Abstract
Objectives: Mesenchymal stem cells have great potential for tissue regeneration, and these cells can be harvested from a variety of tissues; however, up to now it has not been clear whether stem cells could be isolated from cruciate ligaments of the knee joint. The aim of our study was to isolate and characterize stem cells from both anterior and posterior cruciate ligaments (ACL and PCL) of humans.
Materials and methods: Cruciate igaments were obtained from patients receiving total knee arthroplasty for advanced osteoarthritis and plastic‐adherent cells were serially passaged. In vitro chondrogenic, osteogenic and adipogenic abilities of the cells were evaluated by reverse transcriptase–polymerase chain reaction and histological study. Karyotyping and surface immunophenotyping of the cells were performed.
Results: It was found that a population of ligament‐derived cells could be expanded and subcultured extensively. These cells were able to differentiate into osteoblasts, chondrocytes and adipocytes under appropriate inductions. Their phenotypic characteristics were similar to those of bone marrow mesenchymal stem cells. Karyotyping was normal after serial passage.
Conclusions: In summary, our study demonstrates that human multipotent stem cells can be isolated and expanded from human ACL and PCL, which are easily obtained from patients following total knee or cruciate ligament reconstructive surgery. Self‐renewal and mesodermal differentiation potential of these cells make them a viable alternative source for use in regenerative medicine.
Introduction
Anterior cruciate ligament (ACL) and posterior cruciate ligament (PCL) are the two most commonly injured ligaments of the knee (1). Unlike other major ligaments of the knee, the biological properties of the ACL and its intra‐articular environment contribute to its poor healing after injury (2). In a series of clinical and experimental studies, it has been found that even partial ACL damage can lead eventually to complete rupture. Noyes et al. have reported that 38% of partial ACL ruptures progressed to complete ligament deficiencies and tears, and estimated that half‐tears progressed to complete ACL deficiency in 50% of cases (3). Fruensgaard and Johannsen found 21 complete ACL ruptures in a series of 41 patients with previously partial ACL ruptures, and stated that most partial ACL lesions should be considered potential complete ruptures (4). Kleiner et al. found that in a rabbit model, repairs of partial and complete ACL ruptures did not heal, and concluded that the healing potential of ACL is poor (5). Non‐healing of the ligament after injury leads to instability of the knee joint and prevents patients from engaging in sports activities previously enjoyed. Long‐term instability of the joint may also lead to early osteoarthritic changes of the knee (6, 7).
Recently, mesenchymal stem cells (MSC) have been used in various tissue regeneration because of their capacity to differentiate towards various tissue lineages, such as bone, cartilage and ligament. Murphy et al. reported that injected adult MSCs intra‐articularly stimulated regeneration of meniscal tissue in an osteoarthritis‐induced caprine model (8). Agung et al. reported that intra‐articularly injected MSCs mobilized into injured tissues, including the ACL, meniscus and cartilage in a rat model, and contributed to their regeneration (9). Thus, MSCs are a fascinating source for use in regenerative medicine, due to their ease of isolation, propagation in culture for many generations, capacity for multilineage differentiation, and self‐renewal ability. In addition to the most common source of MSCs, bone marrow, there are increasing reports that MSCs can be isolated from other human mesenchymal tissues, such as liposuction fat (10), periosteum (11), skeletal muscle (12), synovium (13), umbilical cord blood (14), and hamstring tendons (15). Although MSCs isolated from these tissues have similar stem cell characteristics irrespective of their original sources, it has also been reported that properties of MSCs can be affected by their preparation as well as by their origin; for instance, stem cells from bone marrow or synovium may have greater potential for osteogenic and chondrogenic differentiation (16). Therefore, choice of an appropriate stem cell source may also play an important role in effectiveness of development of a specific tissue regeneration paradigm.
In the present study, we sought to isolate stem cells from human ACL and PCL and to identify their surface phenotype, differentiation potential and proliferation ability. We have demonstrated that stem cells can, indeed, be isolated from cruciate ligaments of the human knee joint.
Materials and methods
Study design
ACL and PCL tissues were collected aseptically during primary total knee arthroplasty procedures with informed consent of the patients (n = 6); diagnosis was advanced osteoarthritis in all cases (Table 1). Synovial tissues from two of the patients were also harvested for comparison and institutional review board approval was obtained prior to commencement of the study. Growth kinetics of the cells was evaluated by continual subculture and estimation of population doubling time, and capability of anchorage‐independent growth was evaluated by soft agar assay. For differentiation assays, cells were cultured for 21 days in osteogenic, chondrogenic and adipogenic media, and differentiation potentials of ligament‐derived cells were evaluated by histochemical staining and reverse transcriptase–polymerase chain reaction (RT‐PCR). Surface immunophenotype of cells was analysed by flow cytometry, and chromosomal stability after prolonged in vitro culture and serial passage was analysed by karyotyping.
Table 1.
The profile of the donors of ligament‐derived stem cells and synovial‐derived stem cells
| Donor no. | Age (years) and gender of the donor | Source of stem cells |
|---|---|---|
| 1 | 70/male | ACL, PCL |
| 2 | 71/female | ACL, PCL |
| 3 | 56/male | ACL, PCL |
| 4 | 66/male | ACL, PCL |
| 5 | 69/female | ACL, PCL, synovial tissues |
| 6 | 70/female | ACL, PCL, synovial tissues |
ACT, anterior cruciate ligament; PCT, posterior cruciate ligament.
Isolation of ligament‐derived stem cells
ACL‐ and PCL‐derived stem cells were isolated according to previously reported methods for isolating fibroblasts, with modifications (17). The ligaments were washed repeatedly in phosphate‐buffered saline (PBS; Gibco BRL, Grand Island, NY), and the synovial sheath and fat tissue were carefully scraped off, leaving only the core portion of the ligament to be used in the experiment. Tissues were cut into small pieces and placed in culture dishes containing growth medium, which consisted of alpha‐modified Eagle's medium (α‐MEM) (Gibco) supplemented with heat‐inactivated 10% foetal bovine serum (FBS) (Sigma Chemical Co., St Louis, MO, USA), 10 mm HEPES, 100 U penicillin, 1000 U streptomycin, and 2 mm l‐glutamine (Gibco). Medium was changed twice a week. Culture dishes were incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Explant tissues were removed when adequate cell outgrowth had been observed. These cells were referred to as passage 0 cells.
Isolation of synovium‐derived stem cells
Synovial stem cells were isolated as described in the literature (13, 16). Briefly, synovial tissues were washed repeatedly with PBS, minced into small pieces and digested with 0.2% collagenase in α‐MEM (Gibco) containing 10% heat‐inactivated FBS (Sigma Chemical Co.). Following overnight incubation at 37 °C, cells were collected by centrifugation and washed repeatedly in PBS. They were then plated at 50 000 cells/cm2 in T75 flasks with growth medium, as passage 0. Medium was changed twice a week. Culture dishes were incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
Culture maintenance and expansion
Once adherent passage 0 cells from ACL, PCL, and synovium reached semiconfluence, cells were detached with 0.25% trypsin–EDTA (ethylenediaminetetraacetic acid; Gibco), washed twice with PBS, centrifuged at 1000 r.p.m. (200 g) for 4 min, and replated as passage 1 at 50 cells/cm2 in each T75 flask. After an additional 14 days of population growth, cells were harvested and cryopreserved by washing aliquots of the trypsin‐released cells twice with PBS and cryopreserved in liquid nitrogen in FBS with 10% dimethyl sulphoxide (passage 1). To expand the cells, a vial of cryopreserved cells was thawed and plated at 5000 cells/cm2 in a T75 flask. Serial passage of the cells was performed at the same concentration.
Colony‐forming assay
After initial outgrowth of ligament‐derived cells from explant cultures, a portion of the passage 0 cells was diluted and replated onto 100‐mm culture plates at 100 cells/per plate concentration for 14 days, in order to evaluate colony‐forming efficiency of the cells. After 14 days, colonies that had formed from single cells were visualized by light microscopy and then counted after staining with 0.5% crystal violet in methanol for 5 min, after fixing in 4% formaldehyde. Cells were washed twice in distilled water, and number of colonies per dish was determined. Colonies < 2 mm in diameter and faintly stained colonies were ignored (18).
Cell proliferation assay
In order to examine proliferative ability of cells isolated from different tissues with different cell densities, cells from passage 1 were replated at 50 cells/cm2 or 5000 cells/cm2 in six‐well plates in growth medium. Plates were incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air, and cells were serially passaged for more than 50 days until senescence occurred. Medium was changed twice a week. Cells were counted and replated at the same density when confluence was reached, and serial passage of the cells was performed. We calculated population doubling (PD) for each passage using the formula PD = log2[N C/N 0], where N 0 is the inoculum cell population and N C number of cells at confluence; PDs from each passage were added together to obtain population doubling values.
Analysis of anchorage‐independent growth
Anchorage‐independent growth was assessed using a modification of the soft agar assay, the ‘over agar’ assay, described by Dong et al. (19). A total of 50 000 passage 1 cells in 2 ml of complete growth medium were plated on top of prehardened 1% agarose solution in each well of six‐well plates. Plates were then incubated at 37 °C in humidified atmosphere of 5% CO2 and 95% air for 14 days. Medium was changed twice a week. Colonies of eight cells or more were verified using an inverted phase‐contrast microscope.
In vitro differentiation
To induce differentiation, second‐ to seventh‐passage cells were seeded in six‐well plates and cultured by methods previously reported in the literature, with slight modification (13, 14, 16, 20).
Osteogenic differentiation
To induce osteogenic differentiation, cells were seeded at a density of 3000 cells/cm2 and were cultured in osteogenic medium for 3 weeks, with medium changed twice a week. Osteogenic medium consists of Dulbecco's modified Eagle's medium (DMEM; Bio‐Fluid, Rockville, MD, USA) supplemented with 1% FBS, 0.1 µm dexamethasone (Sigma‐Aldrich), 10 mmβ‐glycerol phosphate (Sigma‐Aldrich), and 0.2 mm ascorbic acid (Sigma‐Aldrich). Osteogenesis was assessed at 7‐day intervals.
Chondrogenic differentiation
To induce chondrogenic differentiation, 5 × 105 cells were transferred into 15‐ml polypropylene tubes and centrifuged at 500 r.p.m. for 4 min, to form a pellet micromass at the bottom of the tube, which was treated with chondrogenic medium or control medium for 3 weeks. Medium was changed twice a week, and chondrogenesis was assessed every week. Chondrogenic medium consists of DMEM supplemented with 0.1 µm dexamethasone, 50 µg/ml ascorbic acid, 100 µg/ml sodium pyruvate (Sigma‐Aldrich), 10 ng/ml TGF‐β1 (Becton Dickinson, Flanders, NJ, USA) and 50 mg/ml ITS+ premix (Becton Dickinson; 6.25 µg/ml insulin, 6.25 µg/ml transferrin, 6.25 ng/ml selenious acid, 1.25 ng/ml bovine serum albumin, and 5.35 mg/ml linoleic acid). Control medium consisted of DMEM only.
Adipogenic differentiation
To induce adipogenic differentiation, cells were seeded at a density of 10 000 cells/cm2 and cultured in adipogenic medium for 3 weeks. Medium was changed twice a week, and adipogenesis was assessed weekly. Adipogenic medium consists of DMEM supplemented with 10% FBS, 0.5 mm 3‐isobutyl‐1‐methylxanthine (IBMX; Sigma‐Aldrich), 1 µm dexamethasone, 0.1 mm indomethacin (Sigma‐Aldrich), and 10 µg/ml insulin.
Histological, cytochemical, and immunocytochemical analysis
Cytochemical staining. In order to evaluate the mineralized matrix, cells were fixed with 4% formaldehyde and stained with 1% alizarin red S (Sigma‐Aldrich) solution in water for 10 min. For Oil Red O staining, cells were fixed with 4% formaldehyde, stained with Oil Red O (Sigma‐Aldrich) for 10 min, and then counterstained with Mayer's haematoxylin (Sigma‐Aldrich).
Histological analysis. Chondrogenic differentiation was evaluated after pellets were fixed with 4% formaldehyde, dehydrated in serial ethanol dilutions, and embedded in paraffin wax blocks. Blocks were then cut and sections stained with alcian blue.
Flow cytometry analysis. For cell surface antigen phenotyping, fifth‐ to seventh‐passage cells of three donors were detached and stained with fluorescein‐ or phycoerythrin‐coupled antibodies and analysed using FACSCaliber (Becton Dickinson). Antibodies against human CD13, CD14, CD29, CD34, CD44, CD45, CD73, CD90, CD105, CD166, HLA‐ABC and HLA‐DR were purchased from Becton Dickinson; antibodies against human CD133 were purchased from Miltenyi Biotec (Bergisch Glabach, Germany).
RNA extraction and semiquantitative RT‐PCR analysis. Total RNA from cells was extracted using a commercially available kit (S.N.A.P.; Invitrogen, Groningen, The Netherlands), according to the manufacturer's instructions. Complementary DNA (cDNA) was obtained by RT of 1 µg total RNA using Advantage RT‐for‐PCR (Clontech, Palo Alto, CA, USA) per manufacturer's instructions. cDNA was amplified using ABI GeneAmp PCR system 2400 (PerkinElmer Applied Biosystems, Boston, MA, USA) at 94 °C for 40 s, 56 °C for 50 s, and 72 °C for 60 s for 30 cycles, after initial denaturation at 94 °C for 5 min. Primers used for amplification are listed in Table 2.
Table 2.
Primer used for reverse transcription–polymerase chain reaction analysis
| Gene | Sense primer | Anti‐sense primer |
|---|---|---|
| COMP | CCTTCAATGGCGTGGACTTC | GGTTCGCCTGCCAATACGT |
| AGC1 | AGTATCATCAGTCCCAGAATCTAGCA | GACGCCTCGCCTTCTTGA |
| COL2A1 | TACCCCAATCCAGCAAACGT | TGTTTCGTGCAGCCATCCT |
| COL1 | GTG ATG CTG GTG CTA AAG G‐ | GGT CCA GCA TTT CCA GAG |
| Oct4 | GAC AAC AAT GAG AAC CTT CAG GAG | CTG GCG CCG GTT ACA GAA CCA |
| Nanog | AGC TAC AAA CAG GTG AAG ACC | GTC TGA GTG TTC CAG GAG TG |
| Sox2 | GAG AAC CCC AAG ATG CAC AAC | GGC AGC GTG TAC TTA TCC TTC TTC |
| GAPDH | GAG TCC ACT GGC GTC TTC | GAC TGT GGT CAT GAG TCC TTC |
| FABP | GGA AAG TCA AGA GCA CCA TAA C | CAT GAC GCA TTC CAC CAC |
| PPARγ | CTT CTC CAG CAT TTC TAC TCC | GAA GAA ACC CTT GCA TCC |
| OC | ACA TCT ATC CGG GAG GAA ATC | CTG GCG GTC TCC TCA CTC |
| ON | AGG TAT CTG TGG GAG CTA ATC | ATT GCT GCA CAC CTT CTC |
| OP | GAC CTG ACA TCC AGT ACC C | GTT TCA GCA CTC TGG TCA TC |
COMP, cartilage oligomeric matrix protein; AGC1, Aggrecan 1; COL2A1, collagen type IIA1; COLI, collagen type I; FABP, fatty acid‐binding protein; PPAR, peroxisome proliferator‐activated receptor; OC, osteocalcin; ON, osteonectin; OP, osteopontin.
Karyotyping. Chromosome samples were prepared as described elsewhere (21). Briefly, cells were treated with 0.06 µg/ml colcemid (Invitrogen) for 2–4 h, trypsinized, incubated in 0.075 m of KCl for 10 min, and fixed in Carnoy's fixative. At least 50 spreads were counted for chromosome numbers and 10 banding patterns were analysed at 300–500 band resolution.
Statistical analysis. Statistical analysis of pellet weight from ACL and PCL were performed using the Wilcoxon rank sum test. P‐value of 0.05 was considered significant.
Results
Isolation of stem cells from ACL, PCL and synovium
Stem cells obtained from ACL, PCL and synovial tissues were plastic‐adherent and demonstrated characteristic spindle‐shaped and fibroblast‐like morphology (Fig. 1). These ligament‐derived stem cells (LSC) or synovium‐derived stem cells (SSC) could be culture‐expanded extensively and maintained their proliferative and differentiation abilities after cryopreservation. Doubling times of LSCs and SSCs were estimated to be 32–34 h at low density (50 cells/cm2) and 48–56 h at high density (50 000 cells/cm2), respectively, depending on the origin and donor. Population doubling of cells at low density (more than 30 population doublings) was much greater than that of cells at high density (about 20 population doublings). Cells reached senescence with enlarged cell shape and slower population growth rate, about 60 days at low density and 40 days at high density (Fig. 2).
Figure 1.
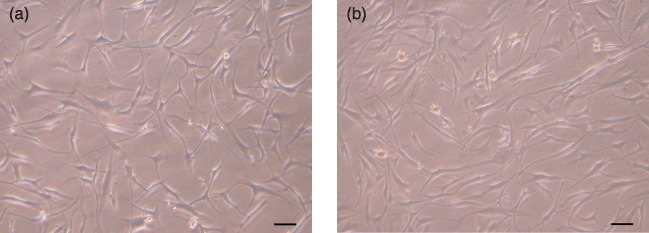
Morphology of ligament‐derived stem cells (LSC). Morphology of clonally expanded LSCs derived from anterior cruciate ligament (a) and posterior cruciate ligament (b). Cells are spindle‐shaped, fibroblast‐like and plastic adherent. Scale bar indicates 50 µm.
Figure 2.

Cell proliferation. Proliferation potentials of cells isolated from anterior (ACL) and posterior cruciate ligament (PCL) and synovial tissue of the same donor at different plating densities. (a) Passage 1 cells derived from the ACL, PCL, and synovial tissue were plated at a density of 50 cells/cm2 on six‐well plates. Cells were replated at 50 cells/cm2 every 14 days. (b) Passage 1 cells were plated at a density of 5000 cells/cm2 on six‐well plates, and then replated at 5000 cells/cm2 every 4–5 days.
Ligament‐derived cells were clonogenic
Commonly used criteria to define stem cells are clonogenicity, multipotency and self‐renewal. In order to assess whether these ligament‐derived cells were clonogenic, we generated and cultured cell suspensions from human ACL and PCL at very low seeding densities. After initial outgrowth of ligament‐derived cells from explant cultures, a portion of the cells was diluted and replated on to 100‐mm culture plates at concentration of 100 cells/per plate. A portion of ligament‐derived cells became attached to the plate and remained quiescent for 4–6 days before they rapidly divided to form adherent colonies. After 14 days, colonies derived from single cells were visible by light microscopy. These colonies were heterogeneous in size and cell density, reflecting differences in rates of cell proliferation. Morphologically, about three different colony types were observed in ligament‐derived cell culture. After staining with crystal violet, colonies on each plate were counted, and average colony‐forming efficiency of LSCs was determined as 20.18 ± 6.06 for ACL and 21.68 ± 5.99 for PCL. Results are shown as mean ± standard deviation of 12 plates in each group. There were no significant differences (Fig. 3).
Figure 3.

Colony‐forming ability of ligament‐derived stem cells (LSC). Cells from anterior (ACL) and posterior cruciate ligament (PCL) were plated at a density of 100 cells in each 100 mm culture plate for 14 days. Around 20% of ligament‐derived cells formed adherent cell colonies. (a) Colonies were stained with 0.5% crystal violet and photographed. Left four plates show colonies formed by single cells of LSCs of ACL and the right four plates colonies formed by single cells of LSCs of PCL. (b) Average colony‐forming efficiency of LSCs was 20.18 ± 6.06 for ACL and 21.68 ± 5.99 for PCL. Results are shown as mean ± standard deviation of 12 culture plates. There were no significant differences. (c) Cell colonies from LSCs of ACL under phase contrast microscopy. (d) Cell colonies from LSCs of PCL. Cells in the colonies were all spindle‐shaped and fibroblast‐like in morphology. These colonies were heterogeneous in size and cell density, partially reflecting differences in the rate of cell proliferation.
Anchorage‐independent growth ability
Stem cells and cancer cells have been shown to possess the capability of anchorage‐independent growth in previous studies (22). In our experiment, LSCs from ACL and PCL were capable of anchorage‐independent viability. These cells began to proliferate to form colonies from single cells in suspension at about 5 days after inoculation. Multiple colonies of cells were present after 14 days of culture in soft agar (Fig. 4).
Figure 4.

Anchorage‐independent growth of ligament‐derived stem cells (LSC). Growth and colony formation of passage 1 LSCs from anterior cruciate ligament (ACL) on soft agar after 14 days of inoculation, photographed using phase contrast microscopy at low (a) and high (b) magnification. Growth and colony formation of passage 1 LSCs from posterior cruciate ligament (PCL) on soft agar after 14 days of inoculation, photographed using phase contrast microscopy at low (c) and high (d) magnification.
Phenotypic characterization of LSCs
Surface immunophenotype of LSCs was positive or high for CD13, CD29, CD44, CD73, CD90, CD105, CD166 and HLA‐ABC, but negative or low for CD14, CD34, CD45, CD133, and HLA‐DPQR (Fig. 5). Average positive ratio of ACL LSCs was 99.69 (CD13), 98.60 (CD29), and 99.71(CD44); average positive ratio of PCL LSCs was 98.62 (CD13), 81.54 (CD29) and 98.90 (CD44). Mean surface immunophenotype expression values and standard deviation of LSCs from ACL and PCL of three donors were measured and are summarized in Table 3.
Figure 5.
Immunophenotype of ligament‐derived stem cells (LSC) isolated from anterior (ACL) and posterior cruciate ligament (PCL). LSCs from ACL and PCL were cultured for 3–5 passages, harvested, and labelled with antibodies against human antigens CD13, CD14, CD29, CD34, CD44, CD45, CD73, CD90, CD105, CD133, CD166, HLAABC, and HLA‐DR, as indicated and analysed by FACS. (a) LSCs from ACL. (b) LSCs from PCL.
Table 3.
The average values of surface immunophenotypes of ligament‐derived stem cells (LSC) from anterior (ACL) and posterior cruciate ligament (PCL) of three different donors
| ACL Mean (SD) | PCL Mean (SD) | ACL Mean (SD) | PCL Mean (SD) | ||
|---|---|---|---|---|---|
| CD13 | 99.69 (0.2553) | 98.62 (2.154) | CD14 | 1.41 (0.026) | 1.36 (0.103) |
| CD29 | 98.60 (1.229) | 81.54 (3.634) | CD34 | 1.40 (0.372) | 1.07 (0.278) |
| CD44 | 99.71 (0.378) | 98.90 (1.830) | CD45 | 1.07 (0.110) | 3.06 (2.279) |
| CD73 | 98.89 (0.856) | 98.91 (1.059) | CD133 | 1.00 (0.146) | 2.17 (1.467) |
| CD90 | 99.98 (0.040) | 99.01 (1.709) | HLA‐DPQR | 1.69 (0.080) | 3.26 (1.780) |
| CD105 | 99.21 (1.121) | 95.99 (6.742) | |||
| CD166 | 93.72 (2.690) | 92.73 (5.150) | |||
| HLA‐ABC | 91.56 (5.736) | 90.40 (2.240) |
In vitro osteogenic differentiation
LSCs from ACL and PCL were stained positively for alkaline‐phosphatase (Alk‐p) after 2 weeks of osteogenic induction, whereas cells cultured in control medium were Alk‐p negative. After 3 weeks of osteogenic induction, cells were positive for alizarin red S in comparison to negative for cells in control medium (Fig. 6a–c). mRNA expression analysis using RT‐PCR of cells from ACL and PCL under osteogenic conditions was performed, and cells revealed increasing expression levels of osteocalcin and osteopontin and stable levels of type I collagen, after 3 weeks under osteogenic culture conditions (Fig. 6d).
Figure 6.

Osteogenic differentiation of ligament‐derived stem cells (LSC). LSCs isolated from anterior (ACL) and posterior cruciate ligament (PCL) after osteogenic differentiation for 3 weeks. (a) Gross pictures of LSCs from PCL after osteogenic differentiation for 2 weeks: upper panel, Alk‐p stain; lower panel, alizarin red stain. Left panel, LSCs of PCL under control medium; middle panel, LSCs of PCL under osteogenic medium for 2 weeks; right panel, FOB (fetal osteoblastic cell line) under osteogenic medium for 2 weeks. (b) Cells, at ×40 magnification: LSCs under osteogenic medium for 2 weeks with Alk‐p stain. Scale bar indicates 50 µm. (c) Cells at ×40 magnification, picture of LSCs under osteogenic medium for 2 weeks with alizarin red S stain. Scale bar indicates 50 µm. (d) Total RNA was analysed by RT‐PCR for mRNA expression. GAPDH was used as the control.
Chondrogenic differentiation
After 3 weeks in chondrogenic induction or control conditions, cells from ACL and PCL formed semitranslucent pellets. All pellets were larger than 1 mm in diameter, and those under chondrogenic conditions were larger in size and smoother in shape than controls (Fig. 7a). Histology of the pellets was positive for alcian blue staining after 3 weeks in chondrogenic medium (Fig. 7b), but weak or negative for alcian blue after 3 weeks in control medium (Fig. 7c). Under chondrogenic conditions, cells in pellets expressed elevated levels of type II collagen, aggrecan and cartilage oligometric protein (COMP) in comparison to those under control conditions (Fig. 7d). Weights of pellets from the first four donors were measured and averaged, and it was found that those from PCL cells were larger and heavier than those from ACL cells (Fig. 7e); however, difference in mean weights of the ACL and PCL cell‐derived pellets was not statistically significant (P = 0.11; Fig. 7f).
Figure 7.

Chondrogenic differentiation of ligament‐derived stem cells (LSC). (a) Gross picture of pellets cultured for 1, 2 or 3 weeks from LSCs of anterior cruciate ligament (ACL) under chondrogenic conditions (left three pellets, the numbers above the pellets indicating number of weeks of culture) and control conditions (right three pellets, the number above the pellets indicating number of weeks of culture). The interval between each two lines of the lower meter is 1 mm. (b) Under chondrogenic conditions for 3 weeks, the histological section of the pellet from LSCs of ACL was positive for Alcian blue stain. Scale bar indicates 200 µm. (c) Under control conditions for 3 weeks, the histological section of the pellet from LSCs of ACL was weakly positive for Alcian blue stain. Scale bar indicates 200 µm. (d) RT‐PCR analysis of chondrogenic‐induced pellet cultures of LSCs from ACL and posterior cruciate ligament (PCL) for 3 weeks. Total RNA was analysed by RT‐PCR for mRNA expression. GAPDH was used as control. COMP, cartilage oligomeric matrix protein. (e) Gross picture of pellets cultured for 3 weeks from LSCs of ACL (upper row) and PCL (lower row). (f) Statistical analysis of the pellet weight from ACL LSCs and PCL LSCs was performed using the Wilcoxon rank sum test. No statistical significance was observed.
Adipogenic differentiation
Light microscopy revealed presence of increasing numbers of lipid vacuoles of cells during the 3‐week period of adipogenic induction (Fig. 8a,b). Lipid vacuoles were positive for Oil Red O stain (Fig. 8c,d). In addition to histological change, culture of LSCs in adipogenic medium demonstrated significantly upregulated expression of fatty acid‐binding protein (FABP) and peroxisome proliferator‐activated receptor‐gamma (PPAR‐γ) as compared to those cultured in control culture medium (Fig. 8e).
Figure 8.

Adipogenic differentiation of ligament‐derived stem cells (LSC). LSCs from anterior (ACL) and posterior cruciate ligament (PCL) were under adipogenic induction for 21 days. Upon adipogenic induction, typical fat droplet appeared 1 week after induction and gradually increased in number. Photographs were taken on day 21 under ×40 magnification. Scale bar indicates 50 µm. (a) Histological picture of ACL‐derived LSCs. (b) Histological picture of PCL‐derived LSCs. (c) Oil Red O staining of PCL‐derived LSCs. (d) Oil Red O staining of PCL‐derived LSCs. (e) RT‐PCR analysis of LSCs from ACL and PCL. Total RNA was analysed by RT‐PCR for mRNA expression. GAPDH was used as the internal control. PPAR‐γ, peroxisome proliferator‐activated receptor‐gamma; FABP, fatty acid‐binding protein.
‘Stemness’ gene expression
To identify expression of genes responsible for maintenance of ‘stemness’ of stem cells, such as Oct‐4, Nanog, and Sox‐2, passage 9 cells from LSCs and SSCs were analysed. Compared to same passage bone marrow MSCs (of which we previously isolated and reported) (23, 24), LSCs showed comparable gene expression (Fig. 9).
Figure 9.

Stemness gene expression. Expression levels of stemness genes, including Oct‐4, Nanog, and Sox‐2, were evaluated. Passage 9 cells from ligament‐derived stem cells (LSC) of the anterior (ACL) and posterior cruciate ligament (PCL) and synovial‐derived stem cells from the same donor were analysed. Compared with the same passage bone marrow MSC, the LSCs shows comparable stemness gene expression. Total RNA was analysed by RT‐PCR for mRNA expression. MSC, bone marrow MSCs.
Karyotyping
To assess chromosome stability of the LSCs after serial passage, passage 9 cells from the ACL of donor 3 and the PCL of donor 5 were collected and karyotyped. After serial passage, such cells showed normal diploid karyotypes (that is, 46XY or 46XX). Chromosomal abnormalities, such as deletion, inversion, translocation, or ring chromosomes, were not observed by karyotyping analysis of G‐banding (Fig. 10).
Figure 10.
Karyotyping. Karyotyping of ligament‐derived stem cells (LSC) from two donors (a, b) after serial passage under standard culture medium (passage 9) was evaluated. No chromosomal abnormality was observed.
Discussion
MSCs have been shown to be useful both preclinically and in clinical trials for regeneration or repair of tissues, not only those of the musculoskeletal system, such as bone and cartilage, but also tissues of the nervous and cardiovascular systems, damage to which involves major organs (25, 26, 27, 28, 29, 30, 31, 32). Thus, stem cells have become more and more important due to their fascinating potential in tissue regeneration. MSCs have been isolated from other mesenchymal tissues (10, 11, 12, 13, 15), and these (MSCs isolated from different tissues) share common characteristics, such as multilineage differentiation, surface immunophenotype, and clonogenic characteristics irrespective of their original sources (33, 34, 35, 36, 37). However, as reported in the literature, MSCs from different origins may still have subtle differences. Therefore, choice of an appropriate stem cell source may play an important role in safety and effectiveness of future developments in tissue regeneration.
As reported in the literature, efforts have been made to isolate fibroblasts from ACL and PCL in order to study their abilities in terms of ligament regeneration (17, 38, 39). However, there have been no previous studies of stem cells isolated from ACL and PCL that can undergo different lineage differentiation, extended ex vivo passage, and expression of high levels of stemness‐related genes. Here, we have adopted methods of stem cells isolation previously reported by Sekiya and Sakaguchi, that is, we isolated passage 0 cells from tissue explants using methods previously described by van Eijk and Nagineni. We obtained a mixture of cells containing fibroblasts, differentiated cells, progenitor cells, and stem cells from ACL and PCL; we then followed the methods of Sekiya and Sakaguchi (16, 18), using very low seeding density of 50 cells/cm2 (less than 1% of normal seeding density of cell cultures). At this cell seeding density, most fibroblasts or differentiated cells die due to loss of cell‐cell contact or fade out due to low proliferation velocity, while cells possessing stem cell or progenitor cell characteristics proliferate and survive. LSCs isolated from ACL and PCL by these methods were able to be culture‐expanded in vitro for more than 30 cumulative population doublings at low seeding density and 20 cumulative population doublings at normal seeding density.
In addition to results described by Sakaguchi et al. (16), LSCs can proliferate more rapidly with less senescence at very low seeding concentrations. Proliferation ability and doubling time of LSCs from ACL and PCL were not significantly different from each other. These cells were able to differentiate into osteoblasts, chondrocytes and adipocytes under appropriate induction in vitro. Cells isolated and passaged in our study showed stable phenotype and gene expression after serial passaging; flow cytometry analysis also showed results comparable with those of stem cells from other sources (40). Surface immunophenotype expression of LSCs from ACL and PCL did not differ either, and histological analysis and gene expression of LSCs from ACL and PCL during osteogenic, adipose and chondrogenic induction were quite similar. LSCs were found to maintain karyotype stability after serial passage.
In the literature, it has been reported that synovial MSCs possess better chondrogenic ability than bone marrow MSCs (16). In this study, it was found that LSCs from ACL and PCL may have the same characteristics as synovial MSCs and could be used in future reconstruction or regeneration of ligaments or osteochondral defects of the knee. There is emerging evidence to suggest that MSCs may contribute more through secretion of soluble factors to help local cells regenerate than by direct differentiation of stem cells to repair damaged tissue (41). In this study, although LSCs can be isolated from ACL and PCL, the role of these cells in maintenance of normal physiology still remains elusive, and further study is necessary in order to answer this question.
Ligaments and tendons have long been considered to be post‐mitotic organs. However, stem cells have been isolated previously from tendons (15), and are isolated from ligaments, here. The question as to why stem cells exist in ACLs but failed to regenerate ruptured ACLs remains unanswered. Researchers have tried to use primary sutures to reattach ruptured ACLs, with varied results (42, 43, 44), possibly due to both mechanical and biological factors. First, instability of ACL‐deficient knee results in excessive anterior‐posterior motion, which is detrimental to healing of the ligament (45, 46, 47, 48). In addition, cytokine composition of synovial fluid in the injured knee joint may also inhibit population growth of stem cells in ACL (49, 50); furthermore, lack of vascular ingrowth after ligament rupture may also inhibit the ligament from healing (51, 52). In a recent report it has been shown that aspirates of haemarthrosis of the knee joint can yield progenitor cells with multidifferentiation potential (53); the source of such cells may be from bone marrow by way of blood, as well as from ruptured ACL. Together, stem cells do exist in the knee joint after ACL injury, but unfavourable biomechanical and biological factors prevent the ligament from healing.
In tissue engineering, the choice of an appropriate cell source plays a critical role. ACL and PCL tissues can be easily obtained from open knee surgery or arthroscopic surgery. Our study demonstrates that multipotent stem cells can be isolated and expanded from human ACL and PCL, and are capable of self‐renewal and mesodermal differentiation. Instead of being discarded as medical wastes, cruciate ligaments can provide an alternative cell source for stem cells for use in regenerative medicine.
Acknowledgement
Grant from Ministry of Education, Aim for the Top University Plan, Taiwan.
References
- 1. Frank CB, Jackson DW (1997) The science of reconstruction of the anterior cruciate ligament. J. Bone Joint Surg. Am. 79, 1556–1576. [DOI] [PubMed] [Google Scholar]
- 2. Feagin JA Jr, Curl WW (1976) Isolated tear of the anterior cruciate ligament: 5‐year follow‐up study. Am. J. Sports Med. 4, 95–100. [DOI] [PubMed] [Google Scholar]
- 3. Noyes FR, Mooar LA, Moorman CT III, McGinniss GH (1989) Partial tears of the anterior cruciate ligament. Progression to complete ligament deficiency. J. Bone Joint. Surg. Br. 71, 825–833. [DOI] [PubMed] [Google Scholar]
- 4. Fruensgaard S, Johannsen HV (1989) Incomplete ruptures of the anterior cruciate ligament. J. Bone Joint Surg. Br. 71, 526–530. [DOI] [PubMed] [Google Scholar]
- 5. Kleiner JB, Roux RD, Amiel D, Woo SL‐Y (1986) Primary healing of the anterior cruciate ligament. Trans. Orthop. Res. Soc. 11, 131. [Google Scholar]
- 6. Barrack RL, Bruckner JD, Kneisl J, Inman WS, Alexander AH (1990) The outcome of non‐operatively treated complete tears of the anterior cruciate ligament in active young adults. Clin. Orthop. Relat. Res. 259, 192–199. [PubMed] [Google Scholar]
- 7. Caborn DN, Johnson BM (1993) The natural history of the anterior cruciate ligament‐deficient knee. A review. Clin. Sports Med. 12, 625–636. [PubMed] [Google Scholar]
- 8. Murphy JM, Fink DJ, Hunziker EB, Barry FB (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48, 3464–3474. [DOI] [PubMed] [Google Scholar]
- 9. Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T et al (2006) Mobilization of bone marrow‐derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg. Sports. Traumatol. Arthrosc. 14, 1307–1314. [DOI] [PubMed] [Google Scholar]
- 10. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA et al (2003) Combined effects of insulin‐like growth factor‐1 and transforming growth factor‐β1 on periosteal mesenchymal cells during chondrogenesis in vitro . Osteoarthritis. Cartilage. 11, 55–64. [DOI] [PubMed] [Google Scholar]
- 12. Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B et al (2003) Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat. Cell Biol. 5, 640–646. [DOI] [PubMed] [Google Scholar]
- 13. De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 44, 1928–1942. [DOI] [PubMed] [Google Scholar]
- 14. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH (2004) Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103, 1669–1675. [DOI] [PubMed] [Google Scholar]
- 15. Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W et al (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 13, 1219–1227. [DOI] [PubMed] [Google Scholar]
- 16. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52, 2521–2529. [DOI] [PubMed] [Google Scholar]
- 17. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH (1992) Characterization of the intrinsic properties of the anterior cruciate and posterior cruciate ligament cells: an in vitro cell culture study. J. Orthop. Res. 10, 465–475. [DOI] [PubMed] [Google Scholar]
- 18. Sekiya I, Larson BL, Smith JR, Pochanmpally R, Cui JG, Prockop DJ (2002) Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 20, 530–541. [DOI] [PubMed] [Google Scholar]
- 19. Dong Z, Cmarik JL, Wendel EJ, Colburn NH (1994) Differential transformation efficiency but not AP‐1 induction under anchorage‐dependent and – independent conditions. Carcinogenesis 15, 1001–1004. [DOI] [PubMed] [Google Scholar]
- 20. De Mos M, Koevoet WJ, Jahr H, Verstegen MM, Heijboer MP, Kops N et al (2007) Intrinsic differentiation potential of adolescent human tendon tissue: an in‐vitro cell differentiation study. BMC Musculoskelet. Disord. 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M et al (2006) Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 97, 744–754. [DOI] [PubMed] [Google Scholar]
- 22. Lin TM, Tsai JL, Lin SD, Lai CS, Chang CC (2005) Accelerated growth and prolonged lifespan of adipose tissue‐derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 14, 92–102. [DOI] [PubMed] [Google Scholar]
- 23. Lee KD, Kuo TK, Whang‐Peng J, Chung YF, Lin CT, Chou SH et al (2004b) In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 40, 1275–1284. [DOI] [PubMed] [Google Scholar]
- 24. Shih YR, Chen CN, Tsai SW, Wang YJ, Lee OK (2006) Growth of mesenchymal stem cells on electrospun type I collagen nanofibers. Stem Cells 24, 2391–2397. [DOI] [PubMed] [Google Scholar]
- 25. Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P (2001) Bone marrow cells regenerate infracted myocardium. Nature 410, 701–705. [DOI] [PubMed] [Google Scholar]
- 26. Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A et al (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 344, 385–386. [DOI] [PubMed] [Google Scholar]
- 27. Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J (2001) Replacement of an avulsed phalanx with tissue‐engineered bone. N. Engl. J. Med. 344, 1511–1514. [DOI] [PubMed] [Google Scholar]
- 28. Min JY, Sullivan MF, Yang Y, Zhang JP, Converso KL, Morgan JP et al (2002) Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann. Thorac. Surg. 74, 1568–1575. [DOI] [PubMed] [Google Scholar]
- 29. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105, 93–98. [DOI] [PubMed] [Google Scholar]
- 30. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M (2002) Human autogenous culture expanded bone marrow mesenchymal stem cell transplantation for repair of cartilage defects in osteoarthritic knee. Osteoarthritis. Cartilage. 10, 199–206. [DOI] [PubMed] [Google Scholar]
- 31. Pittenger MF, Martin BJ (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 95, 9–20. [DOI] [PubMed] [Google Scholar]
- 32. Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmöller M et al (2004) Growth and transplantation of a custom vascularised bone graft in a man. Lancet 364, 766–770. [DOI] [PubMed] [Google Scholar]
- 33. Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD et al (1995) Cultured adherent cells from marrow can serve as long‐lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Acad. Natl. Sci. USA 92, 4857–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M et al (1999) Transplantability and therapeutic effects of bone marrow‐derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 5, 262–264. [DOI] [PubMed] [Google Scholar]
- 35. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- 36. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz‐Gonzalez XR et al (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49. [DOI] [PubMed] [Google Scholar]
- 37. Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ (2004) Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 22, 823–831. [DOI] [PubMed] [Google Scholar]
- 38. Van Eijk F, Saris DB, Riesle J, Willems WJ, Van Blitterswijk CA, Verbout AJ et al (2004) Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng. 10(5–6), 893–903. [DOI] [PubMed] [Google Scholar]
- 39. Ge Z, Goh JC, Lee EH (2005) Selection of cell source for ligament tissue engineering. Cell Transplant. 14, 573–583. [DOI] [PubMed] [Google Scholar]
- 40. Dominici M, Le Blanc K, Mueller I, Slaper‐Cortenbach I, Marini F, Krause D et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- 41. Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells 25, 2896–2902. [DOI] [PubMed] [Google Scholar]
- 42. Kaplan N, Wickiewicz TL, Warren RF (1990) Primary surgical treatment of anterior cruciate ligament ruptures. A long‐term follow‐up study. Am. J. Sports. Med. 18, 354–358. [DOI] [PubMed] [Google Scholar]
- 43. Träger D, Pohle K, Tschirner W (1995) Anterior cruciate ligament suture in comparison with plasty: a 5‐year follow‐up study. Arch. Orthop. Trauma Surg. 114, 278–280. [DOI] [PubMed] [Google Scholar]
- 44. Strand T, Molster A, Hordvik M, Krukhaug Y (2005) Long‐term follow‐up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15–23 years post‐operatively. Arch. Orthop. Trauma Surg. 125, 217–221. [DOI] [PubMed] [Google Scholar]
- 45. Mononen T, Alaranta H, Harilainen A, Sandelin J, Vanhanen I, Osterman K (1997) Instrumented measurement of anterior‐posterior translation of in knees with chronic anterior cruciate ligament tear. Arch. Orthop. Trauma Surg. 116, 283–286. [DOI] [PubMed] [Google Scholar]
- 46. Beynnon BD, Fleming BC, Labovitch R, Parsons B (2002) Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non‐weightbearing to weightbearing. J. Orthop. Res. 20, 332–337. [DOI] [PubMed] [Google Scholar]
- 47. Brandsson S, Karlsson J, Swärd L, Kartus J, Eriksson BI, Kärrholm J (2002) Kinematics and laxity of the knee joint after anterior cruciate ligament reconstruction: pre‐ and postoperative radiostereometric studies. Am. J. Sports. Med. 30, 361–367. [DOI] [PubMed] [Google Scholar]
- 48. Shefelbine SJ, Ma CB, Lee KY, Schrumpf MA, Patel P, Safran MR et al (2006) MRI analysis of in vivo meniscal and tibiofemoral kinematics in ACL‐deficient and normal knees. J. Orthop. Res. 24, 1208–1217. [DOI] [PubMed] [Google Scholar]
- 49. Amiel D, Billings E Jr, Harwood FL (1990) Collagenase activity in anterior cruciate ligament: protective role of the synovial sheath. J. Appl. Physiol. 69, 902–906. [DOI] [PubMed] [Google Scholar]
- 50. Cameron ML, Fu FH, Paessler HH, Schneider M, Evans CH (1994) Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL‐deficient knee. Knee Surg. Sports. Traumatol. Arthrosc. 2, 38–44. [DOI] [PubMed] [Google Scholar]
- 51. Bray RC, Leonard CA, Salo PT (2002) Vascular physiology and long‐term healing of partial ligament tears. J. Orthop. Res. 20, 984–989. [DOI] [PubMed] [Google Scholar]
- 52. Bray RC, Leonard CA, Salo PT (2003) Correlation of healing capacity with vascular response in the anterior cruciate and medial collateral ligaments of the rabbit. J. Orthop. Res. 21, 1118–1123. [DOI] [PubMed] [Google Scholar]
- 53. Lee SY, Miwa M, Sakai Y, Kuroda R, Matsumoto T, Iwakura T et al (2007) In vitro multipotentiality and characterization of human unfractured traumatic hemarthrosis‐derived progenitor cells: a potential cell source for tissue repair. J. Cell. Physiol. 210, 561–566. [DOI] [PubMed] [Google Scholar]




