Summary
Aims
Anxiety disorders are characterized by a deficient extinction of fear memory. Evidence is growing that leptin influences numerous neuronal functions. The aims of this study were to investigate the effects and the mechanism of leptin on fear extinction.
Methods and results
Leptin (1 mg/kg, i.p) was applied to evaluate the anxiolytic effect in rat behavioral tests. Field potentials recording were used to investigate the changes in synaptic transmission in the thalamic‐lateral amygadala (LA) pathway of rat. We found that leptin produced strong anxiolytic effects under basal condition and after acute stress. Systemic administration and intra‐LA infusions of leptin facilitated extinction of conditioned fear responses. The antagonist of NMDA receptor, MK‐801, blocked the effect of leptin on fear extinction completely. Furthermore, these effects of leptin on fear extinction were accompanied by a reversal of conditioning‐induced synaptic potentiation in the LA. Leptin facilitated NMDA receptor‐mediated synaptic transmission, and reversed amygdala long‐term potentiation (LTP) in a dose‐dependent manner in vitro, and this LTP depotentiation effect was mediated by NMDA receptor and MAPK signaling pathway.
Conclusions
These results identify a key role of leptin in dampening fear conditioning‐induced synaptic potentiation in the LA through NMDA receptor and indicate a new strategy for treating anxiety disorders.
Keywords: Amygdala, Anxiety, Fear conditioning, Leptin, Long‐term potentiation
Introduction
Anxiety disorders are one class of the most common psychiatric diseases, and the lifetime morbidity is up to 28%. It is recognized that investigating the mechanisms of fear extinction will improve the clinical treatment for anxiety disorders 1. Fear conditioning is generally accepted as an animal model for anxiety disorders 2.
It has been widely accepted that the lateral amygdala (LA) is a critical region for fear conditioning and the subsequent extinction 3. The responses of neuronal activity in the LA to conditioned stimuli change in proportion to fear responses, that is, increased during fear conditioning and decreased during extinction 4, 5. Recent findings suggest that conditioning‐induced potentiation of synaptic transmission can be depotentiated during fear extinction 6. Extensive studies have shown that NMDA receptor (NMDAR) plays a role in the process of conditioned fear extinction 7. One hallmark of extinction memory is its dependence on NMDARs. Much of the evidence implicates that blockade of NMDAR in the lateral and basolateral amygdale (BLA) prevents the decreased responses (freezing behavior) that normally occur in extinction learning 8.
Leptin is a circulating hormone which is involved in the regulation of the body weight and energy expenditure by activation of leptin receptors (ob) 9. High levels of leptin and mRNA have been observed in different regions in the brain 10. Evidence is growing that leptin exerts widespread effects on CNS function, especially learning, memory, and synaptic plasticity 11. Recent studies have shown that leptin is deeply involved in facilitating hippocampus‐dependent synaptic plasticity (i.e., long‐term potentiation (LTP), and improving the performance of memory in rodents 12, 13.
Leptin receptors are also expressed in limbic structures, indicating it may take part in the control of mood and emotion. Previous studies have shown that leptin not only has antidepressant‐like properties 14, but also has effects on the modulation of anxiety behaviors 15. Meanwhile, leptin‐deficient (ob/ob) or leptin receptor‐selective deleted mice display higher levels of anxiety‐related behavior 16. However, the mechanisms underlying the anxiolytic effects of leptin have not been investigated. PCR and in situ hybridization studies show that the leptin receptor is also expressed in the amygdala 17, 18. Given the important role of amygdala in responses to stress stimuli, we hypothesized that amygdala is the main target region for leptin to produce anxiolytic action.
To address this issue, we identified the action and the underlying mechanisms of leptin on anxiety‐like behaviors. The main discovery is that leptin plays a role in dampening the fear conditioning‐induced synaptic potentiation in the LA through modulation of NMDAR.
Materials and Methods
Chemicals and Reagents
Mouse recombinant leptin was purchased from ProSpec (Ness‐Ziona, Israel). DL‐2‐amino‐5‐phosphonovaleric acid (D‐APV), glycine, picrotoxin, CNQX, and wortmannin were obtained from Sigma (St. Louis, MO, USA). U0126 and LY294002 were bought from Cell Signaling Technology, Inc. (Danvers, MA, USA). PD98059 was from Calbiochem (La Jolla, CA, USA). MK‐801 was provided by NIMH of USA. Other general chemicals were bought from commercial suppliers.
Experimental Animals
C57BL/6J mice (aged 8–10 weeks) were purchased from the Experimental Animal Center of Tongji Medical College. Mice were housed under environmentally controlled conditions (23 ± 1°C) on a 12‐h light/dark cycle with food and water ad libitum. All efforts were made to minimize animal suffering. All the procedures for animal care and experiments were conducted according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Committee of Animal Care of Huazhong University of Science and Technology.
Electrophysiological Recordings
Brain slices were prepared from C57BL/6J mice as described in our previous study 19 with some modification. Briefly, after removed rapidly, the brains were cut into coronal slices (400 μm thickness) containing amygdala with a vibratome in ice‐cold artificial CSF (aCSF) consisting of (mM): 119 NaCl, 3.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 26.2 NaHCO3, 1 NaH2PO4, and 11 glucose (bubbled with 95% O2/5% CO2; pH 7.4). After recovery for 1.5 h at 27°C, a single slice was moved into the recording chamber and placed the glass electrodes filled with 3 M NaCl (2–5 MΩ) in the LA. The bipolar concentric electrode was placed at the internal capsule to evoke fEPSPs by a constant stimulation.
To induce LTP, the stimulation intensity that produced a fEPSPs with about 1/3–1/2 of maximal amplitude was employed during the conditioning stimulation of high‐frequency stimulation (HFS) consisting of five trains at 100 Hz for 1 s, 90 s intermission between trains. Stimulation frequency during baseline recording and after the HFS was always at 0.033 Hz. The initial slopes of fEPSPs of the last 20 min during before HFS were measured and calculated as the control (baseline), and the response was expressed as a percentage of the baseline level. The input–output (I/O) relationship for synaptic transmission was evaluated by stimulation of afferents with increasing intensity during recording fEPSPs in the LA. All the experiments were performed in the aCFS containing 50 μM picrotoxin.
Fear Conditioning
Fear conditioning tests were performed in an observation chamber (32 × 26 × 22 cm) as previously described by our laboratory 20. Mice were placed in the room where conditioning took place for 5 min per day for 3 days. On day 1 (conditioning day), mice were placed into the chamber A. After a 3‐min acclimatizing period, the mice received four times of the tone‐conditioned stimulus (CS; 80 dB, 30 s) co‐terminating with a footshock with 30‐s intervals. Each shock was at 0.5 mA with 2 s duration. Mice were put in the conditioning chamber for 30 s after termination of the procedure, and then returned to the home cage. If the freezing of mice were satisfied the criterion (>50% freezing) during conditioning, they were randomly assigned to either the control or the experimental group, matched for freezing during fear conditioning.
On day 2 (extinction training day), mice were injected intraperitoneally (i.p.) with NS, leptin (0.25 or 1 mg/kg) or MK‐801 (0.1 mg/kg) + leptin (1 mg/kg). The doses were chosen based on our preliminary experiments and previous reports that leptin at 1 mg/kg produced antidepressant‐like activity 14. Moreover, MK‐801 at 0.1 mg/kg impaired the extinction of fear conditioning 19. Thirty minutes after injection, they were exposed to 10 extinction trials in a novel chamber B. The extinction training included 10 presentations of the 30‐s CS with 30‐s intervals. On day 3 (extinction testing day), mice were exposed to four trials (30‐s CS with 30‐s intervals) in the novel chamber B. The time that spent freezing in the tone (cue) was measured during each tone presentation. Freezing was defined as the complete cessation of activity except for respiration.
Data Analysis
All analyses were performed using the software of SPSS 13.0 and data are expressed as mean ± SEM. One‐way ANOVA or two‐way ANOVA were used to evaluate the differences between mean values from different groups. For all one‐way ANOVA, post hoc tests were performed using LSD test. For all two‐way ANOVA, Bonferroni's post hoc tests were used to assess isolated comparisons. When P < 0.05, the difference was determined statistically significant.
Results
Systemic Administration of Leptin Facilitates the Extinction of Conditioned Fear in Mice
There is substantial evidence that the impairment of fear extinction is a major symptom of anxiety disorders. Results in the previous study provide evidence that leptin reduces anxiety‐like behavior caused by acute stress. Thus, what are the effects of leptin on conditioned fear and extinction were investigated.
Firstly, we determined whether the systemic administration of leptin affected the acquisition of fear extinction. Mice were fear‐conditioned with an auditory fear conditioning paradigm. Mice that underwent fear conditioning and matched for freezing scores during fear conditioning were divided into four groups: vehicle, leptin (0.25 mg/kg), leptin (1 mg/kg), and leptin (1 mg/kg) + MK‐801 (0.1 mg/kg). A two‐way ANOVA was performed, with leptin and MK‐801 injection as sources of variance. As shown in Figure 1B, in vehicle control group, mice displayed a high level of conditioned freezing responses upon CS + presentation during the first training session and a continuous decline during nonreinforced CS + exposure during subsequent extinction training sessions, which is defined as fear extinction. Conditioned freezing in leptin‐treated mice was significantly reduced when compared to vehicle‐treated mice (day 2, main effect of leptin), F(2, 30) = 23.7, P < 0.05; post hoc, P < 0.05 versus vehicle, (Figure 1C). This effect persisted in an extinction test on the following day (day 3, main effect of leptin), F(2, 30) = 30.1, P < 0.05; post hoc, P < 0.05 versus vehicle (Figure 1C). These results suggest that leptin facilitates the extinction of conditioned fear dose‐dependently.
Figure 1.
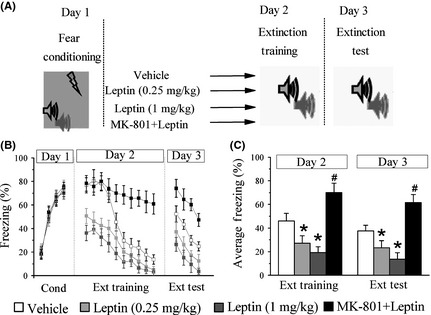
Systemic administration of leptin facilitates the extinction of conditioned fear. (A) Experimental protocols of fear conditioning paradigm. (B) The percentage of freezing during conditioning (4 CS‐footshock trials), trial‐by‐trial extinction training (10 CS‐alone trials), and extinction test (4 CS‐alone trials). Vehicle (white panes), leptin (gray panes), and MK‐801 + leptin (black panes) were intraperitoneal injection 30 min before extinction training. (C) Averaged percentage of freezing across all extinction training and extinction test trials. *P < 0.05 versus vehicle in the same session, # P < 0.05 versus leptin (1 mg/kg) in the same session, n = 9 for each group.
One hallmark for the study of fear extinction is the discovery of that NMDARs mediates the extinction memory 21. Several studies have shown that modulation of NMDAR function underlies the effects of leptin on hippocampus‐dependent synaptic plasticity as well as learning and memory 22, 23. Thus, it is possible that leptin mediates its extinction‐like effects through NMDAR. To address this issue, we performed the following experiments. On day 2, 30 min before extinction training, mice received the following combination treatment: MK‐801 (0.1 mg/kg, i.p.) + leptin (1 mg/kg, i.p.), according to a previous report 19. The mice treated with MK‐801 and leptin displayed a longer freezing response than leptin‐treated mice (day 2, main effect of MK‐801), F(1, 30) = 44.9, P < 0.05 (day 3, main effect of MK‐801), F(1, 30) = 110.4, P < 0.05 (Figure 1C). Moreover, they displayed sustained freezing across multiple extinction training trials, indicating that MK‐801 impairs the acquisition of fear extinction. The freezing response of MK‐801/leptin‐injected mice was significantly higher than that of vehicle‐treated mice (P < 0.05), suggesting that MK‐801 antagonizes the action of leptin on extinction learning.
Intra‐Amygdala Infusions of Leptin Facilitates the Extinction of Conditioned Fear in Mice
To determine whether leptin may act directly on amygdala, we looked for expression of the short (Ob‐Ra) and long (Ob‐Rb) forms of Ob‐R mRNA in the LA. Consistent with previous observations 17, 18, Ob‐Ra and Ob‐Rb were constitutively expressed in LA by RT‐PCR analysis (Figure S4). In addition, leptin mRNA was detected in LA (Figure S4). The presence of the leptin receptor protein in LA was further evaluated by Western blot analysis (Figure 2B). At last, we examined Ob‐R presence in neuronal cells by studying its colocalization with antibody against NeuN protein. In the LA and BLA, the majority of cells labeled by both anti‐NeuN antibody and by anti‐Ob‐R antibody colocalized (Figure 2A).
Figure 2.
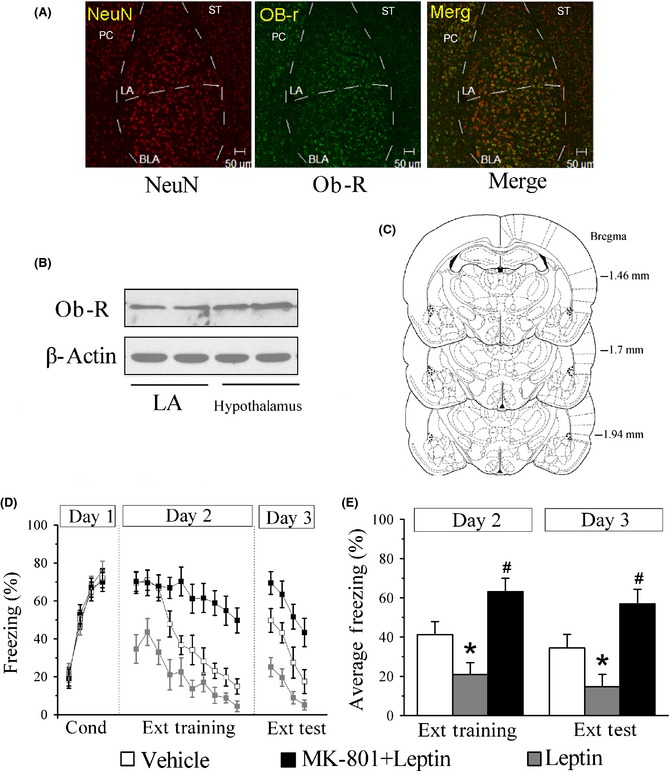
Facilitation of fear extinction by intra‐amygdala infusions of leptin. (A) Ob‐R immunoreactivity was colocalized primarily with NeuN in mice LA and BLA. PC: perirhinal cortex; ST: striatum; Scale bar: 50 μm. (B) Western blot confirmed the expression of Ob‐R in LA. (C) Histological reconstructions showing placements of intralateral amygdala cannula tips of the animals included in the data analysis. (D) The percentage of freezing during conditioning (4 CS‐footshock trials), trial‐by‐trial extinction training (10 CS‐alone trials), and extinction test (4 CS‐alone trials). Vehicle (white panes), leptin (gray panes), and MK‐801 + leptin (black panes) injections were administered 30 min before extinction training. (E) Averaged percentage of freezing across all extinction training and extinction test trials. *P < 0.05 versus vehicle in the same session, # P < 0.05 versus leptin (3 μg/μL/side) in the same session, n = 7 for each group.
Next, we assessed whether infusions of leptin into the LA would affect the acquisition of extinction learning. Mice were subjected to fear conditioning training after 5–7 days of guide cannula implantation, and assigned to different groups after conditioning, matched for CS‐elicited freezing during fear conditioning. The next day (day 2), mice received intra‐amygdala infusions of vehicle, leptin (3 μg/1 μL/side), or MK‐801 (0.1 mg/kg, i.p.) + leptin (3 μg/1 μL/side), 30 min before extinction training. Similar to systemic injections, a significant effect of treatment at the extinction training session was observed (ANOVA), F(2, 18) = 28.5, P < 0.05, and mice that received intra‐amygdala infusion of leptin showed lower freezing across extinction training trials relative to vehicle controls (post hoc analysis, P < 0.05). However, high levels of freezing were observed in MK‐801‐treated rats on the extinction training and test day (P < 0.05 vs. leptin). Leptin‐treated mice displayed lower levels of freezing when compared with saline controls (ANOVA), F(2, 18) = 41.6, P < 0.05, and both the MK‐801 and the leptin groups differed significantly from the saline group and each other (post hoc analysis, P < 0.05). However, intrahippocampus and prefrontal cortex infusions of leptin did not affect fear extinction (Figure S2).
Leptin Induces the Reversal of Conditioning‐Induced Synaptic Potentiation in the LA
Considering that cue‐dependent fear conditioning is mediated by the facilitation of synaptic transmission in the LA 4, and this conditioning‐induced potentiation of synaptic functions may be depotentiated during fear extinction, which is dependent on the extinction protocol and the training duration 6, 24, 25, 26, we therefore investigated whether the facilitation of conditioned fear extinction by leptin was dependent on the reversal of conditioning‐induced potentiation of T‐LA synapses.
The I/O relationships for fEPSPs amplitude were compared among five groups: unpaired (received unpaired tones and shocks), paired, extinction (NS), leptin (1 mg/kg, i.p.) + extinction, and MK801 (0.1 mg/kg, i.p.) + leptin (1 mg/kg, i.p.) + extinction (Figure 3B). Electrophysiological recordings 48 hours after conditioning revealed a significant increase in the amplitude of fEPSPs in the paired group as compared to unpaired group (P < 0.05, Figure 3E) as reported previously 4. Furthermore, the extinction training decreased the amplitude of fEPSPs slightly, and the I/O relationship in the extinction (NS) group did not differ significantly from that of the paired group (Figure 3D,E). The lack of depotentiation in the extinction (NS) group indicates that the extinction protocol used here cannot fully eliminate fear memory. However, pretreatment with leptin for 30 min reduced the amplitude of fEPSPs to near the baseline level during extinction training (P < 0.05 vs. paired, Figure 3E,F), demonstrating that leptin combined with extinction training can reverse fear‐related synaptic strengthening. This reversal was accompanied by the activation of synaptic NMDAR, because MK‐801 blocked the effect of leptin completely (Figure 3E,F).
Figure 3.
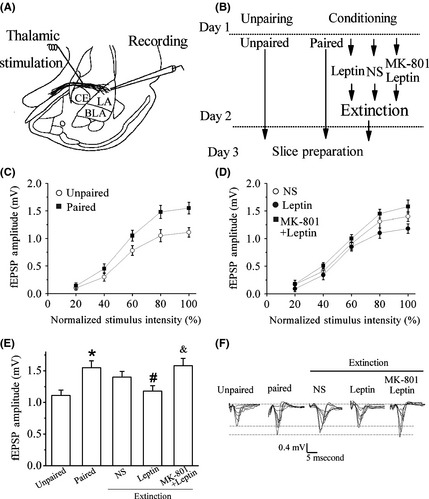
Leptin induces the reversal of conditioning‐induced potentiation in the LA. (A) Diagram of slice preparation with stimulation and recording sites indicated. CE, central nucleus. (B) The behavioral procedure for the electrophysiology experiments shown in C–F. Brain slices were prepared on day 3 for all groups. (C) Fear learning facilitated synaptic transmission in the thalamic‐LA pathway 2 day post‐training. (D) Leptin reversed of conditioning‐induced potentiation in the LA through NMDA receptor. Synaptic strength in slices from NS control, leptin, and MK‐801 + leptin groups after fear extinction training. Leptin facilitated the dampening of conditioning‐induced potentiation in the LA. MK‐801 robustly blocked this effect of leptin. (E) Bar graph showing the maximum amplitude of fEPSPs in slices from mice after behavioral experiments. *P < 0.05 relative to unpaired control; # P < 0.05 relative to paired; & P < 0.05 relative to leptin. (F) Traces of fEPSPs were recorded at increasing stimulus intensities on day 3 in slices from for all five groups. n = 7 for each group.
Leptin Facilitates NMDAR‐Mediated Synaptic Transmission in the LA
Previous studies have proved that leptin rapidly increases NMDAR‐mediated hippocampal synaptic transmission 12. The NMDAR‐mediated fEPSPs in T‐LA synapses were isolated by pharmacological manipulation. In accordance with previous reports 27, to isolate NMDAR‐mediated fEPSPs, the perfusion medium was changed into magnesium‐free ACSF, which contained 10 μM glycine and 10 μM CNQX, an AMPA receptor antagonist (Figure 4A). It was found that bath application of leptin at 50 nM induced an increase in the slope of isolated NMDAR‐mediated synaptic potentials significantly (Figure 4B). We also found that leptin did not affect the basal excitatory synaptic transmission in LA at different concentration (data not shown).
Figure 4.
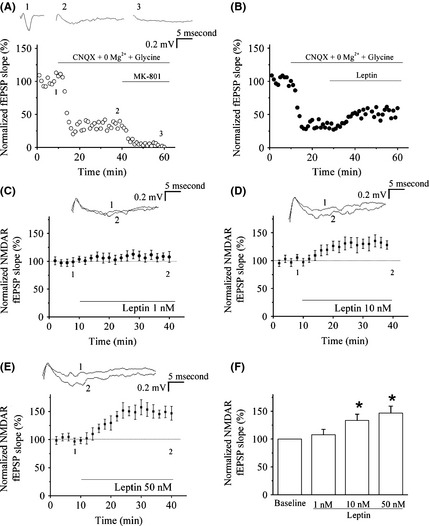
Leptin facilitates NMDA receptor (NMDAR)‐mediated synaptic transmission in the LA (thalamic input). (A) NMDAR‐mediated fEPSPs were isolated by pharmacological manipulation in the LA. D‐APV (50 μM) blocked the NMDAR‐mediated fEPSPs completely. (B) NMDAR‐mediated synaptic transmission was enhanced by leptin (50 nM). (C–E) Effect of leptin (1, 10 and 50 nM) on NMDAR‐mediated fEPSPs in LA. The superimposed fEPSPs in the top portion showed respective recordings from example experiments taken at the time indicated by the number. (F) The histogram showed the level of NMDAR‐mediated fEPSPs 30 min after the presence of various concentrations of leptin. *P < 0.05 versus control. Each point was the normalized mean ± SEM of slices.
We next examined the effect of different concentrations of leptin (1, 10 and 50 nM) on NMDAR‐mediated fEPSPs in T‐LA synapses. As shown in Figure 4C–F, pre‐incubation of brain slices with leptin enhanced the NMDAR‐mediated fEPSPs in a concentration‐dependent manner, with maximal effect observed at 50 nM and persisted for at least 30 min (n = 5, P < 0.05 vs. control baseline).
Leptin Reverses the Synaptic LTP at Thalamic Input to the LA of Mice
Previous studies have demonstrated that leptin induces the reversal of LTP and a new form of LTD at hippocampal CA1 synapses 28, 29. It is not clear whether leptin can produce an effect on the potentiated responses in T‐LA synapses of control mice in vitro. To address this issue, we firstly confirmed that a HFS can produce robust LTP (Figure 5A). We then perfused the slices with leptin (10 or 50 nM; 15 min) at the time point of 30 min after the induction of LTP, as shown in Figure 5, the perfusion of leptin resulted in a rapid and concentration‐dependent reversal of LTP (n = 6; Figure 5C,D, and F). The slopes of fEPSPs were significantly reduced when compared with that of control slices, after the 15 min‐exposure to 10 nM leptin (n = 6). Maximal depotentiation was observed within at least 10 min of leptin washout (n = 6; P > 0.05 vs. basal synaptic transmission). Likewise, the administration of 50 nM leptin at 30 min after LTP induction also elicited a decrease of fEPSPs slopes and this depotentiation reached its maximal level within 10 min of leptin washout (n = 6; P > 0.05 vs. baseline). On the contrary, leptin at a lower concentration (1 nM; 15 min) or boiled leptin (50 nM) did not affect the expression of LTP (n = 6; Figure 5B,F) when applied with same protocol.
Figure 5.
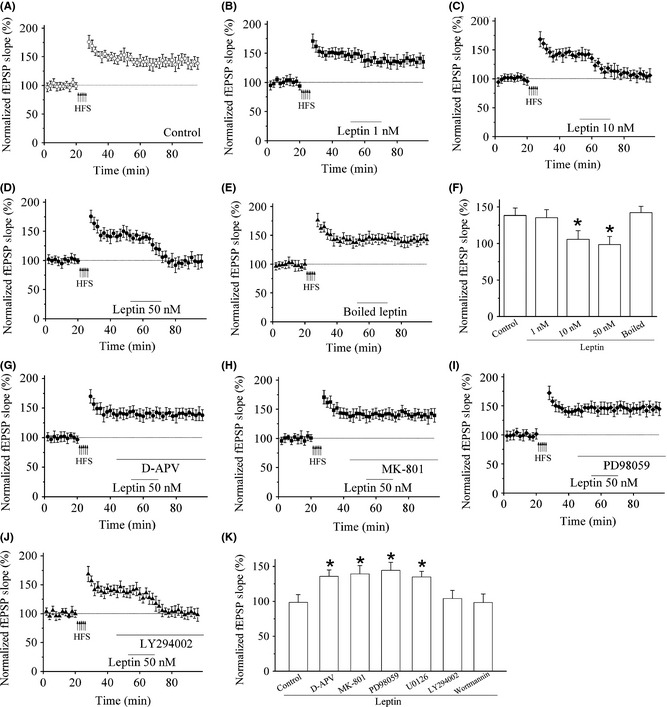
The synaptic depotentiation induced by leptin is mediated by NMDA receptor (NMDAR) and the MAPK signaling pathway. (A) Control LTP was induced electrically by HFS protocol. (B) Application of leptin (1 nM), 30 min after LTP induction did not affect the magnitude of potentiated synapses. n = 5. (C–D) Application of leptin (10 or 50 nM), 30 min after LTP induction reversed synaptic transmission to pre‐LTP levels. n = 5 for each group. (E) Application of boiled leptin (50 nM), 30 min after LTP induction did not affect the magnitude of potentiated synapses. n = 5. (F) Histogram of the pooled data displaying the relative synaptic depressions induced by application of 1, 10, 50 nM, and boiled leptin, respectively. *P < 0.05 versus control. Each point was the normalized mean ± SEM of slices. (G–H) Leptin‐induced depotentiation requires NMDAR activation. n = 5 for each group. Application of the competitive NMDAR antagonist D‐APV (50 μM) and noncompetitive NMDAR antagonist MK‐801 (10 μM), after LTP induction blocked the ability of leptin (50 nM) to reverse amygdala LTP. (I) Leptin‐induced depotentiation is attenuated by the MAPK inhibitor PD98059 (10 μM). n = 5. (J) Application of PI3‐kinase inhibitor LY 294002 (10 μM), after LTP induction did not affect the ability of leptin (50 nM) to reverse amygdala LTP. n = 5. (K) Histogram of the pooled data depicting the effect of NMDAR, MAPK pathway, and PI3‐kinase inhibitor on synaptic depotentiation induced by leptin. *P < 0.05 versus control. Each point was the normalized mean ± SEM of slices.
It was reported that the depotentiation induced by leptin in hippocampus is NMDAR‐dependent process 29. We then examined the effects of the competitive NMDAR antagonist D‐APV (50 μM) and the noncompetitive NMDAR antagonist MK‐801 (10 μM) on the induction of leptin‐induced depotentiation. Figure 5G,H showed that in the presence of D‐APV (50 μM) or MK‐801 (10 μM), leptin (50 nM) failed to reverse LTP (n = 5; P < 0.05 vs. leptin).
It was further reported that the blockade of MAPK pathway occludes leptin‐induced potentiation of NMDA responses in hippocampal neurons 12. We then examined the effects of PD 98059 and U0126, two selective inhibitors of MAPK signaling pathway. Incubation with PD98059 (10 μM) and U0126 (1 μM) obviously blocked the depotentiation induced by leptin (50 nM; n = 5; P < 0.05 vs. leptin; Figure 5I,K). Furthermore, leptin (50 nM) treatment produced significant enhancement of ERK phosphorylation in LA area when compared to control brain slices (P < 0.05 vs. control; Figure S3A,B), without any significant changes in total ERK expression.
PI3‐kinase is an essential molecular of leptin receptor‐mediated signaling pathways 12. We then observed the role of LY 294002 and wortmannin, two specific PI3‐kinase inhibitors, on the effects of leptin. In the presence of the PI3‐kinase inhibitor, leptin still depotentiated the T‐LA synapses. After LTP induction, leptin at 50 nM (15 min) produced a robust depression of fEPSPs to pre‐LTP levels (P > 0.05 vs. leptin; n = 5; Figure 5J,K).
Discussion
This study is to identify the effects and the related mechanisms of leptin on LTP in the amygdala as well as the anxious behavior and fear extinction. We found that leptin, either systemically administrated or locally injected into the LA, produced an anxiolytic‐like effect, displayed an acute reduction in general anxiety, and an accelerated extinction of conditioned fear. Moreover, leptin dampened the fear conditioning‐induced synaptic potentiation and reversed HFS‐induced LTP through NMDAR activation in amygdala.
Neuronal leptin mRNA, ob protein, and leptin immunoreactivity are expressed extensively in the brain 11, 30. This expression pattern is matched with the broad neuronal functions of leptin on neuroendocrine activity, neuroprotection 31, synaptic plasticity 12, and memory processing 22. Furthermore, a great deal of attention has focused on the relationship between leptin and psychiatric disorders. For example, leptin has been found to regulate depression 14, drug addiction 32, and anxiety‐related behaviors in rodents 33.
Growing literatures have revealed that stressful experiences can induce long‐lasting inappropriate anxiety and/or excessive fear 34. Our behavioral results showed that pretreatment with leptin blocked the excessive anxiety behavior induced by stress, suggesting that leptin exhibits strong anxiolytic activity.
Available evidence also implicates the amygdala as crucial nuclei for fear extinction 35. We found that systemic administration of leptin facilitated the extinction of conditioned fear responses. We also showed that leptin and its receptor are distributed in the LA. Next, the behavioral effects of leptin in the amygdala were investigated through bilateral application of leptin to LA. Conditioned freezing in the group of leptin‐treated mice was significantly reduced than that in saline‐infused mice, indicating that leptin promotes fear extinction. Considering that MK‐801 completely blocked the effect of leptin on fear extinction, leptin‐induced reduction in fear is completely depended on NMDARs.
Previous report indicates that extinction is a form of new learning, which prevent the formation of conditioned fear rather than erases the old fear memory 1. While the expression of new memory stands for the popular model of fear extinction, it does not exclude the possibility that there are other mechanisms underlying the extinction of consolidated memory, as for example, erasing conditioned fear responses through synaptic depotentiation 6. Recently, LeDoux and Huganir have reported an extinction protocol that can fully abolish fear memory, named reconsolidation update 24, 36. The result reveals that the changes in the composition of AMPARs in the LA can erase the existing fear memories, even as the latter is maintained by persistent increases in excitatory transmission.
Other group of experiments also examines the depotentiation in the amygdala. It is generally accepted that LTP in the amygdala mediates the acquisition and expression of conditioned fear 4, 35. Depotentiation is a form of plasticity expressed as for the reversal of LTP induced by applying LFS or drugs to the same synapse. Lin et al. 25 demonstrated that the depotentiation in a pathway from the external capsule to the LA in vivo could be blocked by NMDAR antagonists. Choi et al. found that extinction training returned the enhanced T‐LA synaptic efficacy to baseline and blocked the depotentiation 6, 37. These results are in accordance with the application of depotentiation to attenuate the potentiated synaptic inputs onto the LA during extinction 6. Our results demonstrated that the extinction protocol used here cannot fully eliminate fear memory. Furthermore, the extinction training decreased the amplitude of fEPSPs slightly, and the I/O relationship in the extinction (NS) group did not differ significantly from that of paired group. However, pretreatment with leptin reduced the amplitude of fEPSPs to near the baseline level, demonstrating that leptin combined with extinction training reverses fear‐related synaptic strengthening. The depotentiation effect of leptin was mediated by NMDAR because MK‐801 injection completely blocked the effect of leptin. Because the depotentiation in the amygdala is one mechanism underlying the extinction of consolidated memory, we assume that the depotentiation effect of leptin may account for the facilitated extinction of conditioned fear responses. Much evidence indicates that synaptic plasticity within amygdala circuits underlies fear conditioning in animals. Our results indicate facilitation of fear extinction by leptin is dependent on reversal of conditioning‐induced potentiation of thalamic input synapses onto the lateral amygdala (T‐LA synapses) in vivo.
As previously reported that leptin induced a reversal of LTP, expressed as depotentiation, at CA1 synapses 29, in which it was demonstrated that leptin induced a depotentiation by an NMDAR‐dependent manner and through reducing the AMPA receptor rectification but not AMPA receptor‐mediated basal excitatory synaptic transmission. We also found that leptin has the potency to depress HFS‐induced potentiated synapses in T‐LA of control mice in vitro. These results demonstrated that leptin can not only weaken the behavior‐potentiated synaptic transmission but also reverses the HFS‐induced LTP in LA.
There is a close association among food intake, mood regulation, and cognitive function. More and more results have proven a solid relationship between food intake and anxiety 38. Here, we found that leptin has a direct regulatory role in the mediating of anxiety behaviors and fear memory. Consistent with our results, Huang et al. found a direct relationship between stress‐related psychiatric symptoms and the levels of serum leptin 39. Schmid et al. also found that the levels of leptin in patients with PTSD are much higher than those in patients without PTSD 40. In the depressive rat, the circulating leptin levels are low 14, and in some patients with major depression, the leptin levels in serum or cerebrospinal fluid are also low 41. However, another study has showed that leptin evokes the release of corticotropin‐releasing factor in brain slices from amygdala 17. Above results are contradictory and further investigations are needed to evaluate the anxiolytic properties of leptin.
Taken together, the results in the present study imply that leptin has two synergic functions: one is acutely attenuating anxiety‐like response; the other is facilitating the extinction of aversive fear memories. Such synergic effects have potential therapeutic meaning to treat diseases such as PTSD or chronic anxiety disorders.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Appendix S1. Materials and Methods for the Supporting Information.
Figure S1. NMDA receptor antagonist MK‐801 inhibits the extinction of learned fear.
Figure S2. Intra‐hippocampus and prefrontal cortex infusions of leptin did not affect fear extinction.
Figure S3. Effects of leptin treatment on phosphorylation of ERK1/2 in lateral amygdala region of mice.
Figure S4. RT‐PCR detection of leptin, Ob‐Ra and Ob‐Rb mRNAs in LA.
Figure S5. A clearer chart showing placements of intralateral amygdala cannula tips of the animals included in the data analysis.
Acknowledgments
This work was supported by grants from the 973 Program of China (No. 2013CB531303 to Dr. J.G.C.; No. 2014CB744601 to F.W.) and NSFC Projects (No. 81222048 to F.W.; No. 81072720 to S.L.L.). It was also supported by the International Science & Technology Cooperation Program of China (No. 2011DFA32670 to J.G.C.) and PCSIRT (No. IRT13016).
The first two authors contributed to this work equally.
References
- 1. Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry 2007;12:120–150. [DOI] [PubMed] [Google Scholar]
- 2. Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med 2006;73:941–949. [PubMed] [Google Scholar]
- 3. Schafe GE, Doyere V, LeDoux JE. Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J Neurosci 2005;25:10010–10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKernan MG, Shinnick‐Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro . Nature 1997;390:607–611. [DOI] [PubMed] [Google Scholar]
- 5. Hobin JA, Goosens KA, Maren S. Context‐dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci 2003;23:8410–8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim J, Lee S, Park K, et al. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci USA 2007;104:20955–20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amaral OB, Roesler R. Targeting the NMDA receptor for fear‐related disorders. Recent Pat CNS Drug Discov 2008;3:166–178. [DOI] [PubMed] [Google Scholar]
- 8. Falls WA, Miserendino MJ, Davis M. Extinction of fear‐potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 1992;12:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinto S, Roseberry AG, Liu H, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 2004;304:110–115. [DOI] [PubMed] [Google Scholar]
- 10. Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology 1999;140:5995–5998. [DOI] [PubMed] [Google Scholar]
- 11. Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res 2006;45:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci 2001;21:RC186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oomura Y, Hori N, Shiraishi T, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long‐term potentiation and CaMK II phosphorylation in rats. Peptides 2006;27:2738–2749. [DOI] [PubMed] [Google Scholar]
- 14. Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA 2006;103:1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Perez SM, Zhang W, Lodge DJ, Lu XY. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic‐like behavior and increases dopaminergic activity in amygdala. Mol Psychiatry 2011;16:1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finger BC, Dinan TG, Cryan JF. Leptin‐deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety‐related behaviours. Psychopharmacology 2010;210:559–568. [DOI] [PubMed] [Google Scholar]
- 17. Raber J, Chen S, Mucke L, Feng L. Corticotropin‐releasing factor and adrenocorticotrophic hormone as potential central mediators of OB effects. J Biol Chem 1997;272:15057–15060. [DOI] [PubMed] [Google Scholar]
- 18. Udagawa J, Hatta T, Naora H, Otani H. Expression of the long form of leptin receptor (Ob‐Rb) mRNA in the brain of mouse embryos and newborn mice. Brain Res 2000;868:251–258. [DOI] [PubMed] [Google Scholar]
- 19. Baker JD, Azorlosa JL. The NMDA antagonist MK‐801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci 1996;110:618–620. [DOI] [PubMed] [Google Scholar]
- 20. Wang W, Wang F, Yang YJ, et al. The flavonoid baicalein promotes NMDA receptor‐dependent long‐term potentiation and enhances memory. Br J Pharmacol 2011;162:1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu JL, Li M, Dang XR, et al. A NMDA receptor antagonist, MK‐801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PLoS One 2009;4:e7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides 2006;27:1420–1425. [DOI] [PubMed] [Google Scholar]
- 23. Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol 2007;7:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clem RL, Huganir RL. Calcium‐permeable AMPA receptor dynamics mediate fear memory erasure. Science 2010;330:1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol 2003;63:44–52. [DOI] [PubMed] [Google Scholar]
- 26. Lin CH, Lee CC, Huang YC, Wang SJ, Gean PW. Activation of group II metabotropic glutamate receptors induces depotentiation in amygdala slices and reduces fear‐potentiated startle in rats. Learn Mem 2005;12:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Welch JM, Lu J, Rodriguiz RM, et al. Cortico‐striatal synaptic defects and OCD‐like behaviours in Sapap3‐mutant mice. Nature 2007;448:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durakoglugil M, Irving AJ, Harvey J. Leptin induces a novel form of NMDA receptor‐dependent long‐term depression. J Neurochem 2005;95:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moult PR, Milojkovic B, Harvey J. Leptin reverses long‐term potentiation at hippocampal CA1 synapses. J Neurochem 2009;108:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fei H, Okano HJ, Li C, et al. Anatomic localization of alternatively spliced leptin receptors (Ob‐R) in mouse brain and other tissues. Proc Natl Acad Sci USA 1997;94:7001–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen‐glucose deprivation and transient cerebral ischemia. Stroke 2007;38:2329–2336. [DOI] [PubMed] [Google Scholar]
- 32. Fulton S, Pissios P, Manchon RP, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 2006;51:811–822. [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic‐like effects: a comparison with fluoxetine. Psychopharmacology 2009;207:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci 2005;25:8725–8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry 2006;60:322–328. [DOI] [PubMed] [Google Scholar]
- 36. Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction‐reconsolidation boundaries: key to persistent attenuation of fear memories. Science 2009;324:951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hong I, Song B, Lee S, Kim J, Choi S. Extinction of cued fear memory involves a distinct form of depotentiation at cortical input synapses onto the lateral amygdala. Eur J Neurosci 2009;30:2089–2099. [DOI] [PubMed] [Google Scholar]
- 38. Pallister E, Waller G. Anxiety in the eating disorders: under‐standing the overlap. Clin Psychol Rev 2008;28:366–386. [DOI] [PubMed] [Google Scholar]
- 39. Liao SC, Lee MB, Lee YJ, Huang TS. Hyperleptinemia in subjects with persistent partial posttraumatic stress disorder after a major earthquake. Psychosom Med 2004;66:23–28. [DOI] [PubMed] [Google Scholar]
- 40. von Känel R, Begré S, Abbas CC, Saner H, Gander ML, Schmid JP. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. NeuroImmunoModulation 2010;17:39–46. [DOI] [PubMed] [Google Scholar]
- 41. Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord 2006;90:21–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Materials and Methods for the Supporting Information.
Figure S1. NMDA receptor antagonist MK‐801 inhibits the extinction of learned fear.
Figure S2. Intra‐hippocampus and prefrontal cortex infusions of leptin did not affect fear extinction.
Figure S3. Effects of leptin treatment on phosphorylation of ERK1/2 in lateral amygdala region of mice.
Figure S4. RT‐PCR detection of leptin, Ob‐Ra and Ob‐Rb mRNAs in LA.
Figure S5. A clearer chart showing placements of intralateral amygdala cannula tips of the animals included in the data analysis.


