Abstract
Objectives
As mesenchymal stem cells (MSCs) can be isolated easily from adipose tissues while retaining their self‐renewal and multi‐potential differentiation capacities, they hold promising possibilities for being applied extensively in tissue engineering. Bone morphogenetic protein (BMP) family members have been reported to provide instructive signals to MSCs for them to differentiate into several different cell lineages. The study described here aims to investigate whether BMP‐4 could promote adipose‐derived stem cell (ASC) differentiation into adipocytes under various concentrations.
Materials and methods
ASCs were isolated from mouse inguinal adipose pads and cultured in vitro. 10 ng/ml and 50 ng/ml BMP‐4 were added to adipogenic media for 8 days. Oil red‐O staining, reverse transcription/polymerase chain reaction and immunocytofluorescence staining were performed to examine differentiation of the ASCs.
Results
As indicated by increased expression of adipogenic and lipogenic genes (PPAR‐γ, APN and LPL) and proteins, 50 ng/ml BMP‐4 seemed to induce mASCs to differentiate into the adipo‐lineage compared to 10 ng/ml BMP‐4, and control groups. In addition, lipid droplets accumulated within the adipocytes under 50 ng/ml BMP‐4 stimulation, as shown by oil red‐O staining.
Conclusions
Our present study suggests that BMP‐4, as an adipo‐inducing factor, promoted adipogenesis of ASCs at higher concentrations (50 ng/ml) and can perhaps be considered as a candidate for use in adipose tissue engineering.
Introduction
Mesenchymal stem cells (MSCs) as a group of multi‐potential adult stem cells can be isolated from a variety of organs and tissues, including bone marrow, adipose tissue, muscular tissue, dermis, hair follicles and the periodontal ligament 1. Previous studies indicate that stem cells derived from adipose tissues may undergo self‐renewal for several generations while maintaining their capacity to differentiate into different lineages of cells such as osteoblasts, chondrocytes, adipocytes and myoblasts, specially in the adipogenic differentiation process 2, 3, 4, 5. Recently, studies concerning adipose stem cells (ASCs) have spurred further research to make use of potential therapeutic applications of ASCs in regenerative medicine 6, 7. As they are easy to access, simple to isolate and expand in vitro, ASCs are a promising and abundant cell source for adipose tissue engineering to restore large‐sized soft tissue defects resulting from trauma, tumour resection and congenital defects 8, 9, 10.
Bone morphogenetic proteins (BMPs), members of the transforming growth factor β family, are important regulators of various developmental processes including in heart, post‐natal bone, cartilage and the central nervous system 11. Initially named for their ability to induce bone formation, BMP family members (such as BMP‐2/4/7) play crucial roles in osteoblast differentiation and bone formation 12. Interestingly, BMPs may also provide instructive signals for MSCs to become committed to adipogenesis 13, 14, 15.
C3H10T1/2 cells, fibroblast‐like stem cells established from 14‐ to 17‐day‐old embryos of the C3H mouse strain 16, when exposed to BMP‐4, commit to becoming pre‐adipocytes and undergo adipogenesis when further induced by adipogenic differentiation media, while adipogenesis of C3H10T1/2 MSCs was not observed, if BMP‐4 signalling was blocked 13. BMP‐2, similar to BMP‐4, showed the potential to induce MSC adipocytic commitment, but at higher concentration 15. However, so far, the precise role of various BMPs in regulating stem‐cell lineage commitment into various lineages, including osteogenesis versus adipogenesis, and underlying mechanistic pathways, have yet to be fully elucidated.
In our present study we have aimed to investigate effects of BMP‐4 on ASCs, primarily isolated and cultured from mice. We compared (i) accumulation of lipid droplets; (ii) gene expression of adipogenic transcription factors and lipogenic genes after adipogenic induction; and (iii) PPAR‐γ (peroxisome proliferator‐activated receptor‐γ) protein expression after adipogenic induction, to reveal its role in regulating adipose stem‐cell lineage commitment towards adipogenesis under different concentrations, and its potential features for application in regenerative medicine and tissue engineering.
Materials and methods
Isolation and culture of mASCs
We used three‐week‐old Kunming mice from the West China Experimental Animal Center, following all International Guiding Principles for Animal Research (1985). For isolation of mASCs, inguinal adipose tissue was removed, successively cleaned in sterile phosphate buffered saline (PBS), penicillin–streptomycin and PBS. Specimens were then full incised and incubated with 0.075% type I collagenase (Sigma‐Aldrich, St. Louis, MO, USA) in PBS for 60 min at 37 °C with vigorous agitation. After neutralization with serum collagenase, cells were released from adipose specimens then filtered and collected by centrifugation at 1200 g for 10 min. Finally, pellets were re‐suspended and washed three times in medium and cells were seeded in tissue culture‐treated flasks in basic medium (α‐MEM plus 10% FBS and 1% penicillin–streptomycin). mASCs were maintained in a humidified atmosphere of 5% CO2 at 37 °C and passaged 3 times prior to all assays.
Adipogenic induction and BMP‐4 treatment
After culture and expansion in basic medium, third passage mASCs were trypsinized and reseeded into six‐well plates at appropriate initial density; they were then cultured in basic medium for 2 days to reach confluence and adhere to the plates. All wells were then divided into three experimental groups: one control group and two BMP‐4 groups, with at least three parallel wells in each group. For the control group, medium was replaced with adipogenic induction medium containing α‐MEM, 10% FBS, dexamethasone(1 μm), insulin (10 μm), indomethacin (200 μm) and isobutyl‐methylxanthine (0.5 mm) (Sigma‐Aldrich). For BMP‐4 groups, medium was replaced with adipogenic induction medium plus 10 or 50 ng/ml recombinant BMP‐4 (PEPROTECH, INC, Rocky Hill, NJ, USA). Medium was replaced every 2 days. After being induced in adipogenic induction medium or in adipogenic induction medium plus BMP‐4, for 3 or 8 days, cells were harvested for further studies.
Oil red‐O staining
Accumulation of cytoplasmic lipid droplets in differentiated mASCs was assessed using oil red‐O (ORO) staining. After 8 days adipogenic induction, cells were rinsed in phosphate buffered saline and fixed in 4% paraformaldehyde for 15 min at room temperature. After washing in PBS, cells were stained with 1% oil red O for 10 min, then washed several times in distilled water; stained cells were photographed using an Olympus IX 710 microscope (Olympus, Tokyo, Japan). After microscope observation, quantification of lipid accumulation was measured by ORO staining extraction assay. First, we added 400 μl 100% isopropanol to each well, as extraction solution. Thereafter, after being gently mixed for 15 min, extracts were transferred to 96‐well plates. Absorbance at 510 nm was then recorded using a Varioskan Flash spectral scanning multimode reader (Thermo Scientific, Waltham, MA, USA).
Extraction of total RNA, RT‐PCR and real‐time PCR
After 3 or 8 days adipogenic induction, we assessed expression of PPAR‐γ1 (peroxisome proliferator activated receptor‐γ1), LPL (liporotein lipase) and APN (adiponectin) at transcriptional levels in mASCs, by extraction of total RNA, RT‐PCR and real‐time PCR. Total RNA was extracted from each sample by using Simply P total RNA extraction kit (BioFlux, Hangzhou, China) and in the order of 1 μg total RNA was reverse transcribed into cDNA using a PrimeScript RT reagent kit with γDNA Eraser (Takara Bio, Tokyo, Japan), according to the manufacturer's instructions. Total RNA and cDNA were examined using agarose electrophoresis, according to the protocol outlined in ‘Molecular Cloning: A Laboratory Manual’ (2001, 3rd edn). cDNA samples were amplified with primers using an RT‐PCR kit (Tiangen, Beijing, China) to establish the standard curve of a specific certain gene. All primers displayed in Table 1 were designed from established GenBank sequences. For real‐time PCR, expression of target genes in mASCs were quantified using SYBR Premix Ex TaqTM (Perfect Real Time) kit (Takara) carried out on an ABI 7300 system (ABI, Foster City, CA, USA). For each reaction, a melting curve was generated to test primer dimmer formation and false priming. Relative quantification of mRNA levels was carried out by means of a double standard curve method; to assess PCR efficiency, amplification of GAPDH (D‐glyceraldehyde‐3‐phosphate dehydrogenase) was used as control. Real‐time PCR products were electrophoresed on 2% agarose gels, stained with ethidium bromide and photographed with Quantity One software (BIO‐RAD, Hercules, CA, USA).
Table 1.
Primer sequences of target genes and GAPDH for real‐time PCR assay
| Gene (mouse) | Primer sequence (5′–3′) |
|---|---|
| PPAR‐γ1 | F: CCAACTTCGGAATCAGCTCT |
| R: CAACCATTGGGTCAGCTCTT | |
| Lipoproteinlipase | F: AGGGCTCTGCCTGAGTT |
| R: AGAAATCTCGAAGGCCTGGT | |
| Adiponectin | F: GCAGAGATGGCACTCCTGGA |
| R: CCCTTCAGCTCCTGTCATTCC | |
| GAPDH | F: GACGGCCGCATCTTCTTGTGC |
| R: TGCAAATGGCAGCCCTGGTGA |
Immunofluorescence staining of PPAR‐γ and ph‐PPAR‐γ
mASCs were seeded on glass coverslips for immunofluorescence (IF) staining. After 3 days adipogenic induction, cells were washed three times in PBS and fixed in 4% cold paraformaldehyde for 20 min at room temperature. After washing in PBS, they were permeabilized with PBS/0.1% Triton X‐100 and blocked in 1% bovine serum albumin and 1.5% normal goat serum at room temperature for 30 min. Coverslips were then incubated overnight at 4 °C in rabbit polyclonal antibody against PPAR‐γ (1:100) (Abcam, Cambridge, UK) and rabbit polyclonal antibody against PPAR‐γ with phosphoserine at residue 84 (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Then cells on coverslips were incubated at room temperature for 1 h with secondary antibodies conjugated to fluorescein isothiocyanate (Invitrogen, Carlsbad, CA, USA) to detect primary antibody binding. Nuclei were counterstained with DAPI (Molecular Probes, Eugene, OR, USA) for 5 min at room temperature. After washing in PBS, cells were examined by fluorescence microscopy using an Olympus IX 710 microscope (Olympus). Images were analysed using Image‐Pro Plus 6.0.0.260. To evaluate PPAR‐γ and ph‐PPAR‐γ concentrations, integral optical density (IOD) was measured.
Data analysis
All assays were repeated at least three times. ANOVA was used to analyse differences within groups, in all assays and to specify significant differences between experimental groups and controls, Dunnett t‐test was conducted. To determine effectiveness of different BMP‐4 concentrations, data were also analysed using the LSD t‐test. In the figures, representative data are presented as Mean ± standard deviation. P values <0.05 were considered statistically significant.
Results
BMP‐4 promoted accumulation of lipid droplets
After 8 days adipogenic induction, oil red‐O staining was performed to analyse accumulation of lipid droplets (Fig. 1a). At early stages, morphological differentiation of mASCs was observed. First, most cells attached to culture plate surfaces exhibited fibroblast‐like spindle shapes, but subsequently were found to be more spherical. Later, we observed large numbers of small lipid droplets appearing in cells throughout the cytoplasm, so oil red‐O staining was performed. Presence of positively stained adipocytes suggested that BMP‐4 significantly promoted accumulation of lipid droplets (Fig. 1b). In 50 ng/ml BMP‐4 groups, lipid droplets were significantly more numerous than in the control group; oil red‐O spectrophotometer quantification was assessed for all groups (Fig. 1c). Lipid droplets in 50 ng/ml BMP‐4 groups were significantly more concentrated than in controls, while there were no statistical differences between 10 ng/ml BMP‐4 groups and the control group.
Figure 1.
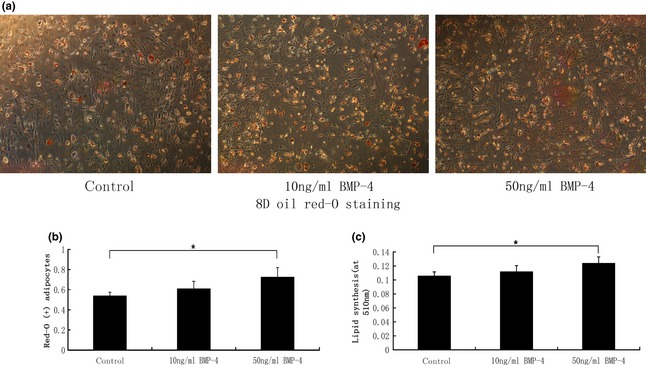
BMP ‐4 promoted lipid accumulation of ASC s. (a) After 8 days being induced by differentiation medium, oil red‐O staining was performed to detect ASC adipogenic differentiation. (b) oil red‐O positively stained adipocytes in each group suggesting that BMP‐4 promoted the process of adipogenesis, as indicated by * (*P < 0.05). (c) Oil red‐O spectrophotometer quantification was carried out for each group. Data demonstrate that lipid accumulation in 50 ng/ml BMP‐4 groups was significantly higher than that of the control group, as indicated by * (*P < 0.05). Magnification ×40.
BMP‐4 promoted PPAR‐γ, APN and LPL transcription
After 3 days adipogenic induction, PPAR‐γ1 and APN mRNAs, and after 8 days, LPL and APN mRNA were detected using real‐time PCR (Fig. 2). Results show that mRNA level of PPAR‐γ1 in 50 ng/ml BMP‐4 groups was significantly higher than in the control group, but there was no statistically significant difference between the 10 ng/ml BMP‐4 groups and the control group. Between 10 ng/ml BMP‐4 and 50 ng/ml BMP‐4 groups, there was also no statistically significant difference (Fig. 2a). Results of LPL gene coding transcription were similar to PPAR‐γ1, as LPL expression was evidently increased in 50 ng/ml BMP‐4 groups compared to the control group. There was no statistically significant difference between 10 ng/ml BMP‐4 groups and the control group or between the 50 ng/ml BMP‐4 and 10 ng/ml BMP‐4 groups (Fig. 2b). After 3 and 8 days adipogenic induction, mRNA level of APN of 50 ng/ml BMP‐4 groups was significantly higher than of 10 ng/ml BMP‐4 groups and the control group, at each time point, and in 50 ng/ml BMP‐4 groups, mRNA level of APN on day 8 was significantly higher than on day 3. However APN transcription in 10 ng/ml BMP‐4 groups was not statistically significant compared to the control group at each time point (Fig. 2c). Results of PPAR‐γ1, APN and LPL transcription were confirmed by AGE (agarose gel electrophoresis) (Fig. 2d).
Figure 2.
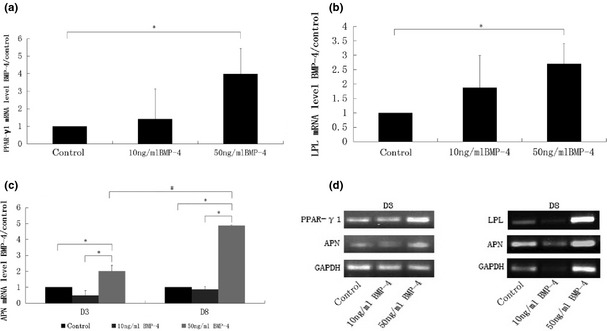
BMP‐4 promoted adiopogenic and lipogenic gene expression. In panel (a), at day 3 adipogenic induction, PPAR‐γ1 expression was highly activated by 50 ng/ml BMP‐4. Panel (b), similar trends seen for LPL expression after 8 days differential induction, as LPL expression was increased in 50 ng/ml BMP‐4 groups compared to the control group. Panel (c), after 3 and 8 days adipogenic induction, mRNA levels of APN in the 50 ng/ml BMP‐4 groups were significantly higher than in the 10 ng/ml BMP‐4 groups and the control group at each time point. For 50 ng/ml BMP‐4 groups, APN expression on day 8 was also obviously enhanced over that of day 3. P values <0.05 were considered statistically significant, as indicated by * and #, * for comparison in different groups on the same day and # for comparison in different groups between day 3 and day 8. Expression of PPAR‐γ1, APN and LPL transcription were confirmed by AGE (agarose gel electrophoresis) in panel (d).
BMP‐4 promoted PPAR‐γ and ph‐PPAR‐γ protein expression
PPAR‐γ is commonly referred to as the master regulator of adipogenesis; it is activated by cytoplasmic dephosphorylation. Dephosphorylated PPAR‐γ moves into nuclei and functions as an intranuclear transcriptional factor. After 3 days adipogenic induction, immunofluorescence staining was performed with anti‐PPAR‐γ (Fig. 3a) and anti‐ph‐PPAR‐γ (Fig. 4a) antibodies, respectively. To evaluate PPAR‐γ and ph‐PPAR‐γ concentrations, integral optical density (IOD) was measured (Figs 3b,4b). We observed that PPAR‐γ was condensed in nuclei, with significantly higher IOD in 50 ng/ml BMP‐4 groups (5.50 ± 0.08%) than in the control group (5.16 ± 0.06%) and 10 ng/ml BMP‐4 groups (5.18 ± 0.07%). ph‐PPAR‐γ expression was also higher in 50 ng/ml BMP‐4 groups (2.54 ± 0.07%) than in the control group (1.95± 0.11%), and in 10 ng/ml BMP‐4 groups (2.23 ± 0.16%). However PPAR‐γ and ph‐PPAR‐γ IOD of 10 ng/ml BMP‐4 groups were not statistically significantly different compared to the control group.
Figure 3.

BMP ‐4 promoted PPAR ‐γ protein expression. (a) After 3 days adipogenic induction, immunofluorescence staining was performed with anti‐PPAR‐γ (green). Cell nuclei were counterstained with 4,6‐diamidino‐2‐phenylindole (blue). (b) Integral optical density (IOD) was measured to evaluate PPAR‐γ concentration. PPAR‐γ was condensed in nuclei, with significantly higher IOD in 50 ng/ml BMP‐4 groups than in the control group and 10 ng/ml BMP‐4 groups. However, PPAR‐γ IOD of the 10 ng/ml BMP‐4 groups was not statistically significant compared to the control group. P values <0.05 were considered statistically significant, as indicated by *. Magnification ×100.
Figure 4.
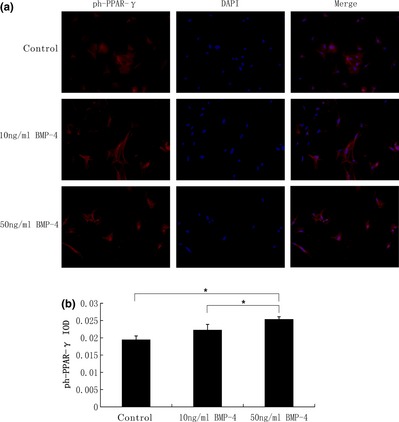
BMP ‐4 promoted ph‐ PPAR ‐γ protein expression. (a) After 3 days adipogenic induction, immunofluorescence staining was performed with anti‐ph‐PPAR‐γ (red). Cell nuclei were counterstained with 4,6‐diamidino‐2‐phenylindole (blue). (b) To evaluate ph‐PPAR‐γ concentration, integral optical density (IOD) was measured. ph‐PPAR‐γ was also expressed more highly in 50 ng/ml BMP‐4 groups than in the control group and in the 10 ng/ml BMP‐4 groups. However, ph‐PPAR‐γ IOD of 10 ng/ml BMP‐4 groups was not statistically significant compared to the control group. P values <0.05 were considered statistically significant, as indicated by *. Magnification ×100.
Discussion
Although adipocyte formation involves multiple steps, adipogenesis is typically described in the context of two major phases – determination and terminal differentiation. In the determination stage, multipotent MSCs become committed to the adipocyte lineage and lose their ability to differentiate into other cell lineages, while during the terminal differentiation process, fibroblastic pre‐adipocytes are converted to spherical, mature adipocytes that can synthesize and transport lipids and secrete adipocyte‐specific proteins 17.
In this study, we demonstrated that compared to the control group and 10 ng/ml concentration BMP‐4, 50 ng/ml BMP‐4 promoted ASCs to differentiate into adipocytes. As MSC lineage commitment is usually assessed by activation of defined transcription factors specific to that lineage, we detected gene expression of adipogenic transcription factors and terminal lipogenic genes. PPAR‐γ predominately expressed in adipose tissue and the immune system 18, has been demonstrated to be a master regulator of adipogenesis, as all critical cell signalling pathways involved in adipogenesis converge on PPAR‐γ, and most factors that stimulate adipogenesis ultimately exert their effect through its regulation 19, 20. The critical role of PPAR‐γ in cell differentiation has also been demonstrated in PPAR‐γ knockout animals 21, 22, 23. Suppression of PPAR‐γ function through RNA interference leads to cell differentiation towards osteoblasts rather than to adipocytes in multi‐potential mesenchymal stem cells 24.
Several reports have indicated that expression of PPAR‐γ is turned on early in adipogenesis, and it is commonly activated by dephosphorylation in the cytoplasm. Dephosphorylated PPAR‐γ moves into nuclei and functions as an intranuclear transcriptional factor 25, 26. In this study, we have shown that PPAR‐γ gene expression was increased 3 days after adipose differential induction. Enhanced expression was more obvious in 50 ng/ml BMP‐4 compared to 10 ng/ml BMP‐4 group and the control group. To analyse the role of BMP‐4 in activation of PPAR‐γ in adipogenesis, active forms of PPAR‐γ (de‐PPAR‐γ) and ph‐PPAR γ were assayed by immunofluorescence.
As the integral optical density (IOD) showed, both expression of PPAR‐γ and ph‐PPAR‐γ markedly increased in 50 ng/ml BMP‐4 groups compared to the control group. Meanwhile, de‐PPAR‐γ was seen to be condensed in nuclei, which may suggest that PPAR‐γ was enormously activated directly or indirectly by 50 ng/ml BMP‐4.
To detect terminal adipose differentiation in our three groups, specific adipocyte differentiation biomarkers were assessed as well. As indicated by our real‐time PCR results, on day 8 after adipogenic induction, expressions of APN and LPL were significantly upregulated in the 50 ng/ml BMP‐4 group compared to 10 ng/ml or control groups.
As shown by RT‐PCR results, as early as 3 days after adipose induction, mASCs expressed enhanced levels of PPAR‐γ, an essential transcription factor for pre‐adipocyte commitment, and mASCs became mature lipid‐laden adipocytes only 8 days after hormonal stimulation. Without BMP‐4 pre‐treatment, C3H10T1/2 adipogenesis usually takes around 14 days or more, as shown in one previous study 27. Activation of defined transcription factors is the crucial step for stem cell lineage commitment. Differentiation to pre‐adipocytes is initiated by C/EBPβ, which activates PPAR‐γ 28, 29. PPAR‐γ and C/EBPα form a positive transcriptional feedback loop orchestrating downstream adipocyte biology 30. Moreover, terminal differentiation of adipocytes is accompanied by lipid accumulation in cytoplasm. As shown by oil red‐O staining, ASCs differentiated and gradually took on adipocyte morphological features as lipid droplets accumulated. After 5 days adipogenic differentiation, large numbers of small lipid droplets began to appear and stained positively with oil red‐O until 8 days differential induction, especially in the 50 ng/ml BMP‐4 group.
Previous studies have mainly focused on elucidating mechanisms of BMP‐mediated osteogenic differentiation 31, 32. While BMP signalling is critical to skeletal development and homeostasis by action on osteoblasts, evidence also exists for an adipogenic role of this signalling pathway. Recent findings indicate that BMP signalling plays important roles in determining commitment of MSCs to the adipocyte lineage. For example, forced commitment of C3H10T1/2 MSC line to a pre‐adipocyte cell lineage (A33) using DNA methyltransferase inhibitor 5‐azacytidine, results in elevated expression of BMP‐4 13, 14. Functionally, this endogenous BMP‐4 expression appears to be critical to further adipose differentiation, as BMP‐4 antagonist noggin treatment inhibited adipocyte differentiation of A33 cells 13. Although differentiation induction media alone fail to stimulate adipogenesis of C3H10T1/2 stem cells, overexpression or application of exogenous BMP‐4 promotes their commitment to the adipocyte lineage and subsequent adipocyte differentiation in response to adipose differentiation inducers 14, 33, 34.
As BMP family members can promote osteogenesis, adipogenesis or chondrogenesis, precise roles of BMPs in regulating stem cell lineage commitment into various lineages, has aroused interest to stimulate further research 35. It has been indicated that BMP treatment favouring MSC differentiation into a specific lineage, may depend on BMP ligands, BMP receptors (BMPRs) involved, concentration of BMP used and the origin of the stem cells. In bone marrow stromal cells, the predominant effect of BMPs, in particular BMP‐2, is to promote osteogenic differentiation and to inhibit adipogenesis 14, 33, 34, 36, 37; however, for the pluripotent mesenchymal cell line C3H10T1/2, low concentrations of BMP‐2 and BMP‐7 induce adipogenic differentiation, whereas high concentrations promote differentiation towards chondrocyte and osteoblast 38, 39. However, in embryonic stem cell‐derived embryoid bodies, BMP‐4 can promote adipogenesis 40.
These BMPs appear to have differential effects on adipogenesis which could be explained in part by the fact that downstream events mediated by BMPs are highly dependent on type of receptor involved, and that this ultimately determines specificity of intracellular signals for adipogenic or osteoblastogenic differentiation. In general, activation of BMPR‐IA is considered adipogenic and BMPR‐IB osteoblastogenic; however, this is by no means exclusive 41. Thus, while interference with BMPR‐IA function blocks adipogenesis in tissue culture, conditional disruption of BMPR‐IA interferes with bone remodelling, in mice 42. Lineage‐determinant effects of BMPs on MSC differentiation also appear to be highly sensitive to dose. For example, differentiation of C3H10T1/2 mouse embryonic stem cells into adipocytes occurs preferentially at lower concentrations of BMP‐2, while osteoblast and chondrocyte differentiation is favoured at higher concentrations 43. A further study has suggested that while 50 or 100 ng/ml BMP‐4 resulted in differentiation of 10T1/2 cells into adipocytes, lower level BMP‐4 (10 ng/ml) was completely ineffective 34. Thus, a threshold of BMP‐4 must be surmounted to induce adipocyte lineage commitment of 10T1/2 cells. This is consistent with our present results that when we stimulated primarily cultured mASCs with 10 ng/ml BMP‐4, no evident lipid accumulation or adipogenic gene expression were found. However, at concentration of 50 ng/ml BMP‐4, mASCs displayed obvious differentiation into adipose lineage as shown by morphological changes, increased adipogenic and lipogenic gene expressions and activated PPAR‐γ expression.
In conclusion, we demonstrated that 50ng/ml other than 10ng/ml BMP‐4 could promote adipogenesis of mouse Adipose‐derived Stem Cells with adipogenic induction medium, via upregulating the gene and protein expressions of PPAR‐γ. As an abundant resource and their easy isolation procedures, ASCs hold promising possibilities for application to adipose tissue engineering for soft tissue defect restoration.
Acknowledgement
This work was funded by National Natural Science Foundation of China (81071273, 31170929, 81201211, 81200810), Foundation for the Author of National Excellent Doctoral Dissertation of China (FANEDD 200977) and Funding for Distinguished Young Scientists in Sichuan (2010JQ0010).
References
- 1. Vats A, Tolley NS, Polak JM, Buttery LD (2002) Stem cells: sources and applications. Clin. Otolaryngol. Allied Sci. 27, 227–232. [DOI] [PubMed] [Google Scholar]
- 2. Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL et al (2001) Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue‐derived stromal cells. Tissue Eng. 7, 729–741. [DOI] [PubMed] [Google Scholar]
- 3. Lin Y, Liu L, Li Z, Qiao J, Wu L, Tang W et al (2006) Pluripotency potential of human adipose‐derived stem cells marked with exogenous green fluorescent protein. Mol. Cell. Biochem. 291, 1–10. [DOI] [PubMed] [Google Scholar]
- 4. Lin Y, Yan Z, Liu L, Qiao J, Jing W, Wu L et al (2006) Proliferation and pluripotency potential of ectomesenchymal cells derived from first branchial arch. Cell Prolif. 39, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen JF, Sugawara A, Yamashita J, Ogura H, Sato S (2011) Dedifferentiated fat cells: an alternative source of adult multipotent cells from the adipose tissues. Int. J. Oral Sci. 3, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jing W, Lin Y, Wu L, Li X, Nie X, Liu L et al (2007) Ectopic adipogenesis of preconditioned adipose‐derived stromal cells in an alginate system. Cell Tissue Res. 330, 567–572. [DOI] [PubMed] [Google Scholar]
- 7. Tsuji W, Inamoto T, Yamashiro H, Ueno T, Kato H, Kimura Y et al (2009) Adipogenesis induced by human adipose tissue‐derived stem cells. Tissue Eng. Part A 15, 83–93. [DOI] [PubMed] [Google Scholar]
- 8. Conrad C, Huss R (2005) Adult stem cell lines in regenerative medicine and reconstructive surgery. J. Surg. Res. 124, 201–208. [DOI] [PubMed] [Google Scholar]
- 9. Yildirim S, Fu SY, Kim K, Zhou H, Lee CH, Li A et al (2011) Tooth regeneration: a revolution in stomatology and evolution in regenerative medicine. Int. J. Oral Sci. 3, 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minguell JJ, Erices A, Conget P (2001) Mesenchymal stem cells. Exp. Biol. Med. (Maywood) 226, 507–520. [DOI] [PubMed] [Google Scholar]
- 11. Anderson GJ, Darshan D (2008) Small‐molecule dissection of BMP signaling. Nat. Chem. Biol. 4, 15–16. [DOI] [PubMed] [Google Scholar]
- 12. Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22, 233–241. [DOI] [PubMed] [Google Scholar]
- 13. Bowers RR, Kim JW, Otto TC, Lane MD (2006) Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP‐4 gene. Proc. Natl. Acad. Sci. USA 103, 13022–13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bowers RR, Lane MD (2007) A role for bone morphogenetic protein‐4 in adipocyte development. Cell Cycle 6, 385–389. [DOI] [PubMed] [Google Scholar]
- 15. Huang H, Song TJ, Li X, Hu L, He Q, Liu M et al (2009) BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 106, 12670–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reznikoff CA, Brankow DW, Heidelberger C (1973) Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 33, 3231–3238. [PubMed] [Google Scholar]
- 17. Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896. [DOI] [PubMed] [Google Scholar]
- 18. Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R et al (1997) The organization, promoter analysis, and expression of the human PPARgamma gene. J. Biol. Chem. 272, 18779–18789. [DOI] [PubMed] [Google Scholar]
- 19. Mueller E, Drori S, Aiyer A, Yie J, Sarraf P, Chen H et al (2002) Genetic analysis of adipogenesis through peroxisome proliferator‐activated receptor gamma isoforms. J. Biol. Chem. 277, 41925–41930. [DOI] [PubMed] [Google Scholar]
- 20. Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W et al (2004) Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc. Natl. Acad. Sci. USA 101, 10703–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz‐Lozano P, Chien KR et al (1999) PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595. [DOI] [PubMed] [Google Scholar]
- 22. Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K et al (1999) PPAR gamma mediates high‐fat diet‐induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4, 597–609. [DOI] [PubMed] [Google Scholar]
- 23. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS et al (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617. [DOI] [PubMed] [Google Scholar]
- 24. Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R et al (2005) TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309, 1074–1078. [DOI] [PubMed] [Google Scholar]
- 25. Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK (1997) Transcriptional activation by peroxisome proliferator‐activated receptor gamma is inhibited by phosphorylation at a consensus mitogen‐activated protein kinase site. J. Biol. Chem. 272, 5128–5132. [DOI] [PubMed] [Google Scholar]
- 26. Lohrke B, Viergutz T, Shahi SK, Pohland R, Wollenhaupt K, Goldammer T et al (1998) Detection and functional characterisation of the transcription factor peroxisome proliferator‐activated receptor gamma in lutein cells. J. Endocrinol. 159, 429–439. [DOI] [PubMed] [Google Scholar]
- 27. Lee JS, Suh JM, Park HG, Bak EJ, Yoo YJ, Cha JH (2008) Heparin‐binding epidermal growth factor‐like growth factor inhibits adipocyte differentiation at commitment and early induction stages. Differentiation 76, 478–487. [DOI] [PubMed] [Google Scholar]
- 28. Clarke SL, Robinson CE, Gimble JM (1997) CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator‐activated receptor gamma 2 promoter. Biochem. Biophys. Res. Commun. 240, 99–103. [DOI] [PubMed] [Google Scholar]
- 29. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM (2000) Transcriptional regulation of adipogenesis. Genes Dev. 14, 1293–1307. [PubMed] [Google Scholar]
- 30. Kim JE, Chen J (2004) regulation of peroxisome proliferator‐activated receptor‐gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 53, 2748–2756. [DOI] [PubMed] [Google Scholar]
- 31. Cordonnier T, Langonne A, Sohier J, Layrolle P, Rosset P, Sensebe L et al (2011) Consistent osteoblastic differentiation of human mesenchymal stem cells with bone morphogenetic protein 4 and low serum. Tissue Eng. Part C Methods 17, 249–259. [DOI] [PubMed] [Google Scholar]
- 32. Shen Q, Zhu S, Hu J, Geng N, Zou S (2009) Recombinant human bone morphogenetic protein‐4 (BMP‐4)‐stimulated cell differentiation and bone formation within the expanding calvarial suture in rats. J. Craniofac. Surg. 20, 1561–1565. [DOI] [PubMed] [Google Scholar]
- 33. Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G (1993) Expression of human bone morphogenetic proteins‐2 or ‐4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 12, 871–880. [DOI] [PubMed] [Google Scholar]
- 34. Tang QQ, Otto TC, Lane MD (2004) Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 101, 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mehlhorn AT, Niemeyer P, Kaschte K, Muller L, Finkenzeller G, Hartl D et al (2007) Differential effects of BMP‐2 and TGF‐beta1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif. 40, 809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jin W, Takagi T, Kanesashi SN, Kurahashi T, Nomura T, Harada J et al (2006) Schnurri‐2 controls BMP‐dependent adipogenesis via interaction with Smad proteins. Dev. Cell 10, 461–471. [DOI] [PubMed] [Google Scholar]
- 37. Sottile V, Seuwen K (2000) Bone morphogenetic protein‐2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone). FEBS Lett. 475, 201–204. [DOI] [PubMed] [Google Scholar]
- 38. Etheridge SL, Spencer GJ, Heath DJ, Genever PG (2004) Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells 22, 849–860. [DOI] [PubMed] [Google Scholar]
- 39. Wang HY, Malbon CC (2004) Wnt‐frizzled signaling to G‐protein‐coupled effectors. Cell. Mol. Life Sci. 61, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hata K, Nishimura R, Ikeda F, Yamashita K, Matsubara T, Nokubi T et al (2003) Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator‐activating receptor gamma during bone morphogenetic protein 2‐induced adipogenesis. Mol. Biol. Cell 14, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ et al (1998) Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J. Cell Biol. 142, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG et al (2004) Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J. Biol. Chem. 279, 27560–27566. [DOI] [PubMed] [Google Scholar]
- 43. Wang EA, Israel DI, Kelly S, Luxenberg DP (1993) Bone morphogenetic protein‐2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 9, 57–71. [DOI] [PubMed] [Google Scholar]


