Abstract.
Intact G0 nuclei from quiescent mammalian cells initiate DNA synthesis asynchronously in Xenopus egg extracts, despite exposure to the same concentration of replication factors. This indicates that individual nuclei differ in their ability to respond to the inducers of DNA replication. Since the induction of DNA synthesis requires the accumulation of replication factors by active nuclear transport, any variation in the rate of transport among nuclei could contribute to the variability of DNA replication. Using the naturally fluorescent protein allophycocyanin (APC) coupled with the nuclear localization sequence (NLS) of SV40 T antigen, as a marker of nuclear uptake, we show here that individual G0 nuclei differ in their rate of transport over a range of more than 20‐fold. Surprisingly, this variation has no direct influence on the timing or extent of DNA synthesis. Similar results were obtained by monitoring the uptake of nucleoplasmin, a nuclear protein present at high levels in egg extracts. These experiments show that the initiation of DNA synthesis is not driven merely by the accumulation of replication factors to some threshold concentration. Instead, some other explanation is needed to account for the timing of initiation.
Introduction
The duration of the cell cycle is highly variable, even for a population of identical cells growing exponentially in steady state (Brooks 1981). Most of this variability arises in the G1 phase and may be related to the mechanisms triggering the G1/S transition (Brooks et al. 1983; Zetterberg & Larsson 1985). Despite its importance, the molecular basis of the variability remains obscure. In order to gain insight into the nature of the factors regulating entry into S phase and the onset of DNA synthesis, we have been exploiting the ability of Xenopus egg extracts to support the initiation and completion of chromosomal DNA replication in vitro (Leno & Laskey 1991a; Hutchison 1993). Although it has been reported that G0 nuclei do not replicate in this system unless first permeabilized (Leno & Munshi 1994, 1997; Wu & Gilbert 1997), we have found that intact nuclei from quiescent Swiss 3T3 cells can be induced to undergo significant levels of DNA replication in Xenopus egg extracts (1994, 1996; Logothetou‐Rella, Sun & Brooks 2000; Sun et al. 2000), though the capacity for replication declines with the duration of quiescence (Sun et al. 2000). Those nuclei that initiate DNA synthesis do so asynchronously, despite exposure to a common level of replication factors (1994, 1996). This suggests that individual nuclei differ in their ability to respond to the inducers of replication. These differences may contribute to the cell cycle variability seen with intact cells (Hola et al. 1996).
When replication begins in a nucleus, it does so at many hundreds of independent foci scattered throughout the nucleus (Mills et al. 1989; Leno & Laskey 1991b). The triggering of replication must therefore be a response to some global signal propagated throughout the nucleus, though the timing of this signal, or the response to it, must vary from nucleus to nucleus. Since replication in Xenopus egg extracts is dependent on active nuclear transport (Cox 1992), one possible explanation for the simultaneous firing of multiple origins within the nucleus could be that initiation is triggered by the accumulation of replication factors to some threshold concentration (Leno & Laskey 1991b). This is an attractive possibility that could also account for the asynchrony of initiation, since any variability in the rate of nuclear transport between nuclei would influence the time required to reach the necessary threshold. For this reason, we have sought to determine the relationship between nuclear uptake and the initiation of DNA synthesis in the same nuclei. We report here for quiescent (G0) 3T3 cells that the rate of nuclear transport is indeed highly variable, as previously shown for asynchronously growing HeLa cells (Adam et al. 1992). Surprisingly, this variation makes little direct contribution to the timing or extent of DNA synthesis. It follows that the initiation of replication is not driven merely by the accumulation of replication factors. Some other explanation is needed to account for the asynchrony of initiation and the co‐ordination of independent replication foci.
METHODS AND MATERIALS
Preparation of Xenopus egg extracts
Low‐speed supernatants (LSS) of Xenopus egg extracts were prepared as described by Blow & Laskey (1986) and Blow (1993). In brief, female frogs were primed by an injection of 30 I.U. of pregnant mare serum gonadotrophin (Sigma, Poole, UK) 2–4 days prior to egg collection (Lohka & Maller 1985; Coppock et al. 1989), and 600 I.U. chorionic gonadotrophin (Sigma) 12–15 h before the desired collection time. Eggs were collected in high salt Barth (110 m m NaCl, 15 m m Tris‐HCl, pH 7.4, 2 m m KCl, 2 m m NaHCO3, 1 m m MgSO4, 0.5 m m Na2HPO4) and dejellied with 2% Cysteine, pH 7.8. Dejellied eggs were washed in modified Barth solution (88 m m NaCl, 2 m m KCl, 1 m m MgCl2, 0.5 m m CaCl2, pH 7.4), and the eggs then activated for 5 min with 0.5 µg/ml calcium ionophore A23187 (Sigma). After removal of unactivated eggs, the rest were packed by centrifugation at 1000 g and subsequently spin‐crushed at 10 000 g for 10 min at 4 °C. The cytoplasmic layer was taken and supplemented with 10 µg/ml cytochalasin B and 1 µg/ml each of aprotinin, pepstatin, and leupeptin (Sigma) before re‐centrifugation at 12 000 g for 15 min at 4 °C. Finally, the resulting extract was supplemented with 2% glycerol and 10 µg/ml cycloheximide before being frozen by dropping 20 µl aliquots into liquid nitrogen.
High‐speed supernatants (HSS) were prepared by centrifuging freshly made low‐speed egg extracts at 100 000 g for 2 h at 4 °C. This fractionated the extract into a clear supernatant (top), a loose membranous layer (middle), and a ribosomal pellet (bottom). The clear supernatant was taken and re‐centrifuged at 100 000 g for 30 min to remove any residual membranous contamination. Aliquots of 20 µl were then frozen by dropping into liquid nitrogen, after addition of 7% glycerol. Control experiments confirmed that the preparations of HSS were unable to support either nuclear envelope repair, nuclear transport or DNA synthesis using permeabilized nuclei.
Cell culture
Swiss 3T3/C5 cells were grown in Dulbecco’s modification of Eagle’s medium (DMEM) supplemented with 10% newborn calf serum, as described previously (Brooks et al. 1984). Quiescent cells used for experiments were seeded in four‐well plates (Nunc) at 5×104 cells per 16 mm diameter well in 0.75 ml DMEM containing 10% newborn calf serum. Cells reached confluence and became quiescent after 7–10 days. Quiescence was confirmed by pulse‐labelling for 2 h with 5′‐bromo‐2′‐deoxyuridine (BrdU), as described (Hola et al. 1994), the labelling index typically being <1%.
Preparation of APC‐NLS conjugates
The APC‐NLS conjugates were prepared as described by Adam et al. (1990). Peptides containing the wild‐type nuclear localization sequence of SV40 large T antigen (CGGGPKKKRKVED) or a transport‐deficient mutant (CGGGPKNKRKVED) were synthesized in‐house by Dr S. Bansal, Pharmaceutical Chemistry Group, King’s College London. The peptides (0.5 mg) were first reduced for 90 min at 37 °C in 100 µl of 50 m m dithiothreitol (DTT) in 100 m m sodium phosphate buffer, pH 7.0. Reduced peptides were separated from DTT by chromatography on Sephadex G‐10. Prior to conjugation, the phycobiliprotein allophycocyanin (APC) (Calbiochem CN Biosciences UK, Nottingham, UK) was activated for 60 min at room temperature with a 20‐fold molar excess of cross‐linker, sulfo‐SMCC (Pierce, Perbio Science UK Ltd, Chester, UK). Activated APC was separated from unlinked sulfo‐SMCC on Sephadex G‐25 and was then mixed with a 50‐fold molar excess of the reduced peptides. After overnight incubation at 4 °C, free peptides were separated from the APC‐NLS conjugates on Sephadex G‐25. The number of NLS peptides conjugated to APC was estimated by mobility shift on SDS‐polyacrylamide gels, and was approximately four to eight peptides per APC molecule. The concentration of the conjugates was determined by absorbance at 650 nm, according to the extinction coefficient, A650 1% = 70.5 with 1 cm light path.
Isolation of intact quiescent nuclei by scrape‐rupture
DNA replication was examined by introducing intact quiescent nuclei into cell‐free extracts of Xenopus eggs. Intact G0 nuclei were isolated by lysis of quiescent cells, utilizing the scraperupture technique (Hola et al. 1994). Swiss 3T3/C5 cells were seeded in four‐well plates. After reaching quiescence (less than 1% of cells in S phase), plates of cells were placed on ice and rinsed twice with replication buffer (110 m m KCl, 20 m m Hepes, pH 7.3, 7 m m MgCl2, 5 m m NaCl, 2 m m DTT, 1 m m EGTA, 0.5 m m spermidine trihydrochloride, 0.15 m m spermine trihydrochloride). To avoid dilution of the extracts, cells were rinsed with 20 µl of Xenopus egg extract per well which was then replaced with fresh extract. Nuclei were then released into the extract by scraping cells with a yellow Gilson pipette tip, after which the suspensions including nuclei and extracts were transferred to 0.5 ml microfuge tubes for analysis of nuclear transport and DNA replication. Nuclear membrane integrity of the scrape‐ruptured nuclei was confirmed by IgG exclusion, as described previously (Hola et al. 1994). In general, approximately 75%~85% of cells were lysed and retained intact nuclei.
Nuclear transport and DNA replication assays
To assay for nuclear transport, Xenopus egg extracts containing intact G0 nuclei were supplemented with an energy regenerating system consisting of phosphocreatine (24 m m, Sigma), and creatine phosphokinase (30 µg/ml, Sigma), together with fluorescein‐12‐dUTP (Fl‐dUTP, 10 µM, Roche Diagnostics) to detect DNA replication, and APC‐NLS (100 n m), as an indicator of nuclear uptake. Such extracts were incubated at 22 °C and samples of 10 µl were taken at the indicated times, these being fixed in 100 µl of 4% formaldehyde in PBSA. Each sample was layered onto 300 µl of 25% glycerol in PBSA in a ‘polo’ (Hola et al. 1994) attached to a coverslip by wax, and centrifuged at 340 g for 15 min at 4 °C. Following centrifugation, the suspensions were removed by aspiration and coverslips detached from the polos and air‐dried. Coverslips were mounted on glass slides in one drop of DABCO (1,4 diazabicyclo‐(2,2,2)‐octane, 2.5% w/v in 1 part PBSA and 9 parts glycerol) (Johnson et al. 1982), containing 1 µg/ml DAPI, and examined by confocal microscopy.
Nucleoplasmin detection
After incubation in egg extracts, 10 µl samples were fixed by addition to 100 µl of 4% formaldehyde in PBSA, and sedimented through 25% glycerol onto coverslips, as above. In some experiments, 4% freshly prepared paraformaldehyde was used in place of formaldehyde, with identical results. After air‐drying, nuclei were permeabilized by addition of 50 µl of ice‐cold methanol for 5 min. The methanol was removed and the coverslips washed twice with PBSA. Nuclei were then stained using a mouse monoclonal antibody to Xenopus nucleoplasmin, PA3C5, kindly supplied by Dr S Dilworth, Royal Postgraduate Medical School, London, as culture supernatant. This antibody is specific for Xenopus nucleoplasmin and gave negligible background staining of control 3T3 nuclei not exposed to Xenopus egg extract. The antibody was diluted 1 : 50 in PBSA and 50 µl used per coverslip. After one hour at 37 °C, the coverslips were washed three times with PBSA, the second wash remaining on the nuclei for 5 min. This was followed by incubation (1 hour, 37 °C) with 20 µl of goat antimouse IgG (Sigma) labelled with tetramethyl‐rhodamine‐isothiocyanate (TRITC), diluted 1–20 in PBSA. Coverslips were then washed as before and finally mounted in DABCO/DAPI, as above.
Quantitative fluorescence microscopy
Samples were examined by confocal microscopy using the BioRad MRC 600 system equipped with a Kr/Ar mixed gas laser, exactly as previously described (Sun et al. 2000). Fluorophores were excited sequentially with either the 488 nm line or the 568 nm line, for FITC or APC/TRITC, respectively. The use of single laser lines ensured negligible bleed‐through of the FITC fluorescence into the APC/TRITC channel, and vice versa. Care was taken to avoid illuminating the same field more than once, to avoid bleaching. For each experiment, the illumination was suitably attenuated using neutral density filters and the gain set manually against the brightest specimen (and thereafter kept constant) so that maximal fluorescence intensity was below the maximum possible 256 units. This ensured that all measurements were within the linear range, allowing quantitative comparisons between samples within an experiment. Measurements from different experiments were not pooled. Four‐frame averaged images were captured using the BioRad COMOS software, and subsequently analysed with the public domain software package NIH‐IMAGE. Elliptical regions of interest placed around individual nuclei were selected for each APC or nucleoplasmin (TRITC) image, and the integrated fluorescence intensity compared with identical regions of the corresponding Fl‐dUTP image. Sample sizes were typically of the order of 150–200 nuclei, for each series of measurements.
RESULTS
APC conjugated with the wild‐type NLS is imported into quiescent nuclei
In an attempt to investigate the relationship between nuclear import and DNA replication, we have used the naturally fluorescent protein allophycocyanin (APC) coupled with the nuclear localization sequence (NLS) of SV 40 T antigen as a substrate for nuclear import (Adam et al. 1990). When intact quiescent nuclei from scrape‐ruptured 3T3 cells were incubated in Xenopus egg extract containing APC conjugated with the wild‐type NLS, the conjugate was rapidly taken up by nuclei giving bright but variable fluorescence (Fig. 1A). No nuclear fluorescence was obtained using APC coupled with a transport‐deficient mutant NLS (Fig. 1B). These observations confirmed that APC can be imported into intact quiescent nuclei only when coupled with the wild‐type NLS, in accord with previous studies using HeLa nuclei in a mammalian cell‐free system (Adam et al. 1990).
Figure 1.
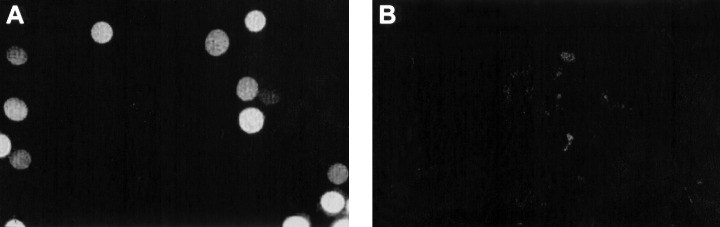
Fluorescence images of nuclei showing uptake of APC‐NLS. Nuclei from scrape‐ruptured quiescent cells were incubated for 1.5 h in Xenopus egg extracts containing APC conjugated with either the wild‐type nuclear localization signal (NLS) of SV40 T antigen (A) or with a transport‐deficient mutant NLS (B). The field in (B) contained 16 nuclei, visible under bright‐field illumination, or as background fluorescence with a more extended exposure.
Uptake of APC‐NLS is variable and declines with quiescence
In order to make quantitative comparisons, relative levels of APC fluorescence were determined for individual nuclei using confocal microscopy. A time‐course of uptake is shown in Fig. 2A. Mean levels of APC fluorescence rose steadily for the first 2 hours, before approaching a plateau (Fig. 2A). For individual nuclei, however, the levels of uptake were remarkably heterogeneous, varying over a range of more than 20‐fold (Fig. 2B).
Figure 2.
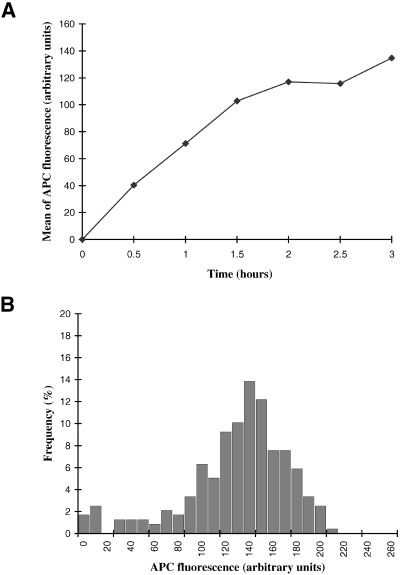
Nuclear accumulation of APC‐NLS. (A) Nuclei from scrape‐ruptured quiescent cells were incubated for the indicated times in Xenopus egg extracts containing APC‐NLS. The APC fluorescence of individual nuclei was quantified by confocal microscopy and the mean fluorescence plotted as a function of time. (B) Frequency histogram of APC‐fluorescence for individual nuclei, for the 3 h time point in (A).
The reasons for the variability in APC uptake are unclear. However, individual cells exit the cell cycle at different times over a period of several days as the population approaches quiescence (Brooks & Riddle 1988). It is therefore possible that the variability in nuclear transport reflects the different times spent by cells in G0. To determine if this explanation is reasonable, the uptake of APC‐NLS by nuclei from cells taken 6 days after attaining confluence was compared with that from nuclei taken 12 days post confluence. In both cases, >99% of the cells were in G0 at the time of the experiment (see Sun et al. 2000). As may be seen from the cumulative distributions presented in Fig. 3, the extent of APC‐NLS uptake declined with quiescence, the median APC fluorescence after 2 h uptake falling from 150 units to 100 units. Variability in the time spent in G0 is thus a plausible reason for the variability in uptake between individual nuclei. However, this could not be tested directly as we are unable to measure uptake in identified nuclei isolated from individual cells of precisely known cell cycle history (e.g. as determined from time‐lapse recordings). An alternative possibility is that the decline in nuclear uptake is a population response to prolonged quiescence affecting all nuclei equally, independent of the source of the heterogeneity in transport rates.
Figure 3.
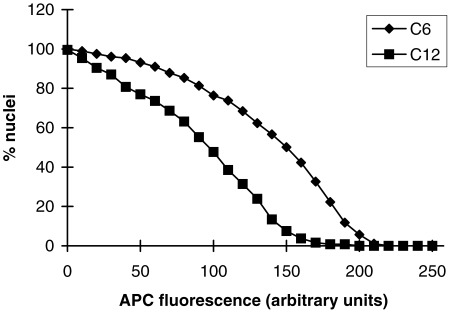
Decline in nuclear uptake with increasing duration of quiescence. Nuclei were isolated by scrape‐rupture of cells taken either 6 or 12 days after the attainment of confluence (C6 or C12, respectively), and incubated in Xenopus egg extracts containing APC‐NLS for 2 h. The APC fluorescence of individual nuclei was quantified by confocal microscopy, and the data presented as cumulative frequency distributions. The ordinate represents the percentage of nuclei with APC‐fluorescence greater than or equal to the value on the abscissa.
Relationship between uptake of APC‐NLS and DNA replication
If the onset of DNA synthesis within a nucleus is driven by the accumulation of replication factors to some threshold, then the decline in nuclear transport with the duration of quiescence and the heterogeneity between nuclei could account for the fall in replication capacity with time and for the asynchrony of initiation. To examine this, the same nuclei were analysed for both APC uptake and incorporation of fluorescein‐12‐dUTP (Fl‐dUTP), a marker of DNA replication. The results of a typical experiment are shown in Fig. 4A, in which nuclei were incubated in egg extracts for 3 h. Surprisingly, there was no obvious correlation between the level of APC uptake and DNA replication. Thus, some nuclei with very low levels of APC accumulation were as brightly labelled with Fl‐dUTP as other nuclei with the highest levels of APC uptake. Conversely, some nuclei with the highest levels of APC had not yet begun replication, as judged by the absence of Fl‐dUTP fluorescence. Nor was there any obvious threshold uptake that must be exceeded before replication could occur.
Figure 4.
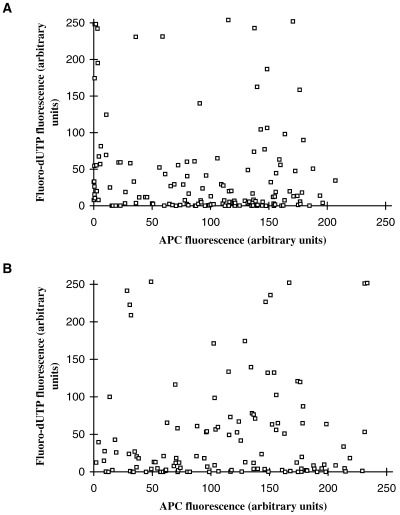
The relationship between DNA synthesis and nuclear uptake. Nuclei from scrape‐ruptured quiescent cells were incubated for 3 h in either (A) low speed supernatants (LSS) or (B) high speed supernatants (HSS) of Xenopus egg extracts containing APC‐NLS and Fl‐dUTP. Levels of APC and Fl‐dUTP fluorescence for the same individual nuclei were then quantified by confocal microscopy and the results presented as scatter plots. Each point represents an individual nucleus.
It seemed possible that some of the heterogeneity in APC uptake might have been due to nuclear damage sustained at the time of scrape‐rupture, coupled with the time required for nuclear repair, leading to delayed nuclear import. Indeed, since transient permeabilization leads to enhanced replication capacity (Leno & Munshi 1994, 1997; Wu & Gilbert 1997; Sun et al. 2000), this could account for the high levels of Fl‐dUTP incorporation shown by some nuclei with low APC uptake. However, importantly, the same results were obtained when the experiment was repeated using high‐speed supernatants of egg extracts (HSS) that lack the membrane vesicles needed for nuclear envelope assembly or repair (Fig. 4B). This confirms our previous findings that the replication of quiescent 3T3 nuclei in Xenopus egg extracts does not require permeabilization of the nuclear envelope (1994, 1996; Sun et al. 2000). It follows that the variability in APC uptake and DNA replication seen here cannot be attributed to the time needed to repair partially damaged nuclei.
The observation of a few nuclei brightly labelled with Fl‐dUTP but poorly labelled with APC raised the possibility that some of the variation in APC levels might have been due to artefactual loss of APC from some nuclei during the process of fixation. To explore this, we tested alternative fixation protocols. Use of other fixatives (e.g. paraformaldehyde instead of formaldehyde), or gentle vs. vigorous addition, made no difference. In particular, dilution of the sample fivefold with replication buffer before addition of fixative, which would be expected to exaggerate any leakage from the nuclei, did not alter the fluorescence intensities. There are therefore no grounds for suspecting that some nuclei might have lost their accumulated APC as a result of the experimental manipulations. However, detergent‐treatment of the nuclei prior to fixation (0.5% Triton X100) led to complete loss of APC fluorescence (not shown), demonstrating that retention of the APC (before fixation) depends on the presence of an intact nuclear envelope.
Although there was little indication of any threshold between DNA synthesis and nuclear uptake after 3 h incubation in egg extracts (Fig. 4), it is possible that the threshold is low and that attainment of it is necessary but not sufficient for the initiation of replication. If so, then the threshold could have been exceeded by all nuclei at 3 h, masking any relationship between uptake and the initiation of DNA synthesis. However, at early times, the first to reach the hypothetical threshold would be the most rapidly transporting nuclei. The first nuclei to begin replication might therefore be expected to be among those with highest rates of uptake. In order to look for this, a time‐course was undertaken, with samples fixed at 30 minute intervals up to 3 h (Fig. 5). In this experiment, the maximum observed background (auto)fluorescence in the FITC channel (used to detect Fl‐dUTP) was less than 30 units, at t = 0 (not shown), so fluorescence levels greater than 50 units can be confidently considered to indicate nuclei positive for Fl‐dUTP incorporation. As may be seen in Fig. 5, the earliest nuclei incorporating Fl‐dUTP (at 0.5 h) were not the nuclei most highly labelled with APC. The same lack of correlation between Fl‐dUTP incorporation and APC uptake was evident in all the other time points in Fig. 5.
Figure 5.
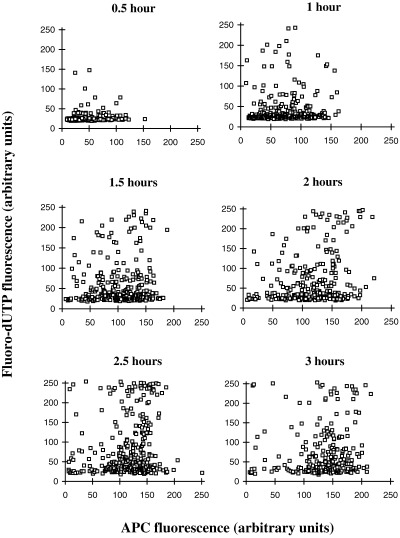
The relationship between DNA synthesis and accumulation of APC‐NLS as a function of time. Nuclei from scrape‐ruptured quiescent cells were incubated for the indicated times in Xenopus egg extracts containing APC‐NLS and Fl‐dUTP. Levels of APC and Fl‐dUTP fluorescence for the same individual nuclei were then quantified by confocal microscopy and the results presented as scatter plots. Each point represents an individual nucleus.
The relationship between nucleoplasmin uptake and DNA replication
In the experiments described so far, we used APC conjugated with the NLS of SV40 T antigen as an indicator of the behaviour of nuclear proteins in general. However, many nuclear proteins have a bipartite NLS, rather than a single, contiguous stretch of amino acids as in SV40 T antigen, and it is possible that such proteins might have different characteristics of nuclear uptake compared to the artificial APC‐NLS conjugates used here. Nucleoplasmin is an example of a protein with a bipartite NLS present at high concentrations in Xenopus egg cytoplasm (Robbins et al. 1991). We therefore used an antibody to Xenopus nucleoplasmin to monitor the uptake of nucleoplasmin from the extracts in relation to the onset of DNA synthesis. The results (Fig. 6) were essentially identical to those obtained with APC‐NLS. The first nuclei to begin DNA synthesis (i.e. Fl‐dUTP‐positive nuclei at early time points) may have been slightly biased towards those with higher levels of nucleoplasmin uptake, but any correlation disappeared at later times (Fig. 6). As with APC, some nuclei did not enter DNA synthesis even when nucleoplasmin accumulated to high levels. Conversely, some nuclei showed extensive DNA replication despite having inefficient nucleoplasmin uptake.
Figure 6.
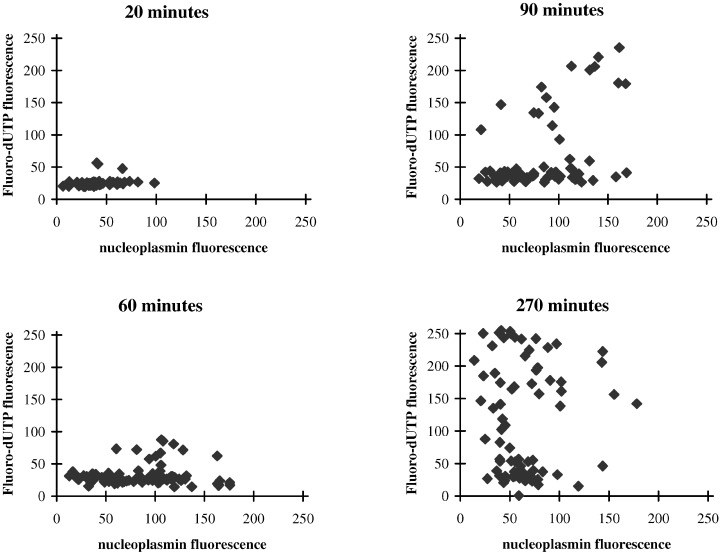
The relationship between DNA synthesis and nucleoplasmin accumulation as a function of time. Nuclei from scrape‐ruptured quiescent cells were incubated for the indicated times in Xenopus egg extracts containing Fl‐dUTP. At the end of incubation, the nuclei were fixed and immunostained for Xenopus nucleoplasmin. Levels of nucleoplasmin‐specific fluorescence were then determined by confocal microscopy, together with Fl‐dUTP incorporation for the same nuclei, and the results presented as scatter plots.
DISCUSSION
Intact G0 nuclei from quiescent 3T3 cells initiate replication asynchronously in Xenopus egg extracts despite exposure to the same concentration of replication factors (1994, 1996). Given that nuclear transport is essential for the initiation of DNA replication (Cox 1992), and in view of reported differences between nuclei in the capacity for nuclear transport (Adam et al. 1992), the aim of the experiments presented here was to determine whether such heterogeneity in nuclear uptake could account for the asynchrony in replication. Using APC conjugated to the NLS of SV40 T Antigen, we have confirmed that the capacity for nuclear transport is indeed highly heterogeneous, varying between nuclei over a range of more than 20‐fold. Surprisingly, these differences in nuclear transport appear to contribute very little to the timing or extent of replication. Similar results were obtained for the relationship between nucleoplasmin (a physiological substrate) and DNA synthesis. For both APC and nucleoplasmin, a few nuclei initiated DNA replication despite having inefficient nuclear transport. Conversely, some nuclei with high rates of nuclear transport failed to enter DNA synthesis.
If it can be assumed that import into the nucleus of APC‐NLS conjugates or nucleoplasmin is typical of nuclear proteins in general, and replication factors in particular, then the experiments reported here show that the initiation of DNA synthesis is not induced merely by the accumulation of replication factors to a common threshold. Instead, some other property of the nucleus must be responsible for the variability in timing, or for the synchronous initiation of replication at multiple independent foci within a nucleus. Remaining possibilities include a global change in nuclear or chromatin structure, in some way propagated throughout the nucleus, though what such a change might represent in molecular terms is hard to visualize. Alternatively, initiation might depend on some soluble nucleoplasmic factor whose activation is highly co‐operative, perhaps analogous to the activation of maturation promoting factor (MPF) at mitosis (King et al. 1994). Alternatively, initiation might depend on the sudden elimination of an inhibitor, perhaps as a result of regulated nuclear export, or targetted proteolysis.
In view of the fact that an intact nuclear envelope and active nuclear transport are necessary for the induction of DNA synthesis in Xenopus egg extracts (Cox 1992), it is surprising that there was little evidence for any threshold level of uptake below which DNA synthesis did not begin. For experiments using LSS, we sometimes observed a few nuclei with very low levels of APC‐NLS uptake and high levels of Fl‐dUTP incorporation (Fig. 4A). These are most likely to be nuclei initially permeabilized during scrape rupture and subsequently repaired during incubation, since permeabilization is known to enhance the replication of quiescent nuclei (Leno & Munshi 1994, 1997; Wu & Gilbert 1997; Sun et al. 2000). If so, then the low level of APC‐NLS uptake shown by these nuclei would be expected since they would have begun transporting (following envelope repair) only towards the end of the incubation. In general, however, all Fl‐dUTP‐positive nuclei showed some uptake of APC‐NLS or nucleoplasmin (albeit sometimes rather faint). Our data are therefore consistent with the view that nuclear transport is necessary for the initiation of DNA synthesis (Cox 1992), even though it is evidently not sufficient.
Regardless of the extent to which the rate of nuclear transport contributes to the timing of DNA synthesis, the fact that these rates are so heterogeneous among nuclei is remarkable. Such heterogeneity was first noted by Adam et al. (1992) for nuclei from exponentially growing HeLa cells, and it was suggested that the variability might be related to cell cycle position. In the simplest sense this cannot be the case here since >99% were in the same phase of the cell cycle (G0). However, since the rate of uptake declined with the duration of quiescence, and since cells exit from the cell cycle at widely differing times (Brooks & Riddle 1988), it is conceivable that the variability in nuclear transport in part reflects the range of times spent by individual cells in G0. The decline in transport rates observed here is consistent with previous reports that proliferating cells, on average, have higher rates of nuclear transport for large particles (diameter 110–270 µ) than do quiescent cells (Feldherr & Akin 1991, 1993), though the uptake of smaller particles and the density of nuclear pores were found to be comparable. The heterogeneity in transport is therefore most likely to reflect differences in the functional activity of nuclear pores. Precisely why this should vary between nuclei drawn from the same population remains to be established. Further work is required to clarify this important issue. Although the heterogeneity in transport rates is not the main determinant of the timing of DNA synthesis, it would nevertheless be expected to influence many other aspects of cell behaviour.
Acknowledgements
We thank Dr S. Bansal for making the NLS peptides and Dr S. Dilworth for the anti‐Xenopus nucleoplasmin antibody. This work was supported by grants from the MRC and the Wellcome Trust.
References
- Adam SA, Sterne‐Marr R, Gerace L (1990) Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 111, 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam SA, Sterne‐Marr R, Gerace L (1992) Nuclear protein import using digitonin‐permeabilized cells. Meth Enzymol. 219, 97. [DOI] [PubMed] [Google Scholar]
- Blow JJ (1993) Preventing re‐replication of DNA in a single cell cycle: evidence for a replication licensing factor. J. Cell Biol. 122, 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ & Laskey RA (1986) Initiation of DNA replication in nuclei and purified DNA by a cell‐free extract of Xenopus eggs. Cell 47, 577. [DOI] [PubMed] [Google Scholar]
- Brooks RF (1981) Variability in the cell cycle and the control of proliferation In: John PCL, ed. The Cell Cycle. p. 35 Cambridge, Cambridge University Press. [Google Scholar]
- Brooks RF, Richmond FN, Riddle PN, Richmond KMV (1984) Apparent heterogeneity in the response of quiescent Swiss 3T3 cells to serum growth factors: implications for the transition probability model and parallels with ‘cellular senescence’ and ‘competence’. J. Cell. Physiol. 121, 341. [DOI] [PubMed] [Google Scholar]
- Brooks RF & Riddle PN (1988) Differences in growth factor sensitivity between individual 3T3 cells arise at high frequency: possible relevance to cell senescence. Exp. Cell Res. 174, 378. [DOI] [PubMed] [Google Scholar]
- Brooks RF, Riddle PN, Richmond FN, Marsden J (1983) The G1 distribution of ‘G1‐less’ V79 Chinese hamster cells. Expl. Cell Res. 148, 127. [DOI] [PubMed] [Google Scholar]
- Coppock DL, Lue RA, Wangh LJ (1989) Replication of Xenopus erythrocyte nuclei in a homologous egg extract requires prior proteolytic treatment. Dev. Biol. 131, 102. [DOI] [PubMed] [Google Scholar]
- Cox LS (1992) DNA replication in cell‐free extracts from Xenopus eggs is prevented by disrupting nuclear envelope function. J. Cell Sci. 101, 43. [DOI] [PubMed] [Google Scholar]
- Feldherr CM & Akin D (1991) Signal‐mediated nuclear transport in proliferating and growth‐arrested BALB/c 3T3 cells. J. Cell Biol. 115, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldherr CM & Akin D (1993) Regulation of nuclear transport in proliferating and quiescent cells. Exp. Cell Res. 205, 179. [DOI] [PubMed] [Google Scholar]
- Hola M, Castleden S, Howard M, Brooks RF (1994) Initiation of DNA synthesis by nuclei from scrape‐ruptured quiescent mammalian cells in high speed supernatants of Xenopus egg extracts. J. Cell Sci. 107, 3045. [DOI] [PubMed] [Google Scholar]
- Hola M, Howard M, Nawaz FN, Castleden S, Brooks RF (1996) Individual nuclei differ in their sensitivity to the cytoplasmic inducers of DNA synthesis: implications for the origin of cell cycle variability. Exp. Cell Res. 229, 350. [DOI] [PubMed] [Google Scholar]
- Hutchison CJ (1993) The use of cell‐free extracts of Xenopus eggs for studying DNA replication in vitro In: Fantes P, Brooks R, eds. The Cell Cycle: a Practical Approach. Oxford: IRL Press, 177. [Google Scholar]
- Johnson GD, Davidson RS, McNamee KC, Russell G, Goodwin D, Holborow EJ (1982) Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J. Immunol. Methods. 55, 231. [DOI] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW (1994) Mitosis in transition. Cell 79, 563. [DOI] [PubMed] [Google Scholar]
- Leno GH & Laskey RA (1991a) DNA replication in cell‐free extracts from Xenopus laevis In: Kay BK, Peng HB, eds. Methods in Cell Biology. San Diego: Academic Press, 561. [DOI] [PubMed] [Google Scholar]
- Leno GH & Laskey RA (1991b) The nuclear membrane determines the timing of DNA replication in Xenopus egg extracts. J. Cell Biol. 112, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leno GH & Munshi R (1994) Initiation of DNA replication in nuclei from quiescent cells requires permeabilization of the nuclear membrane. J. Cell Biol. 127, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leno GH & Munshi R (1997) Reactivation of DNA replication in nuclei from terminally differentiated cells: nuclear membrane permeabilization is required for initiation in Xenopus egg extract. Exp. Cell Res. 232, 412. [DOI] [PubMed] [Google Scholar]
- Logothetou‐Rella H, Sun W‐H, Brooks RF (2000) Induction of DNA synthesis or apoptosis in mammalian nuclei by Xenopus egg extracts that fail to support the replication of sperm chromatin. Cell Biol. International. 24, 129. [DOI] [PubMed] [Google Scholar]
- Lohka MJ & Maller JL (1985) Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell‐free extracts. J. Cell Biol. 101, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A, Blow JJ, White JG, Amos WB, Wilcock D, Laskey RA (1989) Replication occurs at discrete foci spaced throughout nuclei replicating in vitro. J. Cell Sci. 94, 471. [DOI] [PubMed] [Google Scholar]
- Robbins J, Dilworth SM, Laskey RA, Dingwall C (1991) Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64, 615. [DOI] [PubMed] [Google Scholar]
- Sun W‐H, Hola M, Pedley K et al. (2000) The replication capacity of intact mammalian nuclei in Xenopus egg extracts declines with quiescence, but the residual DNA synthesis is independent of Xenopus MCM proteins. J. Cell Sci. 113, 683. [DOI] [PubMed] [Google Scholar]
- Wu J‐R & Gilbert DM (1997) The Replication Origin Decision Point is a mitogen‐independent, 2‐aminopurine‐sensitive, G1‐phase event that precedes restriction point control. Mol. Cell. Biol. 17, 4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg A & Larsson O (1985) Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc. Natl. Acad. Sci. USA. 82, 5365. [DOI] [PMC free article] [PubMed] [Google Scholar]


