Abstract
Abstract. Objective: Dietary conjugated linoleic acids (CLA) have had many health benefits claimed for them, including antineoplastic actions. Materials and methods: The effects of the predominant forms of CLA, namely the c9t11 and t10c12 isomers, or a mixture of these on polyp development, were investigated in the Apc Min/+ mouse. CLAs have also been linked to altered rates of cell renewal and cell proliferation so this was also studied, as was a further means of increasing tissue mass, namely crypt fission. Results: The stomach and small intestine were significantly heavier in the t10c12, and in the mixture‐treated groups (P < 0.001). Crypt fission was increased in the middle small intestine by the t10c12 diet while colonic weight was reduced by c9t11 provision and crypts were 20% shorter. The t10c12 and the mixture significantly reduced polyp number in the proximal small intestine but they increased polyp diameter in the middle and distal small intestine, to an extent that the polyp burden was significantly increased at these sites. All CLAs significantly reduced polyp number in the colon, but the mixture significantly increased polyp diameter in the colon. Conclusion: Increased polyp diameter associated with t10c12 diet and especially with the mixture is a cause of concern, as this is the commercially available form. The naturally occurring isomer, c9t11 decreased colonic polyp number and did not increase diameter, suggesting that this natural isomer is the most likely to be protective.
INTRODUCTION
Conjugated linoleic acids (CLAs) are chemical isomers of the 18‐carbon fatty acid, linoleic acid, in which the two double bonds are conjugated, or contiguous, unlike double bonds in linoleic acid, which are separated by methylene group (Tricon et al. 2005). The most abundant forms are the cis‐9, trans‐11 (c9t11) and trans‐10, cis‐12 (t10c12) isomers.
The main natural source of CLAs is microbial biohydrogenation of linoleic acid in the forestomach of ruminant animals, thus almost all the CLAs in the human diet come from ruminant products such as milk, cheese, yoghurt and meat. Ruminants have a highly specialized foregut fermentation system where the microbes in the rumen convert structural plant carbohydrates (such as cellulose), that are indigestible to mammals, into absorbable energy including the c9t11 CLA isomer, also called rumenic acid (Wahle et al. 2004).
Conjugated linoleic acids have many actions and animal studies have generated considerable interest concerning their role in the control of metabolic rate, body fat and muscle mass. CLAs are now widely available as ‘health supplements’, which are predominantly mixtures of the c9t11 and t10c12 isomers. CLAs have anti‐inflammatory action and may alleviate colitis by activating the peroxisome proliferator‐activated receptor γ (PPARγ) (Bassaganya‐Riera et al. 2004; Sanchez‐Hidalgo et al. 2005; Bassaganya‐Riera & Hontecillas 2006). They are preferentially incorporated into the phospholipids of cell membranes (Bassaganya‐Riera & Hontecillas 2006) and have many actions on fat metabolism and storage, and may increase energy expenditure, thus causing body weight loss (Blankson et al. 2000; DeLany & West 2000). In addition, they may be anti‐atherogenic, alter insulin resistance, have a role in diabetes control (Navarro et al. 2003), and have isomer specific actions on blood lipids in man (Tricon et al. 2005).
Reports of CLA‐induced weight loss have led to their widespread promotion and sale as dietary supplements to improve health and body weight; recent studies have, however, shown that effects on body weight in humans are less pronounced than those seen in rodents (Tricon et al. 2005). While some of the discrepancies in the literature can be attributed to species differences, others may be the result of the use of different forms of CLA; earlier studies used a mixture of isomers while later studies have used purified c9t11 and t10c12 isomers. Most early studies implied a beneficial action but some recent research has been ambivalent and a few have indicated that the CLAs, especially the t10c12 isomer, can be deleterious and may be associated with liver hypertrophy and insulin resistance (via redistribution of fat deposition resembling lipodystrophy) (Larsen et al. 2003; Wahle et al. 2004). There are many reports that CLAs can have an anticancer effect, they were originally discovered in fried ground beef, where contrary to expectation, they were found (by the Ames Salmonella test) to be anticarcinogenic (Ha et al. 1987).
Conjugated linoleic acids have been implicated in the prevention of breast cancer (Kimoto et al. 2001; Hubbard et al. 2003) and colon cancer (Park et al. 2001; Kim & Park 2003; Kohno et al. 2004), perhaps by decreasing cell proliferation and inducing apoptosis (Cho et al. 2003; Park et al. 2004). These antiproliferative and preventative effects may be due to down‐regulation of some target genes of the Apc/β‐catenin/TCF‐4 and PPARδ, and PPARγ signalling pathway (Lampen et al. 2005; , Bassaganya‐Riera & Hontecillas 2006). Not all reports on cancer progression have been positive, as some have found that CLAs, particularly t10c12, have pro‐carcinogenic effects in animal models of colon (Rajakangas et al. 2003) and prostate cancer (Cohen et al. 2003; Goodlad 2007).
Therefore, we decided to investigate effects of the two main isomers of CLA, and a mixture of these, on polyp formation in the multiple intestinal neoplasia, Apc Min/+ mouse, which is heterozygous at the Apc (adenomatous polyposis coli) locus. Loss of the remaining wild‐type allele leads to β‐catenin accumulation and its relocation to the nucleus, where it forms a complex with TCF‐4 leading to transcription of tumour promoting genes. The Apc Min/+ mouse is generally considered to be a relevant model of gut cancer (Boivin et al. 2003; Corpet & Pierre 2003), and although more polyps are seen in the small intestine than in the colon here, this is a common feature of mouse models and polyps seen are similar to those found in humans with the same mutation (familiar adenomatous polyposis; Goodlad 2007).
Altered cell proliferation has been associated with CLAs in the diet (Belury 2002), and we have quantified this effect throughout the gut using the well‐established crypt microdissection method (Alferez & Goodlad 2007). This method is up to six times faster than scoring histological sections and avoids several problems associated with quantifying cell labelling in histological sections (Goodlad & Alferez 2005). As the results of these measures were inconclusive, we also measured metaphase and bromodeoxyuridine (BrdUrd) labelling and distribution within the colonic crypts (Alferez & Goodlad 2007). Crypt fission was also measured as this is an alternate and independent means (Berlanga‐Acosta et al. 2001) of increasing intestinal tissue mass by creating new crypts, which could be a main mechanism for the spread of mutant cells in the gut (Preston et al. 2003; Brittan & Wright 2004).
MATERIALS AND METHODS
Apc Min/+ mice
Apc Min/+ heterozygote mice were originally obtained as a gift from Amy R. Moser (McArdle Laboratory for Cancer Research, University of Wisconsin, Madison, WI, USA) (Moser et al. 1993). Male mice were back‐crossed to female C57BL/6Js and the resultant embryos were transferred by aseptic hysterectomy to foster mothers in specific pathogen‐free isolators. All breeding was subsequently by brother (C57BL/6 J‐Apc Min/+) sister (C57BL/6 J) mating. Genotyping was carried out by a polymerase chain reaction‐based method, using three primers including an internal control for normal mouse DNA. All procedures were approved by the Cancer Research UK Animal Ethics Committees and were covered by the appropriate licences under the Home Office Animal Procedures Act 1986.
Study design
Four groups of 6‐week‐old Apc Min/+ mice were put on the following diets based on a powered version of a standard mouse maintenance diet, RM1 (Special Diets Services no. 1, Stepfield, Witham, Essex, UK; CM8 3AB). There were 20 mice per group.
| Group 1 | Control diet |
| Group 2 | diet + 1% c9/t11 |
| Group 3 | diet + 1% t10/c12 |
| Group 4 | diet + mixture of 0.5% of c9/t11 and 0.5% of t10/c12 |
Conjugated linoleic acids were purchased from Natural ASA, Kjorbokollen 30, N‐1337, Sandvika, Norway, and had the following analysis:
-
•
C18; 2, c9/t11 90.1% plus 4.7% oleic acid, 2.3% t10/c12 and 2.5% other isomers of C18; 2
-
•
C18; 2, t10/c12 92.3% plus 0.8% oleic acid, 4.3% c9/t11 and 2.4% other isomers of C18; 2
-
•
C18; 2, mixture 44.3% c9/t11, 43.5% t10/c12 plus 5.0% oleic acid and 5.1% other isomers of C18; 2
Autopsy
After 8 weeks, mice were injected with 1 mg/kg vincristine (to arrest cells as they enter metaphase) 2 h before and 50 mg/kg BrdUrd 1 h before being killed (Alferez & Goodlad 2007). Small intestines and colons were isolated, rinsed and weighed. The small bowel (SB) was divided into three equal sections (proximal – SB1, middle – SB2 and distal – SB3), dissected longitudinally using a recently described gut cutting device (Rudling et al. 2006) and spread on to filter paper. The entire colon was also dissected and spread on to filter paper. These gut preparations were then fixed in Carnoy's fixative (3 h) and transferred to 70% ethanol. Tissues were assessed later under a stereomicroscope (×20 magnification) for polyp number and diameter, which was measured using digital callipers. Polyp volume was derived from polyp diameter, assuming a hemispherical shape in the small bowel, and a spherical shape in the colon. Tumour burden was calculated as the product of polyp number and polyp volume (Bashir et al. 2004).
Assessment of cell proliferation and crypt fission throughout the gut was performed using the ‘crypt microdissection’ method (Alferez & Goodlad 2007). Representative samples of tissue from the proximal, middle and distal small intestine and colon (taken from positions 10%, 50% and 90% of the total length of the small bowel or colon) were hydrated, hydrolysed and stained with the Feulgen reaction. Mucosal crypts were gently teased apart under a dissection microscope and the numbers of mitoses per crypt (mean of 20 crypts) and crypt fission events per 200 crypts were then determined (Alferez & Goodlad 2007). All samples were counted in a blind.
Distribution and number of BrdUrd‐labelled cells was determined in 4 µm wax‐embedded histological sections. Thirty well‐orientated crypts per mouse from the middle colon were scored. Immunohistochemistry was performed by the streptavidin biotin conjugate method using a mouse monoclonal antibody to BrdUrd; the second antibody was rabbit antimouse‐conjugated biotin, and the third layer was streptavidin–peroxidase complex, which was developed in 3,3′‐diaminobenzidine tetrahydrochloride (DAB) plus hydrogen peroxide, and counterstained with haematoxylin. Distribution of label and metaphases was recorded for each cell position starting from the base of the crypt. Labelling distribution was used to determine the maximum plus the half maximum labelling positions, which were then used to determine the growth fraction (Mandir & Goodlad 1999).
β‐Catenin was detected in histological sections using sequential incubation with mouse anti‐β‐catenin monoclonal primary antibody (1 : 100; DakoCytomation Ltd., Ely, UK), a biotin‐conjugated rabbit antimouse polyclonal secondary antibody (1 : 300; DakoCytomation Ltd.), and a streptavidin–peroxidase reagent that was developed in DAB. After counterstaining with haematoxylin, slides were assessed in semiquantitative manner. Ten high‐power fields (×40) per slide were assigned a score of 0–3 for the proportion and intensity of staining in both the cytoplasm and nuclei.
Statistics
Results are presented as the mean standard error of the mean. Data were tested by analysis of variance using Minitab Statistical Software (release 10.5 Xtra Minitab, Coventry, UK). If an effect of treatment was seen, Dunnet's post hoc analysis was performed using the unsupplemented group as reference comparison.
RESULTS
No differences in body weight gain were seen between the different groups (Fig. 1). Spleens, which are a useful surrogate marker of tumour load (Orner et al. 2002), were somewhat heavier in the t10c12 and mixture‐treated groups but this was not statistically significant.
Figure 1.
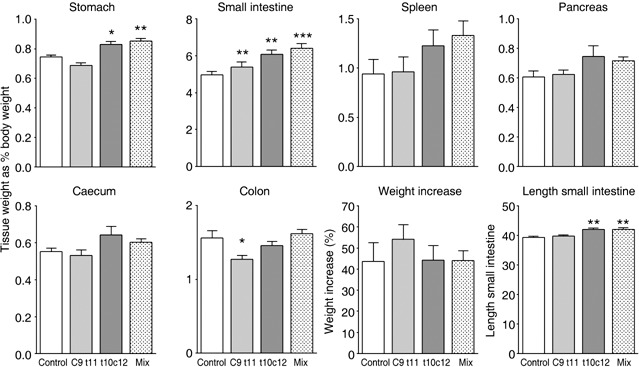
Effects of the various treatments on tissue wet weight and weight change. Tissue weights were normalized by expressing them as a percentage of the body weight at autopsy. Asterisks indicate post hoc comparison against the control groups using Dunnet's test after one‐way anova where *P < 0.05, **P < 0.01 and ***P < 0.001.
Stomachs were significantly heavier in the t10c12 and mixture‐treated groups, with small intestines being heavier in all treated groups. They were also significantly longer in the t10c12 and mixture‐treated groups. Colons were significantly lighter in the c9t11 group. These weight changes must reflect a general change in the mass of normal cells as the sum of all the small bowel polyps (see Fig. 2) would only add an extra 0.065 g to the weight of this tissue, whereas average weight increase in the mixture‐treated group was 0.27 g.
Figure 2.
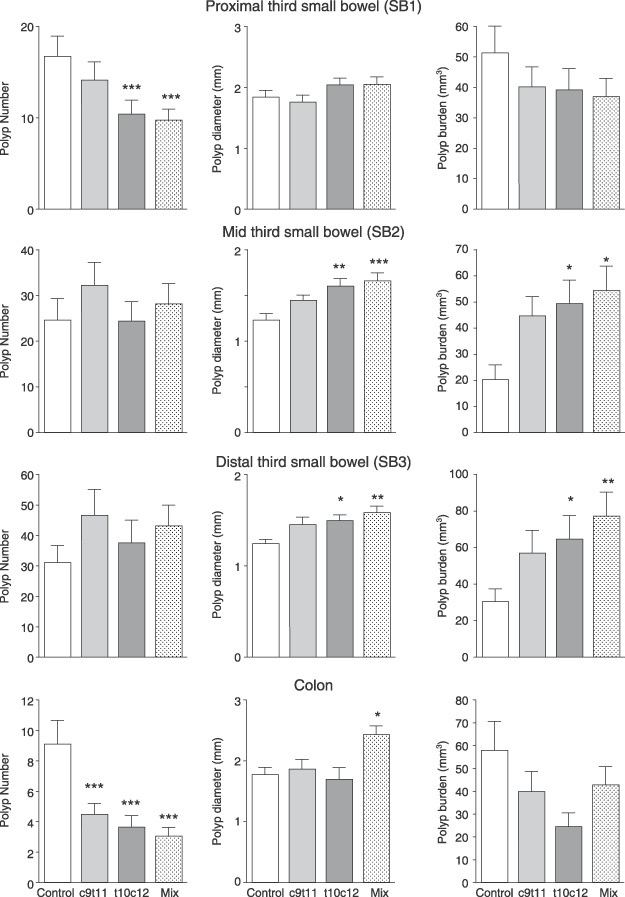
Polyp number, polyp diameter and tumour burden in the three segments of the small intestines (SB1 = the proximal third, SB2 = the middle third and SB3 = the distal third) and the colon. Asterisks indicate post hoc comparison against the control groups using Dunnet's test after one‐way anova where *P < 0.05, **P < 0.01 and ***P < 0.001.
The t10c12 and the mixture significantly reduced polyp number in the proximal small intestine (by 37% and 42%, respectively, P < 0.001, see Fig. 2); however, t10c12 and the mixture increased polyp diameter in the middle small intestine (by 29% and 34%) and distal small intestine by 20% and 27%. Polyp burden (product of number and volume) was thus also more than doubled by these CLA diets in the middle and distal sites.
In the colon, all CLA classes significantly reduced polyp number (from 9.1 ± 1.6 to 4.4 ± 0.7, 3.6 ± 0.8 and 3.0 ± 0.6, P < 0.001). Isomers had no effect on polyp diameter, but the mixture significantly increased polyp diameter in the colon. When burden was calculated for the colon, no significant difference between groups was seen although pure isomers seemed to produce less tumour burden. Very little staining for β‐catenin was seen in nuclei of normal tissue, while nuclei from tumours were highly stained; however, no significant differences between groups were noted (Fig. 3).
Figure 3.
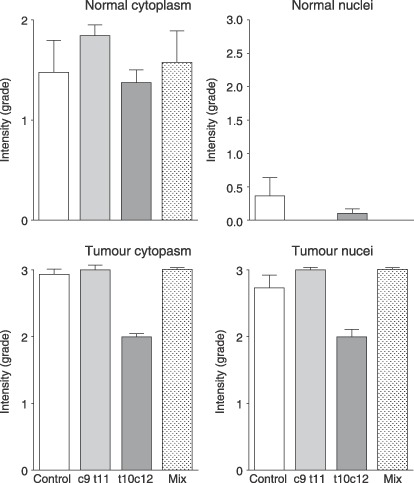
Quantification of β‐catenin immunohistochemical detection in the cytoplasm and nuclei of normal tissue and tumours.
Metaphase arrest in the small intestine showed an indication of increase in proliferation but this was not statistically significant (Fig. 4) and there was no indication of any change in the metaphase counts of the colon. Quantification of histological sections from the colon did not show any differences in number of metaphases nor labelled cells (Fig. 5), nor in metaphase (not shown) nor labelling indices. Number of cells per crypt was significantly lower in the group fed c9t11 and this was reflected in significant reduction in the position of half maximum labelling. There was no change in the proportion of the crypt involved in cell division – the growth fraction, as changes in half maximum labelling and crypt length were concurrent.
Figure 4.
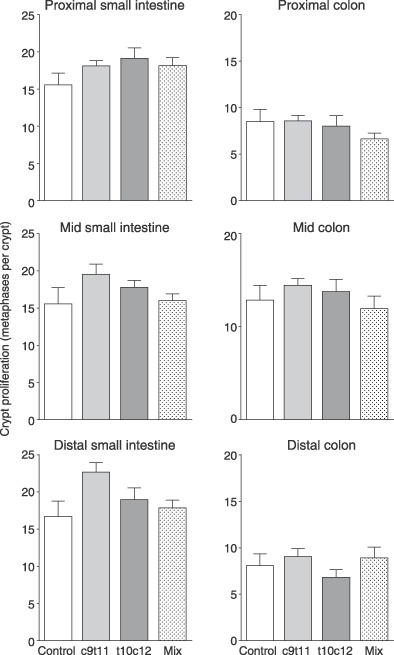
Cell proliferation, assessed by the 2‐h accumulation of vincristine‐arrested metaphases in the proximal, middle and distal small intestines and the colon.
Figure 5.
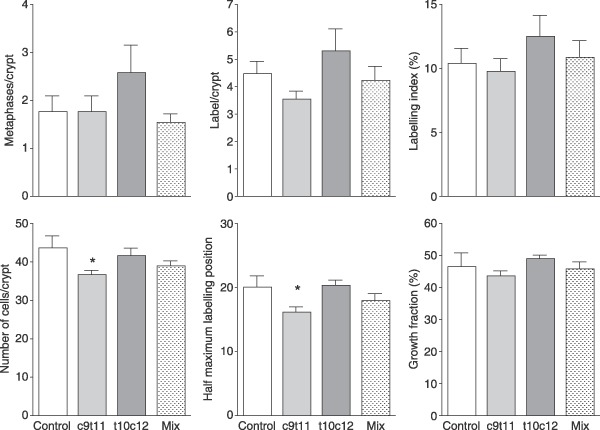
Mitoses and BrdUrd‐labelling scores per crypt and distribution of BrdUrd labelling in the middle colon. Labelled cells per crypt were plotted and the position of half maximum labelling and crypt length (in cells) was used to determine proportion of crypts involved in proliferation (growth fraction). Asterisks indicate post hoc comparison against the control groups using Dunnet's test after one‐way anova where *P < 0.05.
The c9t11 and the mixture had no significant effect on crypt fission in the colon, but t10/c12 significantly increased crypt fission in the middle small bowel and appeared to increase fission in the distal colon (Fig. 6).
Figure 6.
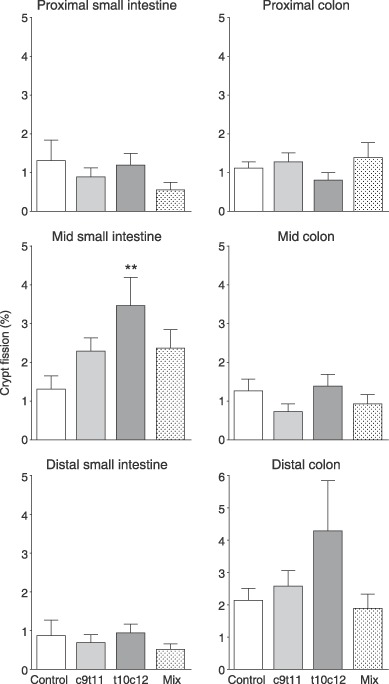
Crypt fission in the proximal, middle and distal small intestines and colon. Asterisks indicate post hoc comparison against control groups using Dunnet's test after one‐way anova where **P < 0.01.
DISCUSSION
The present study has demonstrated that while the pure isomers and the mixed preparation of CLAs could reduce polyp number in the proximal small intestine and especially in the colon, the t10c12 isomer and the mixture increased polyp diameter and tumour burden in the sites with most polyps, namely the middle and distal small bowel. The mixture also was significantly associated with increased polyp diameter in the colon.
No significant effect on polyp burden was observed in the colon, but the burden is a composite measure and as such does not provide as much precision as the other data. Resolution of colonic events in the Apc Min/+ mouse is often rather limited, as there are usually very few polyps in the colon; however, the yield of polyps in our mice was higher, which may explain why we have found, for the first time, a significant reduction in colon polyp number, in contrast to the results of other workers (Rajakangas et al. 2003; Rajakangas et al. 2003). These groups also found that while CLAs did not alter polyp number in the small intestine polyps were larger (as in the present study), especially following administration of t10c12.
Contrary to expectation, we found little effect on cell proliferation using the well‐established metaphase arrest method (Alferez & Goodlad 2007). The more labourious scoring of histological sections showed that while the crypts were 20% shorter in the c9t11 group, few other differences were detectable. The results also showed that despite these crypts being shorter, they were still in proportion, as the proliferative proportion of the crypt (growth fraction) was not different.
Some increases in crypt fission were observed in the small intestine (and there was a hint of this in the distal colon) of the t10c12 fed group. Crypt fission is an alternative means of increasing tissue mass, and is implicated in clonal expansion of mutated crypts (Wasan et al. 1998; Preston et al. 2003; Brittan & Wright 2004). The time course of these events is still unknown; thus, changes in small bowel and colon weight could reflect subtle alterations in proliferation and fission that had occurred at an earlier time.
Tissue weight of the stomach and small intestine and length of the small intestine were all increased with the t10c12 and the mixture provision, suggesting that these had a general trophic action on the small bowel. The c9t11 isomer had less effect on the weight of the small bowel and decreased colon weight, which was substantiated by the observed reduction in colonic crypt size.
The naturally occurring isomer (c9t11) did not significantly increase polyp diameter in the small bowel or in the colon and it has been suggested that this is the form of CLA most likely to have antimalignancy properties. Several reports of adverse effects with use of the t10c12 isomer have been reported, such as increased LDL:HDL cholesterol ratio and total HDL cholesterol in man, whereas c9t11 CLA decreased these (Tricon et al. 2004). The t10c12 has been shown to increase levels of C‐reactive protein, which is a marker of inflammation (Riserus et al. 2002) and inflammation can enhance tumourigenesis (Rhodes & Campbell 2002).
Fermentation of resistant carbohydrates in ruminants produces the c9t11 isomer (Wahle et al. 2004), but the content of this is considerably less in modern intensive production systems than in traditional husbandry (where animals mainly consume grass rather than concentrates) (Wijesundera et al. 2003). CLA content can also be increased by feeding ruminants supplemental feed oils, particularly fish oil (Wahle et al. 2004) and the European Union is funding a large agro‐food project for alteration of the metabolism of dairy cows to reduce levels of saturates in milk fat and increase CLA levels by changing the bioflora of cows (LipGene 2004). If this isomer of CLA is indeed protective, it would cast some doubt on the tentative link between red meat consumption of and cancer (Willett 2001). Epidemiological studies on the effects of ‘dietary fibre’ have been hampered by the use of such a broad term to define a wide range of substances (Goodlad & Englyst 2001) and in a similar vein it may no longer be enough to talk of ‘red meat’, rather one may need to determine what sort of meat and animal husbandry was involved in the dietary investigations. These changes in animal feeding are relatively recent and may help to account for differences in the literature concerning risks associated with eating red meat (Truswell 2002). Most studies showing harmful effects have come from the USA (Norat et al. 2002; Fung et al. 2003) while studies showing no ill effects are mainly non‐American (Mathew et al. 2004; Tiemersma et al. 2004; Sato et al. 2006).
Conjugated linoleic acids are involved in the cyclooxygenase and prostaglandin inflammatory pathways, and can inhibit the eicosanoid pathways (Kim & Park 2003). Considerable interest has been generated by the finding that they can ameliorate chemically and genetically induced colitis, perhaps through nuclear pathways involving PPARγ activation (Bassaganya‐Riera et al. 2004; Greicius et al. 2004). Little PPARγ is expressed in the small bowel while it is heavily expressed in the caecum and colon, which could perhaps explain different actions seen in the small intestine and colon (Saez et al. 1998).
The present study has confirmed that the two main isomers used and their mixture of CLAs can have different actions on tumour formation in the gastrointestinal tract, with the t10c12 isomer increasing polyp size in some sites and the mixture causing a more general increase on tumour size. These actions appeared to be associated with a general trophic effect on the small bowel, which may be attributable to increased crypt fission. The c9t11 isomer did not alter tumour diameter, but still markedly decreased polyp number in the colon. In addition, c9t11 fed mice had reduced colon weights, which seemed to be the result of a modest antiproliferative effect. These results suggest that naturally occurring c9t11 isomer is the form most likely to be beneficial in the diet. The ‘worst’ effects in our study were seen with the mixture of t10c12 and c9t11 isomers, which is a cause for concern as the mixture is the version commercially available in dietary supplements.
ACKNOWLEDGEMENTS
We would like to thank all the staff at the Biological Services Unit (BSU) Clare Hall for their help and Ian Rosewell for the genotyping.
REFERENCES
- Alferez D, Goodlad RA (2007) To best measure cell proliferation in samples from the intestine. Cell Prolif. 40, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir O, Fitzgerald AJ, Goodlad RA (2004) Both suboptimal and elevated vitamin intake increase intestinal neoplasia and alter crypt fission in the Apc Min/+ mouse. Carcinogenesis 25, 1507–1515. [DOI] [PubMed] [Google Scholar]
- Bassaganya‐Riera J, Hontecillas R (2006) CLA and n‐3 PUFA differentially modulate clinical activity and colonic PPAR‐responsive gene expression in a pig model of experimental IBD. Clin. Nutr. 25, 454–465. [DOI] [PubMed] [Google Scholar]
- Bassaganya‐Riera J, Reynolds K, Martino‐Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R (2004) Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 127, 777–791. [DOI] [PubMed] [Google Scholar]
- Belury MA (2002) Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J. Nutr. 132, 2995–2998. [DOI] [PubMed] [Google Scholar]
- Berlanga‐Acosta J, Playford RJ, Mandir N, Goodlad RA (2001) Gastrointestinal cell proliferation and crypt fission are separate but complementary means of increasing tissue mass following infusion of epidermal growth factor in rats. Gut 48, 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O (2000) Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J. Nutr. 130, 2943–2948. [DOI] [PubMed] [Google Scholar]
- Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ (2003) Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124, 762–777. [DOI] [PubMed] [Google Scholar]
- Brittan M, Wright NA (2004) Stem cell in gastrointestinal structure and neoplastic development. Gut 53, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH (2003) Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT‐29 human colon cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G996–1005. [DOI] [PubMed] [Google Scholar]
- Cohen LA, Zhao Z, Pittman B, Scimeca J (2003) Effect of soy protein isolate and conjugated linoleic acid on the growth of Dunning R‐3327‐AT‐1 rat prostate tumors. Prostate 54, 169–180. [DOI] [PubMed] [Google Scholar]
- Corpet DE, Pierre F (2003) Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol. Biomarkers Prev. 12, 391–400. [PMC free article] [PubMed] [Google Scholar]
- DeLany JP, West DB (2000) Changes in body composition with conjugated linoleic acid. J. Am. Coll. Nutr. 19, 487S–493S. [DOI] [PubMed] [Google Scholar]
- Fung T, Hu FB, Fuchs C, Giovannucci E, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC (2003) Major dietary patterns and the risk of colorectal cancer in women. Arch. Intern. Med. 163, 309–314. [DOI] [PubMed] [Google Scholar]
- Goodlad RA (2007) Mouse models of cancer In: Alison MR, ed. Cancer Handbook, p. 243–262. London: John Wiley & Sons Ltd. [Google Scholar]
- Goodlad RA, Alferez D (2005) Defective denominators. Gut 54, 1502–1503. [PMC free article] [PubMed] [Google Scholar]
- Goodlad RA, Englyst HN (2001) Redefining dietary fibre: potentially a recipe for disaster. Lancet 358, 1833–1834. [DOI] [PubMed] [Google Scholar]
- Greicius G, Arulampalam V, Pettersson S (2004) A CLA's act: feeding away inflammation. Gastroenterology 127, 994–996. [DOI] [PubMed] [Google Scholar]
- Ha YL, Grimm NK, Pariza MW (1987) Anticarcinogens from fried ground beef: heat‐altered derivatives of linoleic acid. Carcinogenesis 8, 1881–1887. [DOI] [PubMed] [Google Scholar]
- Hubbard NE, Lim D, Erickson KL (2003) Effect of separate conjugated linoleic acid isomers on murine mammary tumorigenesis. Cancer Lett. 190, 13–19. [DOI] [PubMed] [Google Scholar]
- Kim KH, Park HS (2003) Dietary supplementation of conjugated linoleic acid reduces colon tumor incidence in DMH‐treated rats by increasing apoptosis with modulation of biomarkers. Nutrition 19, 772–777. [DOI] [PubMed] [Google Scholar]
- Kimoto N, Hirose M, Futakuchi M, Iwata T, Kasai M, Shirai T (2001) Site‐dependent modulating effects of conjugated fatty acids from safflower oil in a rat two‐stage carcinogenesis model in female Sprague‐Dawley rats. Cancer Lett. 168, 15–21. [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Yasui Y, Hosokawa M, Miyashita K, Tanaka T (2004) Pomegranate seed oil rich in conjugated linoleic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 95, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen A, Leifheit M, Voss J, Nau H (2005) Molecular and cellular effects of cis‐9, trans‐11‐conjugated linoleic acid in enterocytes: effects on proliferation, differentiation, and gene expression. Biochim. Biophys. Acta 1735, 30–40. [DOI] [PubMed] [Google Scholar]
- Larsen TM, Toubro S, Astrup A (2003) Efficacy and safety of dietary supplements containing CLA for the treatment of obesity: evidence from animal and human studies. J. Lipid Res. 44, 2234–2241. [DOI] [PubMed] [Google Scholar]
- LipGene (2004) Welcome to the LipGene project website. http://www.lipgene.tcd.ie/[accessed on 10 February 2008].
- Mandir N, Goodlad RA (1999) The effects of glutamine on intestinal epithelial cell proliferation in parenterally fed rats. Gut 44, 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A, Sinha R, Burt R, Caan B, Paskett E, Iber F, Kikendall W, Lance P, Shike M, Weissfeld J, Schatzkin A, Lanza E (2004) Meat intake and the recurrence of colorectal adenomas. Eur. J. Cancer Prev. 13, 159–164. [DOI] [PubMed] [Google Scholar]
- Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN (1993) ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc. Natl. Acad. Sci. USA 90, 8977–8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro V, Zabala A, Macarulla MT, Fernandez‐Quintela A, Rodriguez VM, Simon E, Portillo MP (2003) Effects of conjugated linoleic acid on body fat accumulation and serum lipids in hamsters fed an atherogenic diet. J. Physiol. Biochem. 59, 193–199. [DOI] [PubMed] [Google Scholar]
- Norat T, Lukanova A, Ferrari P, Riboli E (2002) Meat consumption and colorectal cancer risk: dose‐response meta‐analysis of epidemiological studies. Int. J. Cancer 98, 241–256. [DOI] [PubMed] [Google Scholar]
- Orner G, Dashwood W, Blum C, Diaz G, Li Q, Al‐Fageeh M, Tebbutt N, Heath J, Ernst M, Dashwood R (2002) Response of Apc (min) and A33 (deltaNbeta‐cat) mutant mice to treatment with tea, sulindac, and 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP). Mutat. Res. 30, 121–127. [DOI] [PubMed] [Google Scholar]
- Park HS, Cho HY, Ha YL, Park JH (2004) Dietary conjugated linoleic acid increases the mRNA ratio of Bax/Bcl‐2 in the colonic mucosa of rats. J. Nutr. Biochem. 15, 229–235. [DOI] [PubMed] [Google Scholar]
- Park HS, Ryu JH, Ha YL, Park JH (2001) Dietary conjugated linoleic acid (CLA) induces apoptosis of colonic mucosa in 1,2‐dimethylhydrazine‐treated rats: a possible mechanism of the anticarcinogenic effect by CLA. Br. J. Nutr. 86, 549–555. [DOI] [PubMed] [Google Scholar]
- Preston SL, Wong WM, Chan AO, Poulsom R, Jeffery R, Goodlad RA, Mandir N, Elia G, Novelli M, Bodmer WF, Tomlinson IP, Wright NA (2003) Bottom‐up histogenesis of colorectal adenomas: origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res. 63, 3819–3825. [PubMed] [Google Scholar]
- Rajakangas J, Basu S, Salminen I, Mutanen M (2003) Adenoma growth stimulation by the trans‐10, cis‐12 isomer of conjugated linoleic acid (CLA) is associated with changes in mucosal NF‐kappa B and cyclin D1 protein levels in the min mouse. J. Nutr. 133, 1943–1948. [DOI] [PubMed] [Google Scholar]
- Rhodes JM, Campbell BJ (2002) Inflammation and colorectal cancer: IBD‐associated and sporadic cancer compared. Trends Mol. Med. 8, 10–16. [DOI] [PubMed] [Google Scholar]
- Riserus U, Basu S, Jovinge S, Fredrikson GN, Arnlov J, Vessby B (2002) Supplementation with conjugated linoleic acid causes isomer‐dependent oxidative stress and elevated C‐reactive protein: a potential link to fatty acid‐induced insulin resistance. Circulation 106, 1925–1929. [DOI] [PubMed] [Google Scholar]
- Rudling R, Kitau J, Hassan AB, Mandir N, Goodlad RA (2006) A simple device to rapidly prepare whole mounts of murine intestine. Cell Prolif. 39, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM (1998) Activators of the nuclear receptor PPAR gamma enhance colon polyp formation. Nat. Med. 4, 1058–1061. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Hidalgo M, Martin AR, Villegas I, Alarcon De La Lastra C (2005) Rosiglitazone, an agonist of peroxisome proliferator‐activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem. Pharmacol. 69, 1733–1744. [DOI] [PubMed] [Google Scholar]
- Sato Y, Nakaya N, Kuriyama S, Nishino Y, Tsubono Y, Tsuji I (2006) Meat consumption and risk of colorectal cancer in Japan: the Miyagi Cohort Study. Eur. J. Cancer Prev. 15, 211–218. [DOI] [PubMed] [Google Scholar]
- Tiemersma EW, Voskuil DW, Bunschoten A, Hogendoorn EA, Witteman BJ, Nagengast FM, Glatt H, Kok FJ, Kampman E (2004) Risk of colorectal adenomas in relation to meat consumption, meat preparation, and genetic susceptibility in a dutch population. Cancer Causes Control 15, 225–236. [DOI] [PubMed] [Google Scholar]
- Tricon S, Burdge GC, Williams CM, Calder PC, Yaqoob P (2005) The effects of conjugated linoleic acid on human health‐related outcomes. Proc. Nutr. Soc. 64, 171–182. [DOI] [PubMed] [Google Scholar]
- Truswell AS (2002) Meat consumption and cancer of the large bowel. Eur J. Clin. Nutr. 56 (Suppl. 1), S19–S24. [DOI] [PubMed] [Google Scholar]
- Wahle KW, Heys SD, Rotondo D (2004) Conjugated linoleic acids: are they beneficial or detrimental to health? Prog. Lipid Res. 43, 553–587. [DOI] [PubMed] [Google Scholar]
- Wasan HS, Park HS, Liu KC, Mandir NK, Winnett A, Sasieni P, Bodmer WF, Goodlad RA, Wright NA (1998) APC in the regulation of intestinal crypt fission. J. Pathol. 185, 246–255. [DOI] [PubMed]
- Wijesundera C, Shen Z, Wales WJ, Dalley DE (2003) Effect of cereal grain and fibre supplements on the fatty acid composition of milk fat of grazing dairy cows in early lactation. J. Dairy Res. 70, 257–265. [DOI] [PubMed] [Google Scholar]
- Willett WC (2001) Diet and cancer: one view at the start of the millennium. Cancer Epidemiol. Biomarkers Prev. 10, 3–8. [PubMed] [Google Scholar]


