Abstract
Abstract. Embryonic stem cells have huge potential in the field of tissue engineering and regenerative medicine as they hold the capacity to produce every type of cell and tissue in the body. In theory, the treatment of human disease could be revolutionized by the ability to generate any cell, tissue, or even organ, ‘on demand’ in the laboratory. This work reviews the history of murine and human ES cell lines, including practical and ethical aspects of ES cell isolation from pre‐implantation embryos, maintenance of undifferentiated ES cell lines in the cell culture environment, and differentiation of ES cells in vitro and in vivo into mature somatic cell types. Finally, we discuss advances towards the clinical application of ES cell technology, and some of the obstacles which must be overcome before large scale clinical trials can be considered.
INTRODUCTION
Murine embryonic stem (ES) cells were first described over 20 years ago, when they were isolated from the inner cell mass of the developing blastocyst and grown in vitro (Evans & Kaufman 1981; Martin 1981). ES cells have since been shown to contribute to all cell lineages, including the germ line, when incorporated into chimeras with intact mouse embryos (Bradley et al. 1984; Nagy et al. 1990). In vitro, murine ES cells can be propagated indefinitely in the undifferentiated state, but retain the capacity to differentiate to all mature somatic phenotypes when induced by the appropriate signals. The initial isolation of murine ES cell lines in 1981 heralded a major breakthrough for developmental biology as it provided a simple model system to study the basic processes of early embryonic development and cellular differentiation. However, it also promised something else. If ES cells could be derived from human blastocysts, their capacity for multilineage differentiation might be exploited for cell‐based therapies in which virtually any tissue or cell type could be produced ‘to order’ in the laboratory. This would provide a radical new approach to the treatment of a wide variety of diseases where organ damage or dysfunction exceeds the body's capability for natural repair. Human ES cells were eventually derived in 1998 (Thomson et al. 1998; Reubinoff et al. 2000), finally making regenerative medicine and tissue engineering a real possibility for the future treatment of human disease. This article reviews the current status of ES cell research, with particular emphasis on progress towards the development of therapies for human disease.
WHAT IS A STEM CELL?
Two properties are generally considered to define a stem cell, the capacity for long‐term self‐renewal without senescence and pluripotency, the ability to differentiate into one or more specialized cell types. These cells could therefore provide a theoretically inexhaustible supply of cells for transplantation. Totipotent stem cells, which have an ability to generate all tissue types, play a critical role in human development, providing the raw material for the development of all tissues and organs in the embryo and all the extra‐embryonic tissues. However, tissue‐specific stem cells are also deposited in various niches throughout the body, such as bone marrow, brain, liver and skin, as a mechanism for tissue maintenance, growth and repair in later life (Lavker & Sun 2000; Uchida et al. 2000; Vessey & de la Hall 2001; Wagers et al. 2002). These ‘adult’ stem cells were originally thought to be committed to regenerating only a very restricted set of cell lineages, however, it is becoming increasingly evident that they can show considerably more plasticity (Blau et al. 2001; Morrison 2001; Prockop et al. 2003). In theory, these cells could be harvested from a patient, differentiated in the laboratory and transplanted back into the same individual for tissue repair, thus bypassing the need for immunosuppression. However, for some stem cell types, low frequency, difficulties in accessing the niche and isolating the cells, restricted lineage potential and poor growth in cell culture may render their use impractical for tissue engineering purposes (Vogel 2001). In these cases, ES cells are likely to provide a more appropriate cell source.
EMBRYONIC STEM CELLS
The first pluripotent cell lines to be established were embryonic carcinoma (EC) cell lines, derived from the undifferentiated compartment of murine and human germ cell tumours (Finch & Ephrussii 1967; Andrews 2002). These cells could be expanded continuously in culture and could also be induced to differentiate into derivatives of all three embryonic germ layers; endoderm, ectoderm and mesoderm (Kleinsmith & Cochran 1964). Murine EC cells can also contribute extensively to all the normal tissues of chimaeric mice generated by the injection of EC cells into mouse blastocysts (Illmensee & Mintz 1976). However, being cancer‐derived and usually aneuploid, EC cells are not suitable for clinical application, although they have proven to be a very useful model system in the laboratory. Murine ES cells were first isolated in 1981, using culture techniques based on experience with the culture of EC cells (Evans & Kaufman 1981; Martin 1981). ES cells are derived from the pre‐implantation blastocyst, a hollow sphere of cells containing an outer layer of trophoblast cells which give rise to the placenta and the inner cell mass (ICM), from which ES cells are derived. Cells of the ICM ultimately go on to form the embryo proper and therefore have the capacity to form all the tissues in the body. Although these truly pluripotent cells are relatively short‐lived in the embryo in vivo, they can be propagated indefinitely in culture in an undifferentiated state, by growth in the presence of leukaemia inhibitory factor (LIF) and/or on a feeder layer of murine embryonic fibroblasts (MEF) (Smith et al. 1988; Williams et al. 1988). Murine ES cells have had an enormous impact on many fields of research over the last 20 years. In particular, ES cells can be used to reconstitute early mouse embryos (Bradley et al. 1984), and this has formed the basis of genome manipulation technology that has produced hundreds of ‘knock out’ and ‘knock in’ transgenic animals for the investigation of gene expression and regulation in vivo (Thomas & Capecchi 1987). ES cells also allow studies of the initial stages of mammalian development in vitro, without the need to harvest peri‐implantation embryos, and continue to be used to dissect the basic mechanisms underlying pluripotency and cell lineage specification. Not surprisingly, significant efforts have been made to isolate ES cells from other species and, to date, ES cell lines are available from rodents (Evans & Kaufman 1981; Martin 1981; Doetschman et al. 1988; Iannaconne et al. 1994), rabbits (Graves & Moreadith 1993), pigs (Li et al. 2003), primates (1995, 1996) and, significantly, an ever‐increasing number from humans (Thomson et al. 1998; Amit et al. 2000; Reubinoff et al. 2000; Richards et al. 2002; Hovatta et al. 2003; Mitalipova et al. 2003), (summarized in Orive et al. 2003).
The generation of human ES cell lines has sparked a great deal of controversy in the media, with particularly strong objections being raised to the use of human embryos in scientific research by certain religious communities (Annas et al. 1999; De Wert & Mummery 2003; Orive et al. 2003). Legislation governing the use of human embryos to produce ES cell lines varies between countries and, at the time of writing, is still under debate in many cases. The initial human ES cell lines were derived from ‘spare’ embryos produced by in vitro fertilization and donated with the informed consent of the parents (Anonymous 2002). This source is widely held to be the most acceptable source of embryos for research, the embryos being originally created for reproductive, not scientific, purposes. Many people consider it to be ethically superior to use these embryos for medical research from which the human population as a whole is likely to benefit, than to simply destroy them or store them indefinitely. An alternative method of deriving human ES cells is somatic nuclear transfer, or cloning, which bypasses the need to destroy an embryo produced ‘naturally’ by the fusion of sperm and egg, which could otherwise develop to term if implanted. This procedure was first described in sheep, where a somatic cell nucleus was transferred into an enucleated oocyte leading to apparently normal embryonic development in a proportion of cases (Campbell et al. 1996). Cloned embryos have since been generated in a number of species, using a variety of somatic cell types (McGrath & Solter 1983; Wilmut et al. 2002). The clinical development of regenerative medicine could be markedly expedited by the use of autologous cloned human embryos as it would circumvent any potential problems with the rejection of foreign cells, which remains the bane of transplantation medicine. However, the creation of a cloned human embryo specifically for the purposes of research is even more ethically loaded than the use of ‘spare’ embryos from IVF treatment. In all cases where animal embryos have been successfully cloned using this technique, a small proportion of embryos have survived and developed to become live young after implantation (1997, 2002). The even remote possibility of generating a live human clone has been sufficient to ban the technique of somatic nuclear transfer from being applied to human cells in most western countries (Andorno 2002; Bosch 2002). Although there has been a single scientific report of the cloning of a human embryo (Cibelli et al. 2001), these results have met widespread scepticism as the embryos were only allowed to develop to the 6‐cell stage, before nuclear DNA begins to regulate embryonic development (Stix 2001). However, a report of the cloning of non‐human primate embryos suggests that cloning by nuclear transfer is technically possible in humans (Mitalipov et al. 2002), and ES cells have been recently developed by somatic nuclear transfer of human nuclei into rabbit oocytes (Chen et al. 2003). Although this latter work was carried out to avoid the use of human oocytes, which are in short supply for clinical use and difficult to obtain for scientific research, the creation of a ‘human‐rabbit hybrid’ added fuel to the fire of an already acrimonious debate. Nevertheless, the universally extreme inefficiency of somatic nuclear transfer in generating viable embryos (less than 1% development to blastocysts of cloned rhesus money embryos) is likely to render it impractical for human use, even if the cloning of human embryos were to become less morally dubious (Colman & Kind 2000).
Human ES cells show several important differences from murine ES cells in culture. They grow more slowly, tend to form flat rather than spherical colonies and are more easily dissociated into single cells than their mouse counterparts (Laslett et al. 2003). Human ES cells are also unresponsive to LIF and require culture on MEF feeder layers in the presence of basic fibroblast growth factor (Thomson et al. 1998; Amit et al. 2000; Reubinoff et al. 2000; Laslett et al. 2003), or on matrigel or laminin in MEF‐conditioned medium (Xu et al. 2001). The two sources also differ in some antigenic phenotypes, for example, undifferentiated murine ES cells express the embryonic antigen SSEA‐1A but not SSEA‐3 and 4, but undifferentiated human ES cells have precisely the opposite phenotype (Thomson et al. 1998; Reubinoff et al. 2000). The demonstration of pluripotency for murine ES cells usually involves reconstitution of embryos and the generation of chimaeric mice, however, for human cells this test cannot be applied for obvious reasons. Instead, for human ES cells, the ‘gold standard’ test for pluripotency which has evolved is the demonstration that cells can differentiate into all three germ layers in vivo as teratomas when implanted into immunodeficient mice (Thomson et al. 1998; Amit et al. 2000; Reubinoff et al. 2000). A growing number of human ES cell lines are now available from several research groups and commercial companies, including some that have been clonally derived (summarized in Annas et al. 1999). For therapeutic purposes, it is unlikely that human ES cells grown on murine feeder layers, or in cell culture medium containing animal‐derived products, will be acceptable due to the risk of transferring animal pathogens into the human population. For this reason, human ES cells are now beginning to be derived on human feeder cells, or without feeder layers entirely, under completely animal‐free conditions (Richards et al. 2002; Amit et al. 2003; Hovatta et al. 2003; Richards et al. 2003).
DIFFERERENTIATION OF EMBRYONIC STEM CELLS IN VITRO
One of the most intriguing and important aspects of ES cell lines is their ability to differentiate into multiple mature somatic cell types in cell culture, presumably via precursor cells, when the appropriate stimuli are applied. The most common method for initiating differentiation in culture is the formation of 3‐D spherical structures in suspension culture, termed embryoid bodies (EBs) (Fig. 1a) due to their similarity to post‐implantation embryonic tissue in vivo. These aggregated structures contain derivatives of all three embryonic germ layers (Fig. 1b) (Abe et al. 1996; Leahy et al. 1999; Itskovitz‐Eldor et al. 2000), and their formation is a prerequisite for the generation of most mature somatic cell lineages as ES cells differentiated in monolayer culture alone form large quantities of an endodermal‐like cell which is identified by ES cell colonies flattening and developing a ‘rough’ appearance. It should be noted, however, that EB differentiation does not reconstitute the full array of embryonic development, having no form of polarity or ‘body plan’. As such, ES cells cannot form viable human embryos. EB formation can be achieved in a number of ways following the withdrawal of LIF and/or the feeder layer (Keller 1995). Cells can be transferred to suspension culture at high density (Doetschman et al. 1995) or in methylcellulose‐containing medium (Wiles & Keller 1991), to form EBs of a range of sizes and shapes; alternatively ‘hanging drop’ cultures provide a more controlled method of generating single EBs from a defined cell number in individual droplets of culture medium (Wobus et al. 1991; Boheler et al. 2002). The length of EB culture time is dependent upon the ultimate target cell type and EB differentiation appears to correlate well temporally with the post‐implantation development of embryos (Keller 1995). Mesodermal and ectodermal precursors form within a few days, whereas some endodermal cell types may benefit from more extended culture time (up to 10 days) to a stage where most EBs have cavitated and become cystic (Abe et al. 1996; Leahy et al. 1999).
Figure 1.
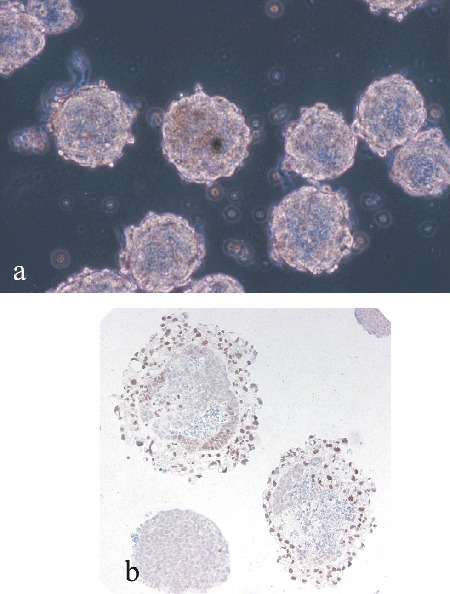
(a) Murine embryoid bodies, derived spontaneously from differentiating embryonic stem cells, floating in culture. (b) Sections of murine embryoid bodies after 8 days of differentiation immunostained for the transcription marker hepatocyte nuclear factor 3β, demonstrating the formation of endoderm on the outside of the bodies (ABC method).
Following the formation of EBs, they are returned to adherent culture conditions upon which specialized cells develop in the areas of outgrowth. Not surprisingly, progress in directing and characterizing the in vitro differentiation of murine ES cells is significantly more advanced than for human ES cells. One of the most visually impressive phenotypes of differentiated ES cells is the appearance of primitive cardiomyocytes in the culture, which can initially be observed as areas of ‘twitching’ in EBs after several days in suspension culture, and then later as large patches of synchronously contracting cells in adherent culture. Murine ES cell‐derived cardiomyocytes have been shown to express tissue‐specific markers, including structural cardiac proteins, cardiac receptors and cardiac transcription factors (summarized in Wobus 2001 and Boheler et al. 2002). Within the population of contractile cells, proportions representing a variety of specialized functions of the heart have also been identified by electrophysiology, including atrial‐like, ventricular‐like, purkinje‐like and nodal‐like (Wobus 2001; Boheler et al. 2002). Preliminary data on engraftment of mouse ES cell‐derived cardiomyocytes into mouse models has also indicated that these cells are capable of integrating into endogenous heart tissue, vascularizing, and continuing to differentiate in vivo (Klug et al. 1996; Johkura et al. 2003). Towards the production of cardiomyocytes for tissue engineering, work is now proceeding on techniques to scale‐up the differentiation process (Zandstra et al. 2003), strategies to select out the appropriate cell type from the differentiated population (Muller et al. 2000), and cardiomyocytes are now being generated from human ES cells based on protocols optimized for murine ES cells (Kehat et al. 2001; He et al. 2003; Mummery et al. 2003). Tissue engineering of the heart is undoubtedly the aspect of regenerative medicine closest to a clinical application; however, there is also a word of warning. As well as issues surrounding immunocompatibility which will affect any cell transplantation technology, questions have also been raised about the differentiation state of ES cell‐derived cardiomyocytes, which tend to recapitulate the phenotype of the fetal heart rather than adult cells (Doevendans et al. 2000; Fijnvandraat et al. 2003a). It has been suggested that implanting spontaneously beating cells may, in fact, cause serious cardiac arrhythmias which could not be corrected once the cells had engrafted into the endogenous heart tissue (Fijnvandraat et al. 2003b). ES cell‐based therapies still have many hurdles to overcome before they can be transferred from the laboratory to the clinic, and it will be critical to validate all types of differentiated progeny of ES cells for the ability to integrate fully and functionally with host tissue.
Many other somatic cell types have also been derived from both murine and human ES cells. As well as cardiac differentiation, other mesodermal cell types that have been obtained from ES cells include chondrocytes (Kramer et al. 2000), adipocytes (Dani et al. 1997) and endothelial cells (Risau et al. 1988; Wang et al. 1994; Yamashita et al. 2000). In our own laboratory, a robust system has been developed for osteoblast differentiation, which involves dispersal of murine EBs into a culture medium designed for the maintenance and growth of explanted osteoblasts (Buttery et al. 2001). Differentiation is further augmented sevenfold by the addition of dexamethasone 14 days after EB dispersal, illustrating that, not only the stimulus itself, but also the time at which it is administered, can be significant. Osteoblasts have now also been derived at high yield from human ES cells using a similar protocol (Sottile et al. 2003).
Neuroectodermal differentiation is also relatively easy to achieve from murine ES cells and high yields of neuron‐, astrocyte‐ and oligodendrocyte‐like cells have been achieved with several, very different protocols (Stavridis & Smith 2003). These protocols have since been further simplified to remove the EB stage and differentiate murine and human ES cells into neuroectodermal lineages in monolayer, the only somatic cell type for which this is known to be possible (Reubinoff et al. 2001; Pachernik et al. 2002; Ying et al. 2003). Preliminary studies of the implantation of ES cell‐derived neurons into mouse models have also indicated that these cells are functionally active (i.e. form synapses and can generate action potentials), integrate into the brain and, in at least one case, have corrected the phenotype of a neurodegenerative disease (Barberi et al. 2003; Benninger et al. 2003; Chiba et al. 2003).
As well as deriving osteoblasts, our laboratory has also focused on the differentiation of ES cells to alveolar epithelium. Lung tissue is formed from the definitive endoderm during embryogenesis, and the picture gradually emerging from ES cell research is that endodermal cell lineages are substantially more difficult to derive in significant yields than either ectoderm or mesodermal cell types. Using a similar approach to that which was used for osteoblast differentiation, we demonstrated that differentiated murine ES cell cultures could be enriched for type II alveolar epithelial‐like cells by the application of SAGM (small airway growth medium), a medium designed for the maintenance of primary distal lung epithelium in culture (Ali et al. 2002). Type II cells were identified by their expression of a cell‐specific gene product, surfactant protein C (Fig. 2), and by the presence of a cell‐specific organelle, lamellar bodies. However, despite the enrichment step, these cells were present only at very low frequency and work is ongoing to increase the yield of the target cell type by modulating the composition of the culture medium. Other endodermal cell types that have been obtained from ES cells include hepatocytes (Chinzei et al. 2002; Kuai et al. 2003; Rambhatla et al. 2003) and pancreatic islet cells (Lumelsky et al. 2001; Kim et al. 2003). However, progress in deriving endodermal lineages from ES cells is lagging significantly behind that of the other two germ layers at the present time.
Figure 2.
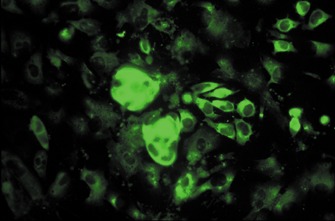
Clusters of cells derived from murine embryonic stem cells immunostained for surfactant protein C, a specific marker for type II pneumocytes (indirect immunofluorescence).
The majority of the reports of ES cell differentiation in vitro have so far focused on differentiation of cells in adherent culture following EB formation. However, for many tissue engineering applications, the incorporation of differentiated cells into higher‐order structures will be essential for implants to be functional, and the acquisition of an appropriate 3‐D structure may also further direct the maturation and specialization of differentiated cell types. In general, differentiated progeny of ES cells have been difficult to sustain in 3‐D culture due to problems in controlling cell proliferation and survival, and the presence of a highly mixed population of cell types. Recently, human ES cells have been differentiated on a polymeric scaffold designed for the support of complex tissue structures rather than via EB formation, and these were observed to form structures with the characteristics of neural tissues, cartilage and liver, as well as a network of blood vessel‐like tubules (Levenberg et al. 2003). Organization of the structures could be enhanced by conditioning the scaffold with specific growth factors and by implantation into severe combined immunodeficient (SCID) mice, in which the constructs were observed to be viable for at least 2 weeks. Most intriguingly, in implanted constructs, the vessel‐like structures were shown to contain intraluminal red blood cells, suggesting that they had anastamosed with the host vascular system. ES cells might therefore provide a means to generate histologically complete tissue constructs, which would not rely entirely on full vascularization by the host circulatory system alone for survival. In addition to the potential for transplantation, in the shorter term, such constructs could also provide extremely useful in vitro models to recapitulate the physiological state sufficiently for the evaluation of new drugs and identification of new therapeutic targets in tissue culture.
EMBRYONIC STEM CELLS FOR REGENERATIVE MEDICINE
Although the potential of ES cells in transplantation medicine is vast, before any clinical application of ES cells can succeed there are a number of obstacles that must be overcome. Firstly, no approach to the differentiation of ES cells has yet yielded a 100% pure population of mature progeny. It will be essential to avoid implanting undifferentiated ES cells or inappropriate cell lineages because of the risk of teratoma formation or further perturbation of tissue function, therefore there must be an efficient means to purify the required population. Methods such as fluorescence‐activated cell sorting (FACS) or magnetic‐activated cell sorting (MACS) allow such purification but are dependent on the cell type of interest expressing a surface marker that can be recognized by a fluorescent or magnetic microbead‐tagged antibody and, to be fully effective, the marker needs to be absolutely cell‐type specific. In many cases, such a marker is not presently available, and sorting methods then rely on genetic modification of the ES cells with a marker gene under the control of a lineage‐specific promoter. Alternatively, cells could be transduced with a drug‐resistance gene instead of a marker, to allow for preferential selection of subpopulations.
Finally, as with all transplants, there is a risk that allogeneic ES cell‐derived implants could be rejected by the host. Although the immunogenicity of the transplant can be contained through the lifelong use of immunosuppressive drugs, they are associated with many unpleasant side‐effects and render the patient extremely susceptible to infection. As discussed, the production of autologous ES cells by somatic nuclear transfer is unlikely to provide a satisfactory solution, however, the amenability of ES cells to genetic modification provides a means to reduce their immunogenicity. This could be achieved by the insertion of immunosuppressive molecules such as Fas ligand, or by deleting immunoreactive molecules such B7 antigens (Walker et al. 1997; Harlan & Kirk 1999). More ambitiously, foreign major histocompatability complex (MHC) genes could be replaced by the recipient's MHC genes, increasing the immunocompatability of the cells.
CONCLUSIONS
The potential of human ES cells to generate any cell, tissue or organ ‘on demand’ for tissue repair or replacement promises to revolutionize the treatment of human disease. In the shorter term, 3‐D ES cell‐derived ‘organ’ cultures could be used for pharmacological testing, streamlining the drug‐testing process and reducing the number of animals used, and also for the detailed mechanistic investigation of differentiation itself. However, although it is over 20 years since murine ES cells were first isolated, human ES cell technology is still in its infancy and many technical and ethical hurdles must be cleared before clinical trials can begin.
REFERENCES
- Abe K, Niwa H, Iwase K, Takiguchi M, Mori M, Abe SI, Abe K, Yamamura KI (1996) Endoderm‐specific gene expression in embryonic stem cells differentiated to embryoid bodies. Exp. Cell Res. 229, 27. [DOI] [PubMed] [Google Scholar]
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE (2002) Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 8, 541. [DOI] [PubMed] [Google Scholar]
- Amit M, Carpenter MK, Inokuma MS, Chiu C‐P, Harris CP, Waknitz MA, Itskovitz‐Eldor J, Thomson JA (2000) Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271. [DOI] [PubMed] [Google Scholar]
- Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, Itskovitz‐Eldor J (2003) Human feeder layers for human embryonic stem cells. Biol. Reprod. 68, 2150. [DOI] [PubMed] [Google Scholar]
- Andorno R (2002) Biomedicine and international human rights law: in search of a global consensus. Bull. WHO 80, 959. [PMC free article] [PubMed] [Google Scholar]
- Andrews PW (2002) From teratocarcinomas to embryonic stem cells. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 357, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annas GJ, Caplan A, Elias S (1999) Stem cell politics, ethics and medical progress. Nat. Med. 5, 1339. [DOI] [PubMed] [Google Scholar]
- Anonymous (2002) Donating spare embryos for embryonic stem‐cell research. Fertil. Steril. 78, 957. [DOI] [PubMed] [Google Scholar]
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L (2003) Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat. Biotechnol. 21, 1200. [DOI] [PubMed] [Google Scholar]
- Benninger F, Beck H, Wernig M, Tucker KL, Brustle O, Scheffler B (2003) Functional integration of embryonic stem cell‐derived neurons in hippocampal slice cultures. J. Neurosci. 23, 7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Brazelton TR, Weimann JM (2001) The evolving concept of a stem cell: entity or function? Cell 105, 829. [DOI] [PubMed] [Google Scholar]
- Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM (2002) Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ. Res. 91, 189. [DOI] [PubMed] [Google Scholar]
- Bosch X (2002) United nations debates human cloning ban. Lancet 360, 1574. [DOI] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E (1984) Formation of germ‐line chimeras from embryo‐derived teratocarcinoma cell lines. Nature 309, 255. [DOI] [PubMed] [Google Scholar]
- Buttery LDK, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SPF, Episkopou V, Polak JM (2001) Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 7, 89. [DOI] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Ritchie WA, Wilmut I (1996) Sheep cloned by nuclear transfer from a cultured cell line. Nature 380, 64. [DOI] [PubMed] [Google Scholar]
- Chen T, He ZX, Liu A, Wang K, Mao WW, Chu JX, Lu Y, Fang ZF, Shi YT, Yang QZ, Chen Da Y, Wang MK, Li JS, Huang SL, Kong XY, Shi YZ, Wang ZQ, Xia JH, Long ZG, Xue ZG, Ding WX, Sheng HZ (2003) Embryonic stem cells generated by nuclear transfer of human somatic nuclei into rabbit oocytes. Cell Res. 13, 251. [DOI] [PubMed] [Google Scholar]
- Chiba S, Iwasaki Y, Sekino H, Suzuki N (2003) Transplantation of motoneuron‐enriched neural cells derived from mouse embryonic stem cells improves motor function of hemiplegic mice. Cell Transplant. 12, 457. [DOI] [PubMed] [Google Scholar]
- Chinzei R, Tanaka Y, Shimizu‐Saito K, Hara Y, Kakinuma S, Watanabe M, Teramoto K, Arii S, Takase K, Sato C, Terada N, Teraoka H (2002) Embryoid‐body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology 36, 22. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Kiessling AA, Cunniff K, Richards C, Lanza RP, West MD (2001) Somatic cell nuclear transfer in humans: pronuclear and early embryonic development. Ebiomed: J. Regen. Med. 2, 25. [Google Scholar]
- Colman A, Kind A (2000) Therapeutic cloning: concepts and practicalities. Trends Biotechnol. 18, 192. [DOI] [PubMed] [Google Scholar]
- Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, Darimont C, Ailhard G (1997) Differentiation of embryonic stem cells into adipocytes in vitro . J. Cell Sci. 110, 1279. [DOI] [PubMed] [Google Scholar]
- De Wert G, Mummery C (2003) Human embryonic stem cells: research, ethics and policy. Hum. Reprod. 18, 672. [DOI] [PubMed] [Google Scholar]
- Doetschman T, Williams P, Maeda N (1988) Establishment of hamster blastocyst‐derived embryonic stem cells. Dev. Biol. 127, 224. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter HR, Katz M, Schmidt W, Kemler R (1995) The in vitro development of blastocyst‐derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morph. 87, 27. [PubMed] [Google Scholar]
- Doevendans PA, Kubalak SW, An RH, Becker DK, Chien KR, Kass RS (2000) Differentiation of cardiomyocytes in floating embryoid bodies is comparable to fetal cardiomyocytes. J. Mol. Cell Cardiol. 32, 839. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman M (1981) Establishment in culture of pluripotent cells from mouse embryos. Nature 292, 154. [DOI] [PubMed] [Google Scholar]
- Fijnvandraat AC, Van Finneken ACG, De Boer PAJ, Ruijter JM, Christoffels VM, Moorman AFM, Lekanne Deprez RH (2003a) Cardiomyocytes derived from embryonic stem cells resemble cardiomyocytes of the embryonic heart tube. Cardiovasc. Res. 58, 399. [DOI] [PubMed] [Google Scholar]
- Fijnvandraat AC, Lekanne Deprez RH, Moorman AFM (2003b) Development of heart muscle cell diversity: a help or a hindrance for phenotyping embryonic stem cell‐derived cardiomyocytes. Cardiovasc. Res. 58, 303. [DOI] [PubMed] [Google Scholar]
- Finch BW, Ephrussii B (1967) Retention of multiple developmental potentialities by cells of a mouse testicular teratocarcinoma during prolonged culture in vitro and their extinction upon hybridization with cells of permanent lines. Proc. Natl Acad. Sci. USA 57, 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves KH, Moreadith RW (1993) Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol. Reprod. Dev. 36, 424. [DOI] [PubMed] [Google Scholar]
- Harlan DM, Kirk AD (1999) The future of organ and tissue transplantation: can T‐cell co‐stimulatory pathway modifiers revolutionize the prevention of graft rejection? JAMA 282, 1076. [DOI] [PubMed] [Google Scholar]
- He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ (2003) Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterisation. Circ. Res. 93, 32. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Mikkola M, Gertow K, Stromberg A‐M, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andang M, Ahrlund‐Richter L (2003) A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum. Reprod. 18, 1404. [DOI] [PubMed] [Google Scholar]
- Iannaconne PM, Taborn GU, Garton RL, Caplice MD, Brenin DR (1994) Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev. Biol. 163, 288. [DOI] [PubMed] [Google Scholar]
- Illmensee K, Mintz B (1976) Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc. Natl Acad. Sci. USA 73, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskovitz‐Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N (2000) Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 6, 88. [PMC free article] [PubMed] [Google Scholar]
- Johkura K, Cui L, Suzuki A, Teng R, Kamiyoshi A, Okamura S, Kubota S, Zhao X, Asanuma K, Okouchi Y, Ogiwara N, Tagawa Y, Sasaki K (2003) Survival and function of mouse embryonic stem cell‐derived cardiomyocytes in ectopic transplants. Cardiovasc. Res. 58, 435. [DOI] [PubMed] [Google Scholar]
- Kehat I, Kenyagin‐Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz‐Eldor J, Gepstein L (2001) Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest 108, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GM (1995) In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 7, 862. [DOI] [PubMed] [Google Scholar]
- Kim D, Gu Y, Ishii M, Fujimiya M, Qi M, Nakamura N, Yoshikawa T, Sumi S, Inoue K (2003) In vivo functioning and transplantatble mature pancreatic islet‐like cell clusters differentiated from embryonic stem cell. Pancreas 27, E34. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Cochran NA (1964) Multipotentiality of single embryocarcinoma cells. Cancer Res. 24, 1544. [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY, Field LJ (1996) Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J. Clin. Invest 98, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J, Hegert C, Guan K, Wobus A, Muller PK, Rohwedel J (2000) Embryonic stem cell‐derived chondrogenic differentiation in vitro: activation by BMP‐2 and BMP‐4. Mech. Dev. 92, 193. [DOI] [PubMed] [Google Scholar]
- Kuai XL, Cong XQ, Li XL, Xiao SD (2003) Generation of hepatocytes from cultured mouse embryonic stem cells. Liver Transplant. 9, 1094. [DOI] [PubMed] [Google Scholar]
- Laslett AL, Filipczyk AA, Pera MF (2003) Characterization and culture of human embryonic stem cells. Trends Cardiovasc. Med. 13, 295. [DOI] [PubMed] [Google Scholar]
- Lavker RR, Sun TT (2000) Epidermal stem cells: properties, markers and location. Proc. Natl Acad. Sci. USA 97, 13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy A, Xiong JW, Kuhnert F, Stuhlmann H (1999) Use of developmental marker genes to define temporal and spatial patterns of differentiation during embryoid body formation. J. Exp. Zool. 284, 67. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz‐Eldor J, Langer R (2003) Differentiation of human embryonic stem cells on three‐dimensional polymer scaffolds. Proc. Natl Acad. Sci. USA 100, 12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang D, Hou Y, Jiao L, Zheng X, Wang WH (2003) Isolation and culture of embryonic stem cells from porcine blastocysts. Mol. Reprod. Dev. 65, 429. [DOI] [PubMed] [Google Scholar]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R (2001) Differentiation of embryonic stem cells to insulin‐secreting structures similar to pancreatic islets. Science 292, 1389. [DOI] [PubMed] [Google Scholar]
- Martin G (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma cells. Proc. Natl Acad. Sci. USA 78, 7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Solter D (1983) Nuclear transplantation in mouse embryos by microsurgery and cell fusion. Science 220, 1300. [DOI] [PubMed] [Google Scholar]
- Mitalipov SM, Yeoman RR, Nusser KD, Wolf DP (2002) Rhesus monkey embryos produced by nuclear transfer from embryonic blastomeres or somatic cells. Biol. Reprod. 66, 1367. [DOI] [PubMed] [Google Scholar]
- Mitalipova M, Calhoun J, Shin S, Wininger D, Schulz T, Noggle S, Venable A, Lyons I, Robins A, Stice S (2003) Human embryonic stem cell lines derived from discarded embryos. Stem Cells 21, 521. [DOI] [PubMed] [Google Scholar]
- Morrison SJ (2001) Stem cell potential: can anything make anything? Curr. Biol. 11, R7. [DOI] [PubMed] [Google Scholar]
- Muller M, Fleischmann BK, Selbert S, Ji GJ, Endl E, Middeler G, Muller OJ, Schlenke P, Frese S, Wobus AM, Hescheler J, Katus HA, Franz WM (2000) Selection of ventricular‐like cardiomyocytes from ES cells in vitro . FASEB J. 14, 2540. [DOI] [PubMed] [Google Scholar]
- Mummery C, Oostwaard DW, Doevendans P, Spijker R, Van Den Brink S, Hassink R, Van Der Heyden M, Opthof T, Pera M, De La Riviere AB, Passier R, Tertoolen L (2003) Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm‐like cells. Circulation 107, 2733. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gocza E, Diaz EM, Prideaux VR, Ivanyi E, Marrkula M, Rossant J (1990) Embryonic stem cells alone are able to support fetal development in the mouse. Development 110, 815. [DOI] [PubMed] [Google Scholar]
- Orive G, Hernandez RM, Gascon AR, Igartua M, Pedraz JL (2003) Controversies over stem cell research. Trends Biotechnol. 21, 109. [DOI] [PubMed] [Google Scholar]
- Pachernik J, Esner M, Bryja V, Dvorak P, Hampl A (2002) Neural differentiation of mouse embryonic stem cells grown in monolayer. Reprod. Nutr. Dev. 42, 317. [PubMed] [Google Scholar]
- Prockop DJ, Gregory CA, Spees JL (2003) One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc. Natl Acad. Sci. USA 100, 11917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK (2003) Generation of hepatocyte‐like cells from human embryonic stem cells. Cell Transplant. 12, 1. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A (2000) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro . Nat. Biotechnol. 18, 399. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben Hur T (2001) Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 19, 1134. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong C‐Y, Chan W‐K, Wong P‐C, Bongso A (2002) Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 20, 933. [DOI] [PubMed] [Google Scholar]
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A (2003) Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells 21, 546. [DOI] [PubMed] [Google Scholar]
- Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschmen T (1988) Vasculogenesis and angiogenesis in embryonic stem cell‐derived embryoid bodies. Development 102, 471. [DOI] [PubMed] [Google Scholar]
- Smith A, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D (1988) Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336, 688. [DOI] [PubMed] [Google Scholar]
- Sottile V, Thomson A, McWhir J (2003) In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells 5, 149. [DOI] [PubMed] [Google Scholar]
- Stavridis M, Smith AG (2003) Neural differentiation of mouse embryonic stem cells. Biochem. Soc. Trans. 31, 45. [DOI] [PubMed] [Google Scholar]
- Stix G (2002) What clones? Scientific American 286, 18. [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR (1987) Site‐directed mutagenesis by gene targeting in mouse embryo‐derived stem cells. Cell 51, 503. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP (1995) Isolation of a primate embryonic stem cell line. Proc. Natl Acad. Sci. USA 92, 7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP (1996) Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol. Reprod. 55, 254. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cells derived from human blastocysts. Science 282, 1145. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL (2000) Direct isolation of human central nervous system stem cells. Proc. Natl Acad. Sci. USA 97, 14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey CJ, De La Hall PM (2001) Hepatic stem cells: a review. Pathology (Phila.) 33, 130. [PubMed] [Google Scholar]
- Vogel G (2001) Can adult stem cells suffice? Science 292, 1820. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Christensen JL, Weissman IL (2002) Cell fate determination from stem cells. Gene Ther. 9, 606. [DOI] [PubMed] [Google Scholar]
- Walker PR, Saas P, Dietrich PY (1997) Role of Fas ligand (CD95L) in immune escape: the tumour cell strikes back. J. Immunol. 158, 4521. [PubMed] [Google Scholar]
- Wang R, Clark R, Bautch VL (1994) Embryonic stem cell‐derived cystic embryoid bodies form vascular channels: an in vitro model of blood vessel development. Development 114, 303. [DOI] [PubMed] [Google Scholar]
- Wiles MV, Keller G (1991) Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development 111, 259. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CI, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM (1988) Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 36, 684. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schneike AE, McWhir J, Kind AJ, Campbell KHS (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Beaujean N, De Sousa PA, Dinnyes A, King TJ, Paterson LA, Wells DN, Young LE (2002) Somatic cell nuclear transfer. Nature 419, 583. [DOI] [PubMed] [Google Scholar]
- Wobus AM (2001) Potential of embryonic stem cells. Mol. Aspects Med. 22, 149. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Wallukat G, Hescheler J (1991) Pluripotent embryonic stem cells are able to differentiate into cardiomyocytes express chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation 48, 173. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokumi MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK (2001) Feeder‐free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa SI (2000) Flk‐1 positive cells derived form embryonic stem cells serve as vascular progenitors. Nature 408, 92. [DOI] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A (2003) Conversion of embryonic stem cells in neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183. [DOI] [PubMed] [Google Scholar]
- Zandstra PW, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, Pasumarthi KBS, Field LJ (2003) Scalable production of embryonic stem cell‐derived cardiomyocytes. Tissue Eng. 9, 767. [DOI] [PubMed] [Google Scholar]


