Kopan R, Weintraub H (1993) Mouse notch: expression in hair follicles correlates with cell fate determination. J. Cell Biol. 121, 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Artavanis‐Tsakonas S, Simpson P (1991) Choosing a cell fate: a view from the Notch locus. Trends Genet. 7, 403–408. [DOI] [PubMed] [Google Scholar]
- 12. Greenwald I (1994) Structure/function studies of lin‐12/Notch proteins. Curr. Opin. Genet. Dev. 4, 556–562. [DOI] [PubMed] [Google Scholar]
- 13. Mitsiadis TA, Lardelli M, Lendahl U, Thesleff I (1995a) Expression of Notch 1, 2 and 3 is regulated by epithelial‐mesenchymal interactions and retinoic acid in the developing mouse tooth and associated with determination of ameloblast cell fate. J. Cell Biol. 130, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebay I, Fehon RG, Artavanis‐Tsakonas S (1993) Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell 74, 319–329. [DOI] [PubMed] [Google Scholar]
- 15. Struhl G, Fitzgerald K, Greenwald I (1993) Intrinsic activity of the Lin‐12 and Notch intracellular domains in vivo. Cell 74, 331–345. [DOI] [PubMed] [Google Scholar]
- 16. Grottkau BE, Chen XR, Friedrich CC, Yang XM, Jing W, Wu Y et al. (2009) DAPT enhances the apoptosis of human tongue carcinoma cells. Int. J. Oral Sci. 1, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katoh M, Katoh M (2007) Notch signaling in gastrointestinal tract (review). Int. J. Oncol. 30, 247–251. [PubMed] [Google Scholar]
- 18. Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MA et al. (1990) Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF‐homologous genes in Drosophila. Cell 61, 523–534. [DOI] [PubMed] [Google Scholar]
- 19. Gray GE, Mann RS, Mitsiadis E, Henrique D, Carcangiu ML, Banks A et al. (1999) Human ligands of the Notch receptor. Am. J. Pathol. 154, 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leong KG, Karsan A (2006) Recent insights into the role of Notch signaling in tumorigenesis. Blood 107, 2223–2233. [DOI] [PubMed] [Google Scholar]
- 21. Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis‐Tsakonas S (1991) Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell 67, 687–699. [DOI] [PubMed] [Google Scholar]
- 22. Katoh M, Katoh M (2006a) Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int. J. Mol. Med. 17, 681–685. [PubMed] [Google Scholar]
- 23. Lindsell CE, Shawber CJ, Boulter J, Weinmaster G (1995). Jagged: a mammalian ligand that activates Notch1. Cell 80, 909–917. [DOI] [PubMed] [Google Scholar]
- 24. Luo B, Aster JC, Hasserjian RP, Kuo F, Sklar J (1997) Isolation and functional analysis of a cDNA for human Jagged2, a gene encoding a ligand for the Notch1 receptor. Mol. Cell. Biol. 17, 6057–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitsiadis TA, Regaudiat L, Gridley T (2005) Role of the Notch signalling pathway in tooth morphogenesis. Arch. Oral Biol. 50, 137–140. [DOI] [PubMed] [Google Scholar]
- 26. Eiraku M, Hirata Y, Takeshima H, Hirano T, Kengaku M (2002) Delta/notch‐like epidermal growth factor (EGF)‐related receptor, a novel EGF‐like repeat‐containing protein targeted to dendrites of developing and adult central nervous system neurons. J. Biol. Chem. 277, 25400–25407. [DOI] [PubMed] [Google Scholar]
- 27. Cui XY, Hu QD, Tekaya M, Shimoda Y, Ang BT, Nie DY et al. (2004) NB‐3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J. Biol. Chem. 279, 25858–25865. [DOI] [PubMed] [Google Scholar]
- 28. Kluppel M, Wrana JL (2005) Turning it up a Notch: cross‐talk between TGF beta and Notch signaling. Bioessays 27, 115–118. [DOI] [PubMed] [Google Scholar]
- 29. Miele L (2006) Notch signaling. Clin. Cancer Res. 12, 1074–1079. [DOI] [PubMed] [Google Scholar]
- 30. Miele L, Golde T, Osborne B (2006) Notch signaling in cancer. Curr. Mol. Med. 6, 905–918. [DOI] [PubMed] [Google Scholar]
- 31. Osipo C, Golde TE, Osborne BA, Miele LA (2008) Off the beaten pathway: the complex cross talk between Notch and NF‐kappaB. Lab. Invest. 88, 11–17. [DOI] [PubMed] [Google Scholar]
- 32. Oswald F, Tauber B, Dobner T, Bourteele S, Kostezka U, Adler G et al. (2001) p300 acts as a transcriptional coactivator for mammalian Notch‐1. Mol. Cell. Biol. 21, 7761–7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wallberg AE, Pedersen K, Lendahl U, Roeder RG (2002) p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by notch intracellular domains in vitro. Mol. Cell. Biol. 22, 7812–7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baron M (2003) An overview of the Notch signalling pathway. Semin. Cell Dev. Biol. 14, 113–119. [DOI] [PubMed] [Google Scholar]
- 35. Kojika S, Griffin JD (2001) Notch receptors and hematopoiesis. Exp. Hematol. 29, 1041–1052. [DOI] [PubMed] [Google Scholar]
- 36. Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T et al. (1995) Disruption of the mouse RBP‐J kappa gene results in early embryonic death. Development 121, 3291–3301. [DOI] [PubMed] [Google Scholar]
- 37. Sullivan DC, Bicknell R (2003) New molecular pathways in angiogenesis. Br. J. Cancer 89, 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 194, 237–255. [DOI] [PubMed] [Google Scholar]
- 39. Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K et al. (2002) Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 298, 840–843. [DOI] [PubMed] [Google Scholar]
- 40. Ronchini C, Capobianco AJ (2001) Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic). Mol. Cell. Biol. 21, 5925–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H et al. (2001) Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ge W, Martinowich K, Wu X, He F, Miyamoto A, Fan G et al. (2002) Notch signaling promotes astrogliogenesis via direct CSL‐mediated glial gene activation. J. Neurosci. Res. 69, 848–860. [DOI] [PubMed] [Google Scholar]
- 43. Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R et al. (2003) Notch signaling regulates left‐right asymmetry determination by inducing Nodal expression. Genes Dev. 17, 1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oakley F, Mann J, Ruddell RG, Pickford J, Weinmaster G, Mann DA (2003) Basal expression of IkappaBalpha is controlled by the mammalian transcriptional repressor RBP‐J (CBF1) and its activator Notch1. J. Biol. Chem. 278, 24359–24370. [DOI] [PubMed] [Google Scholar]
- 45. Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Simel EJ et al. (2001) Notch‐1 regulates NF‐kappaB activity in hemopoietic progenitor cells. J. Immunol. 167, 4458–4467. [DOI] [PubMed] [Google Scholar]
- 46. Oswald F, Liptay S, Adler G, Schmid RM (1998) NF‐kappaB2 is a putative target gene of activated Notch‐1 via RBP‐Jkappa. Mol. Cell. Biol. 18, 2077–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lecourtois M, Schweisguth F (1995) The neurogenic suppressor of hairless DNA‐binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev. 9, 2598–2608. [DOI] [PubMed] [Google Scholar]
- 48. Bush G, diSibio G, Miyamoto A, Denault JB, Leduc R, Weinmaster G (2001) Ligand‐induced signaling in the absence of furin processing of Notch1. Dev. Biol. 229, 494–502. [DOI] [PubMed] [Google Scholar]
- 49. Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, Hayward D et al. (1996) Notch signaling inhibits muscle cell differentiation through a CBF1‐independent pathway. Development 122, 3765–3773. [DOI] [PubMed] [Google Scholar]
- 50. Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE et al. (2006) Notch1 augments NF‐kappaB activity by facilitating its nuclear retention. EMBO J. 25, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sriuranpong V, Borges MW, Strock CL, Nakakura EK, Watkins DN, Blaumueller CM et al. (2002) Notch signaling induces rapid degradation of achaete‐scute homolog 1. Mol. Cell. Biol. 22, 3129–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lovschall H, Tummers M, Thesleff I, Fuchtbauer EM, Poulsen K (2005) Activation of the Notch signaling pathway in response to pulp capping of rat molars. Eur. J. Oral Sci. 113, 312–317. [DOI] [PubMed] [Google Scholar]
- 53. Mitsiadis TA, Fried K, Goridis C (1999) Reactivation of Delta‐Notch signaling after injury: complementary expression patterns of ligand and receptor in dental pulp. Exp. Cell Res. 246, 312–318. [DOI] [PubMed] [Google Scholar]
- 54. Mucchielli ML, Mitsiadis TA (2000) Correlation of asymmetric Notch2 expression and mouse incisor rotation. Mech. Dev. 91, 379–382. [DOI] [PubMed] [Google Scholar]
- 55. Pouyet L, Mitsiadis TA (2000) Dynamic Lunatic fringe expression is correlated with boundaries formation in developing mouse teeth. Mech. Dev. 91, 399–402. [DOI] [PubMed] [Google Scholar]
- 56. Thesleff I, Aberg T (1997) Tooth morphogenesis and the differentiation of ameloblasts. Ciba Found. Symp. 205, 3–12; discussion 12–17. [PubMed] [Google Scholar]
- 57. Lumsden AG (1988) Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 103(Suppl), 155–169. [DOI] [PubMed] [Google Scholar]
- 58. Mina M, Kollar EJ (1987) The induction of odontogenesis in non‐dental mesenchyme combined with early murine mandibular arch epithelium. Arch. Oral Biol. 32, 123–127. [DOI] [PubMed] [Google Scholar]
- 59. Jernvall J, Keranen SV, Thesleff I (2000) Evolutionary modification of development in mammalian teeth: quantifying gene expression patterns and topography. Proc. Natl. Acad. Sci. USA 97, 14444–14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laurikkala J, Mikkola M, Mustonen T, Aberg T, Koppinen P, Pispa J et al. (2001) TNF signaling via the ligand‐receptor pair ectodysplasin and edar controls the function of epithelial signaling centers and is regulated by Wnt and activin during tooth organogenesis. Dev. Biol. 229, 443–455. [DOI] [PubMed] [Google Scholar]
- 61. Vaahtokari A, Aberg T, Jernvall J, Keranen S, Thesleff I (1996) The enamel knot as a signaling center in the developing mouse tooth. Mech. Dev. 54, 39–43. [DOI] [PubMed] [Google Scholar]
- 62. Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I (1994) Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non‐dividing cells express growth stimulating Fgf‐4 gene. Int. J. Dev. Biol. 38, 463–469. [PubMed] [Google Scholar]
- 63. Mustonen T, Tummers M, Mikami T, Itoh N, Zhang N, Gridley T et al. (2002) Lunatic fringe FGF, and BMP regulate the Notch pathway during epithelial morphogenesis of teeth. Dev. Biol. 248, 281–293. [DOI] [PubMed] [Google Scholar]
- 64. Harada H, Ichimori Y, Yokohama‐Tamaki T, Ohshima H, Kawano S, Katsube K et al. (2006) Stratum intermedium lineage diverges from ameloblast lineage via Notch signaling. Biochem. Biophys. Res. Commun. 340, 611–616. [DOI] [PubMed] [Google Scholar]
- 65. Gridley T (2003). Notch signaling and inherited disease syndromes. Hum. Mol. Genet. 12 Spec No 1 , R9–R13. [DOI] [PubMed] [Google Scholar]
- 66. Lewis J (1998) Notch signalling and the control of cell fate choices in vertebrates. Semin. Cell Dev. Biol. 9, 583–589. [DOI] [PubMed] [Google Scholar]
- 67. Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I (1999) Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J. Cell Biol. 147, 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mitsiadis TA, Henrique D, Thesleff I, Lendahl U (1997). Mouse Serrate‐1 (Jagged‐1): expression in the developing tooth is regulated by epithelial‐mesenchymal interactions and fibroblast growth factor‐4. Development 124, 1473–1483. [DOI] [PubMed] [Google Scholar]
- 69. Weinmaster G (1997) The ins and outs of notch signaling. Mol. Cell. Neurosci. 9, 91–102. [DOI] [PubMed] [Google Scholar]
- 70. About I, Mitsiadis TA (2001) Molecular aspects of tooth pathogenesis and repair: in vivo and in vitro models. Adv. Dent. Res. 15, 59–62. [DOI] [PubMed] [Google Scholar]
- 71. Mitsiadis TA, Romeas A, Lendahl U, Sharpe PT, Farges JC (2003) Notch2 protein distribution in human teeth under normal and pathological conditions. Exp. Cell Res. 282, 101–109. [DOI] [PubMed] [Google Scholar]
- 72. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 97, 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Honda MJ, Shinohara Y, Sumita Y, Tonomura A, Kagami H, Ueda M (2006) Shear stress facilitates tissue‐engineered odontogenesis. Bone 39, 125–133. [DOI] [PubMed] [Google Scholar]
- 74. Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC (2002) Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J. Dent. Res. 81, 695–700. [DOI] [PubMed] [Google Scholar]
- 75. Bray S (1998) A Notch affair. Cell 93, 499–503. [DOI] [PubMed] [Google Scholar]
- 76. Carlesso N, Aster JC, Sklar J, Scadden DT (1999) Notch1‐induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood 93, 838–848. [PubMed] [Google Scholar]
- 77. Fortini ME, Artavanis‐Tsakonas S (1993) Notch: neurogenesis is only part of the picture. Cell 75, 1245–1247. [DOI] [PubMed] [Google Scholar]
- 78. Morsczeck C, Moehl C, Gotz W, Heredia A, Schaffer TE, Eckstein N et al. (2005) In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol. Int. 29, 567–575. [DOI] [PubMed] [Google Scholar]
- 79. Grottkau BE, Purudappa PP, Lin YF (2010) Multilineage differentiation of dental pulp stem cells from green fluorescent protein transgenic mice. Int. J. Oral Sci. 2, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peng L, Ye L, Zhou XD (2009) Mesenchymal stem cells and tooth engineering. Int. J. Oral Sci. 1, 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lovschall H, Mitsiadis TA, Poulsen K, Jensen KH, Kjeldsen AL (2007) Coexpression of Notch3 and Rgs5 in the pericyte‐vascular smooth muscle cell axis in response to pulp injury. Int. J. Dev. Biol. 51, 715–721. [DOI] [PubMed] [Google Scholar]
- 82. Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M (2006) Side population cells isolated from porcine dental pulp tissue with self‐renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 24, 2493–2503. [DOI] [PubMed] [Google Scholar]
- 83. Kawanabe N, Murakami K, Takano‐Yamamoto T (2006) The presence of ABCG2‐dependent side population cells in human periodontal ligaments. Biochem. Biophys. Res. Commun. 344, 1278–1283. [DOI] [PubMed] [Google Scholar]
- 84. Matsuzaki Y, Kinjo K, Mulligan RC, Okano H (2004) Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity 20, 87–93. [DOI] [PubMed] [Google Scholar]
- 85. Honda MJ, Nakashima F, Satomura K, Shinohara Y, Tsuchiya S, Watanabe N et al. (2007) Side population cells expressing ABCG2 in human adult dental pulp tissue. Int. Endod. J. 40, 949–958. [DOI] [PubMed] [Google Scholar]
- 86. Kumano K, Chiba S, Shimizu K, Yamagata T, Hosoya N, Saito T et al. (2001). Notch1 inhibits differentiation of hematopoietic cells by sustaining GATA‐2 expression. Blood 98, 3283–3289. [DOI] [PubMed] [Google Scholar]
- 87. Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT (2002) Notch1 activation increases hematopoietic stem cell self‐renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 99, 2369–2378. [DOI] [PubMed] [Google Scholar]
- 88. Aberg T, Wozney J, Thesleff I (1997) Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev. Dyn. 210, 383–396. [DOI] [PubMed] [Google Scholar]
- 89. Adams JC, Watt FM (1993) Regulation of development and differentiation by the extracellular matrix. Development 117, 1183–1198. [DOI] [PubMed] [Google Scholar]
- 90. Mitsiadis TA, Muramatsu T, Muramatsu H, Thesleff I (1995b) Midkine (MK), a heparin‐binding growth/differentiation factor, is regulated by retinoic acid and epithelial‐mesenchymal interactions in the developing mouse tooth, and affects cell proliferation and morphogenesis. J. Cell Biol. 129, 267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mitsiadis TA, Salmivirta M, Muramatsu T, Muramatsu H, Rauvala H, Lehtonen E et al. (1995c) Expression of the heparin‐binding cytokines, midkine (MK) and HB‐GAM (pleiotrophin) is associated with epithelial‐mesenchymal interactions during fetal development and organogenesis. Development 121, 37–51. [DOI] [PubMed] [Google Scholar]
- 92. Ruch JV, Lesot H, Begue‐Kirn C (1995) Odontoblast differentiation. Int. J. Dev. Biol. 39, 51–68. [PubMed] [Google Scholar]
- 93. Vaahtokari A, Vainio S, Thesleff I (1991) Associations between transforming growth factor beta 1 RNA expression and epithelial‐mesenchymal interactions during tooth morphogenesis. Development 113, 985–994. [DOI] [PubMed] [Google Scholar]
- 94. Vainio S, Karavanova I, Jowett A, Thesleff I (1993) Identification of BMP‐4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75, 45–58. [PubMed] [Google Scholar]
- 95. Nakashima M (1994) Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins‐2 and ‐4 with collagen matrix. Arch. Oral Biol. 39, 1085–1089. [DOI] [PubMed] [Google Scholar]
- 96. Tziafas D, Smith AJ, Lesot H (2000) Designing new treatment strategies in vital pulp therapy. J. Dent. 28, 77–92. [DOI] [PubMed] [Google Scholar]
- 97. van Mullem PJ (1991) Healing of the guinea pig incisor after partial pulp removal. Endod. Dent. Traumatol. 7, 164–176. [DOI] [PubMed] [Google Scholar]
- 98. Begue‐Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D et al. (1992) Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int. J. Dev. Biol. 36, 491–503. [PubMed] [Google Scholar]
- 99. Melin M, Joffre‐Romeas A, Farges JC, Couble ML, Magloire H, Bleicher F (2000) Effects of TGFbeta1 on dental pulp cells in cultured human tooth slices. J. Dent. Res. 79, 1689–1696. [DOI] [PubMed] [Google Scholar]
- 100. Faux CH, Turnley AM, Epa R, Cappai R, Bartlett PF (2001) Interactions between fibroblast growth factors and Notch regulate neuronal differentiation. J. Neurosci. 21, 5587–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Uyttendaele H, Soriano JV, Montesano R, Kitajewski J (1998) Notch4 and Wnt‐1 proteins function to regulate branching morphogenesis of mammary epithelial cells in an opposing fashion. Dev. Biol. 196, 204–217. [DOI] [PubMed] [Google Scholar]
- 102. Hansson EM, Lendahl U, Chapman G (2004) Notch signaling in development and disease. Semin. Cancer Biol. 14, 320–328. [DOI] [PubMed] [Google Scholar]
- 103. Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U et al. (2003) Cross‐talk between the Notch and TGF‐beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J. Cell Biol. 163, 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF et al. (2003) Functional Notch signaling is required for BMP4‐induced inhibition of myogenic differentiation. Development 130, 6089–6099. [DOI] [PubMed] [Google Scholar]
- 105. Itoh F, Itoh S, Goumans MJ, Valdimarsdottir G, Iso T, Dotto GP et al. (2004) Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. EMBO J. 23, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Henderson AM, Wang SJ, Taylor AC, Aitkenhead M, Hughes CC (2001) The basic helix‐loop‐helix transcription factor HESR1 regulates endothelial cell tube formation. J. Biol. Chem. 276, 6169–6176. [DOI] [PubMed] [Google Scholar]
- 107. Campbell KJ, Perkins ND (2006) Regulation of NF‐kappaB function. Biochem. Soc. Symp. 73, 165–180. [DOI] [PubMed] [Google Scholar]
- 108. Ohazama A, Hu Y, Schmidt‐Ullrich R, Cao Y, Scheidereit C, Karin M et al. (2004) A dual role for Ikk alpha in tooth development. Dev. Cell 6, 219–227. [DOI] [PubMed] [Google Scholar]
- 109. Bash J, Zong WX, Banga S, Rivera A, Ballard DW, Ron Y et al. (1999) Rel/NF‐kappaB can trigger the Notch signaling pathway by inducing the expression of Jagged1, a ligand for Notch receptors. EMBO J. 18, 2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Moran ST, Cariappa A, Liu H, Muir B, Sgroi D, Boboila C et al. (2007) Synergism between NF‐kappa B1/p50 and Notch2 during the development of marginal zone B lymphocytes. J. Immunol. 179, 195–200. [DOI] [PubMed] [Google Scholar]
- 111. Guan E, Wang J, Laborda J, Norcross M, Baeuerle PA, Hoffman T (1996) T cell leukemia‐associated human Notch/translocation‐associated Notch homologue has I kappa B‐like activity and physically interacts with nuclear factor‐kappa B proteins in T cells. J. Exp. Med. 183, 2025–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang J, Shelly L, Miele L, Boykins R, Norcross MA, Guan E (2001) Human Notch‐1 inhibits NF‐kappa B activity in the nucleus through a direct interaction involving a novel domain. J. Immunol. 167, 289–295. [DOI] [PubMed] [Google Scholar]
- 113. Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R (2002) FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(−/−) mice. Genes Dev. 16, 3173–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sarkar L, Sharpe PT (1999) Expression of Wnt signalling pathway genes during tooth development. Mech. Dev. 85, 197–200. [DOI] [PubMed] [Google Scholar]
- 115. Katoh M (2005a) Epithelial‐mesenchymal transition in gastric cancer (Review). Int. J. Oncol. 27, 1677–1683. [PubMed] [Google Scholar]
- 116. Katoh M (2005b) WNT2B: comparative integromics and clinical applications (Review). Int. J. Mol. Med. 16, 1103–1108. [PubMed] [Google Scholar]
- 117. Swain RK, Katoh M, Medina A, Steinbeisser H (2005) Xenopus frizzled‐4S, a splicing variant of Xfz4 is a context‐dependent activator and inhibitor of Wnt/beta‐catenin signaling. Cell Commun. Signal. 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Katoh M, Katoh M (2006b) NUMB is a break of WNT‐Notch signaling cycle. Int. J. Mol. Med. 18, 517–521. [PubMed] [Google Scholar]
- 119. Strutt D, Johnson R, Cooper K, Bray S (2002) Asymmetric localization of frizzled and the determination of notch‐dependent cell fate in the Drosophila eye. Curr. Biol. 12, 813–824. [DOI] [PubMed] [Google Scholar]
- 120. Ross DA, Kadesch T (2001) The notch intracellular domain can function as a coactivator for LEF‐1. Mol. Cell. Biol. 21, 7537–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Foltz DR, Santiago MC, Berechid BE, Nye JS (2002) Glycogen synthase kinase‐3beta modulates notch signaling and stability. Curr. Biol. 12, 1006–1011. [DOI] [PubMed] [Google Scholar]
- 122. Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M et al. (2003) Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 33, 416–421. [DOI] [PubMed] [Google Scholar]
- 123. Bitgood MJ, McMahon AP (1995) Hedgehog and Bmp genes are coexpressed at many diverse sites of cell‐cell interaction in the mouse embryo. Dev. Biol. 172, 126–138. [DOI] [PubMed] [Google Scholar]
- 124. Dassule HR, McMahon AP (1998) Analysis of epithelial‐mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev. Biol. 202, 215–227. [DOI] [PubMed] [Google Scholar]
- 125. Hardcastle Z, Mo R, Hui CC, Sharpe PT (1998). The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development 125, 2803–2811. [DOI] [PubMed] [Google Scholar]
- 126. Zhang Y, Zhao X, Hu Y, St Amand T, Zhang M, Ramamurthy R et al. (1999) Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev. Dyn. 215, 45–53. [DOI] [PubMed] [Google Scholar]
- 127. Dassule HR, Lewis P, Bei M, Maas R, McMahon AP (2000) Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775–4785. [DOI] [PubMed] [Google Scholar]
- 128. Ingram WJ, McCue KI, Tran TH, Hallahan AR, Wainwright BJ (2008) Sonic Hedgehog regulates Hes1 through a novel mechanism that is independent of canonical Notch pathway signalling. Oncogene 27, 1489–1500. [DOI] [PubMed] [Google Scholar]


