Abstract
Objectives: Four adult non‐human primates Papio ursinus were used to study induction of bone formation by recombinant human transforming growth factor‐β2 (hTGF‐β2) together with muscle‐derived stem cells.
Materials and methods: The hTGF‐β2 was implanted in rectus abdominis muscles and in calvarial defects with and without addition of morcellized fragments of striated muscle, harvested from the rectus abdominis or temporalis muscles. Expression of osteogenic markers including osteogenic protein‐1, bone morphogenetic protein‐3 and type IV collagen mRNAs from generated specimens was examined by Northern blot analysis.
Results: Heterotopic intramuscular implantation of 5 and 25 μg hTGF‐β2 combined with 100 mg of insoluble collagenous bone matrix yielded large corticalized mineralized ossicles by day 30 with remodelling and induction of haematopoietic marrow by day 90. Addition of morcellized rectus abdominis muscle to calvarial implants enhanced induction of bone formation significantly by day 90.
Conclusions: In Papio ursinus, in marked contrast to rodents and lagomorphs, hTGF‐β2 induced large corticalized and vascularized ossicles by day 30 after implantation into the rectus abdominis muscle. This striated muscle contains responding stem cells that enhance the bone induction cascade of hTGF‐β2. Induction of bone formation by hTGF‐β2 in the non‐human primate Papio ursinus may occur as a result of expression of bone morphogenetic proteins on heterotopic implantation of hTGF‐β2; the bone induction cascade initiated by mammalian TGF‐β proteins in Papio ursinus needs to be re‐evaluated for novel molecular therapeutics for induction of bone formation in clinical contexts.
Introduction
There are three transforming growth factor‐β (TGF‐β) isoforms in mammals, which are structurally related cytokines with overlapping and distinct functions in regulating proliferation and differentiation of mesenchymal precursor cells as well as osteoblasts, osteoclasts and chondrocytes (1, 2). Effects of TGF‐βs in combination with other growth factors on osteoblast proliferation or differentiation, depends on concentration, duration of treatment and status of cell differentiation (3, 4). In recent years, several different studies have cast a new light on the specific role of TGF‐β isoforms within bone matrix. Cabiling et al. (1) demonstrated that induction of osteoblast differentiation or apoptosis by TGF‐βs in mouse dural cells was isoform‐specific, and further propose that the role of TGF‐β isoforms is species‐specific. A combinatorial approach by Balooch et al. (5) to assess properties of the bone matrix, independently of bone mass and architecture, showed that TGF‐β signalling regulates mechanical properties and composition of the bone matrix.
Systematic studies in the non‐human primate Papio ursinus have shown a previously unknown function of mammalian TGF‐β isoforms, that is, induction of endochondral bone formation, defined as de novo generation of endochondral bone in heterotopic, intramuscular sites (6, 7, 8, 9, 10). This is in marked contrast to studies in rodents and lagomorphs where only promotive effects were observed (11, 12). Isoforms of the TGF‐β superfamily control tissue induction and morphogenesis of bone as well as disparate organs and tissues (4, 5). These include central and peripheral nervous systems, tooth morphogenesis and induction of cementum with newly formed periodontal ligament fibres, highlighting pleiotropic activity of osteogenic proteins of the TGF‐β superfamily (9, 13, 14, 15, 16, 17). Each of the mammalian TGF‐β isoforms differs in their mode of activation and in receptor binding affinities, as well as having differential effects in relation to their local concentrations (2).
Rapid induction of endochondral bone formation by human transforming growth factor‐β3 (hTGF‐β3) in heterotopic rectus abdominis and orthotopic calvarial sites of Papio ursinus, accompanied by expression of TGF‐β1, bone morphogenetic protein‐3 (BMP‐3) and osteogenic protein‐1 (OP‐1) mRNAs. These with hypercellular osteoblastic activity, osteoid synthesis, angiogenesis and capillary sprouting has suggested a novel molecular and morphological basis for induction of bone formation in clinical contexts (10). Regenerative phenomena by hTGF‐β3‐treated calvarial defects in Papio ursinus are modulated by a regenerative response controlled by expression of inhibitory Smad‐6 and ‐7 proteins (10). The rectus abdominis muscle of adult Papio ursinus is endowed with a stem cell niche (18), which provides a large number of differentiating stem cells including osteogenic progenitors and myoendothelial stem cells, which contribute to postnatal tissue regeneration (18, 19, 20).
It is noteworthy that at least in Papio ursinus, TGF‐β isoforms induce endochondral bone formation in spite of differences in their receptor binding affinities, structure and modes of activation (2). While TGF‐β isoforms are highly homologous, particularly in the carboxy terminal domain, there are significant differences in their regulation and expression, as well as their activities in vitro (2). To further our knowledge concerning their site/tissue specificity, four Papio ursinus individuals were used to study induction of bone formation by recombinant human transforming growth factor‐β2 (hTGF‐β2) isoform when implanted into heterotopic rectus abdominis muscles and orthotopic calvarial defects. We also tested effects of the addition of morcellized fragments of striated muscle tissue, harvested either from the temporalis or rectus abdominis muscles when preparing the orthotopic and heterotopic implantation sites respectively.
Materials and methods
Preparation of hTGF‐β2 osteogenic devices
Recombinant hTGF‐β2 was a kind gift from Genzyme Corporation (Framingham, MA, USA). Stock solutions of the morphogen were prepared by aliquoting required amounts in 20 mm sodium succinate, 4% mannitol, pH 4.0. Allogeneic insoluble collagenous bone matrix was used for local delivery of hTGF‐β2 as optimal tissue induction is dependent on combinatorial action of a molecular signal, hTGF‐β2 protein, with a complementary substratum or carrier matrix (21, 22, 23).
Demineralized bone matrix, prepared from diaphyseal segments of baboon cortical bone, was dissociatively extracted in 4 m guanidinium–HCl containing protease inhibitors (21). The resulting insoluble collagenous bone matrix, inactive after extraction of osteogenic proteins (21), was washed three times in distilled water, dehydrated in ethanol and ether, and used as carrier for hTGF‐β2. The collagenous matrix is an optimal substratum for cell attachment, proliferation and differentiation (21, 22, 23, 24). Implants for heterotopic implantation in the rectus abdominis were prepared in sterile polypropylene tubes by adding 5 and 25 μg hTGF‐β2 to 100 mg of insoluble collagenous bone matrix (8, 10). Identically prepared implants of 5 and 25 μg hTGF‐β2 were mixed with finely minced fragments of temporalis muscle before implantation in the rectus abdominis muscle. For preparation of samples suitable for calvarial implantation, 100 and 250 μg hTGF‐β2 in 500 μl of liquid vehicle were combined with 1 g of collagenous matrix per implant in 50 ml sterile Falcon tubes and lyophilized (9, 10).
Primate models for tissue induction
Four clinically healthy adult Chacma baboons (Papio ursinus), with mean weight of 16.3 ± 2.3 kg, were selected from the primate colony of the University of the Witwatersrand, Johannesburg. Comparative histomorphometric studies between iliac crest biopsies of human and Papio ursinus show a remarkable degree of similarity (25); adult Papio ursinus species are thus suited for study of comparative bone physiology and repair with relevance to humans. Criteria for selection, housing conditions and diets were as described previously (26). Research protocols were approved by the Animal Ethics Screening Committee and conducted according to ‘Guidelines for the Care and Use of Experimental Animals’ prepared by the university and in compliance with the National Code for Animal Use in Research, Education and Diagnosis in South Africa (27).
Lyophilized pellets of 100 mg of collagenous matrix combined with 5 and 25 μg hTGF‐β2 were implanted bilaterally in quadruplicate, in eight ventral intramuscular pouches created by sharp and blunt dissection in the rectus abdominis muscle of each animal (Fig. 1a) (6, 7, 8, 9, 10). After heterotopic implantation, the calvariae were exposed and on each side of the calvarium, two full‐thickness defects, 25 mm in diameter, each separated by 2.5–3 cm of intervening calvarial bone, were created using a craniotome, under saline irrigation (9, 10). An ipsilateral Latin square block design was used to allocate position of the 100 and 250 μg TGF‐β2 osteogenic devices, in 16 calvarial defects in the four adult baboons with balanced distribution of the two treatment modalities between anterior and posterior regions (Fig. 1b) (9, 10).
Figure 1.
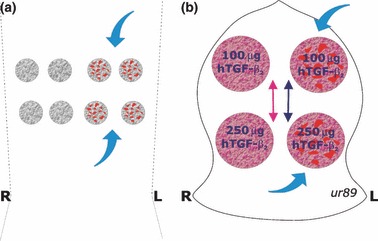
Heterotopic rectus abdominis and orthotopic calvarial implantation designs for bone induction and morphogenesis by doses of recombinant human transforming growth factor‐β2 (hTGF‐β2). (a) Heterotopic intramuscular model and implantation design in the rectus abdominis muscle. Quadruplicate samples of 5 and 25 μg hTGF‐β2 are implanted, along with four specimens additionally treated with morcellized fragments of autogenous temporalis muscle (blue arrows). (b) The calvarial Latin block design results in the ipsilateral implantation of 100 and 250 μg hTGF‐β2 reconstituted with insoluble collagenous bone matrix with a balanced distribution between anterior and posterior defects with a total of eight 100 and eight 250 μg hTGF‐β2 osteogenic implants in four animals at two time periods. Two hTGF‐β2 specimens per animal are additionally treated with morcellized fragments of autogenous rectus abdominis muscle (blue arrows).
Reconstitution of the hTGF‐β2 osteogenic device with minced fragments of rectus abdominis and temporalis muscles
A series of morphological and molecular analyses has shown that addition of minced fragments of rectus abdominis muscle results in induction of greater calvarial osteogenesis when compared to hTGF‐β3 osteogenic devices implanted without minced fragments of rectus abdominis muscle (10, 28). Because of lack and/or limited induction of bone formation in calvarial defects of Papio ursinus, it has been mandatory to study expression patterns of inhibitory Smad‐6 and ‐7 proteins in orthotopic calvarial defects, to unravel segregated osteogenic induction by hTGF‐β3 isoform when implanted in calvarial defects versus ;rectus abdominis intramuscular sites (10). Different results achieved by mammalian TGF‐β osteogenic devices when implanted into the rectus abdominis muscle versus calvarial defects covered by the temporalis muscle have pointed to different and opposite biological functions of respective striated muscles, that is, profoundly osteogenic cellular elements of the rectus abdominis muscle versus inhibitory cellular elements and/or secreted cellular products in the temporalis muscle. To further mechanistically clarify segregated osteogenic induction by the hTGF‐β isoforms in defectes at these sites, it was esssential to harvest fragments of temporalis muscle, which were minced then added to lyophilized hTGF‐β2 osteogenic devices and implanted into heterotopic rectus abdominis sites (Fig. 1a). At the same time, harvested fragments of autogenous rectus abdominis muscle were finely minced and added to hTGF‐β2 osteogenic devices just before implantation into calvarial defects (Fig. 1b).
Tissue harvest, histology and histomorphometry
After harvesting heterotopic specimens for molecular analyses, anaesthetized animals were subjected to bilateral buffered saline carotid perfusion and killed by intravenous overdose of sodium pentobarbitone, on days 30 and 90, two animals per observation period (6, 7, 8, 9, 10). Orthotopic specimen blocks were cut along the sagittal one‐third of the implanted defects and embedded, undecalcified, in a methyl‐methacrylate plastic embedding resin (K‐Plast; Dia Tec Diagnostic Systems, Hallstadt, Germany) (6, 7, 8, 9, 10). Heterotopic specimens were fixed, processed and embedded as described above.
Serial sections, cut at 6 μm (Leica SM2500 Polycut‐S; Reichert, Heidelberg, Germany), were stained free‐floating in modified Goldner’s trichrome (9). Sections were examined using a Provis AX70 research microscope (Olympus Optical Co., Tokyo, Japan) equipped with a calibrated Zeiss Integration Platte II (Carl Zeiss International, Oberkochen, Germany) with 100 lattice points for determination by the point‐counting technique of mineralized bone, osteoid and residual collagenous matrix volumes (in %) (6, 7, 8, 9, 10, 29). Calvarial sections were analysed at 40×, superimposing the Zeiss graticule over five sources (8, 10, 30) selected for histomorphometry, and defined as follows: anterior and posterior interfacial regions (AIF and PIF), anterior and posterior internal regions (AIN and PIN), and a central region (CEN) (6, 7, 8, 9, 10). Each source represented a field of 7.84mm2. Undecalcified sections generated from heterotopic specimens were evaluated by the point‐counting technique for mineralized bone, osteoid, and residual collagenous matrix volumes (in %) (29) superimposing the Zeiss graticule over corticalized outer levels, and traversing to internal regions of the ossicles (8, 9, 10).
Northern blot analyses
Samples of 100/200 mg from replicate specimens of heterotopic ossicles harvested from the rectus abdominis on days 30 and 90 were pooled and total RNA was isolated using TriPure™ isolation reagent (Roche Molecular Biochemicals, Mannheim, Germany), according to the manufacturers’ protocols (8, 10). Purity and yield of RNA was judged by A 260/A 280 absorbance ratio and by γ‐actin signals from Northern blots. Quality of ribosomal RNA bands was resolved on 1% agarose gels incorporating 2.2 m formaldehyde, and was transferred to nylon membrane filters (Hybond™‐N+; Amersham, Amersham Biosciences, UK Ltd, Little Chalfont, Bucks, UK). Linearized vectors containing cDNA of γ‐actin, type IV collagen, BMP‐3 and OP‐1 as described (19) were radiolabelled to high specific activity with α32 P‐dCTP by random prime labelling, using a DNA Megaprime labeling kit (RPN 1606; Amersham), and washed under conditions of high stringency (68 °C) with 0.1% × SSC with 0.1% SDS (26). Membranes were exposed to Kodak Biomax MS film (Eastman Kodak Co., Rochester, NY, USA) with intensifying screens for 6 days. Signals were quantified relative to their respective γ‐actin signals on the same blots by densitometric analysis (GelDoc, Vacutec, Germany) (8, 10).
Statistical analyses
Histomorphometric data of heterotopic and orthotopic tissue sections were analysed using Graph Pad Prism software with one‐way analysis of variance procedure using Bonferroni’s multiple comparison test (8, 10) and are presented in Fig. 6.
Figure 6.
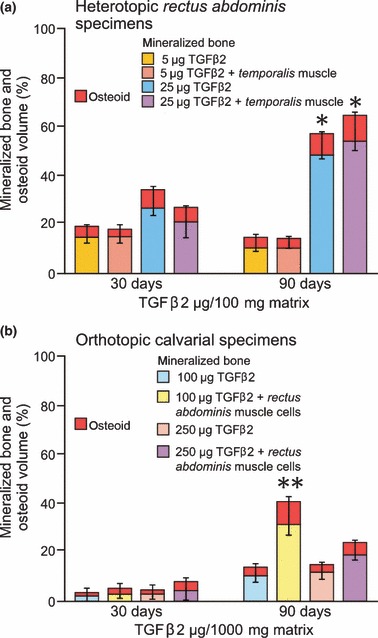
Effects of hTGF‐β2 on bone induction on day 30 and 90 after heterotopic rectus abdominis (a) and orthotopic calvarial implantation (b). (a) Histomorphometric results of induced mineralized bone and osteoid volumes (in %) in the newly formed heterotopic ossicles (*P < 0.05 vs. 5 μg hTGF‐β2 specimens with or without morcellized temporalis muscle). (b) Induced mineralized bone and osteoid seams in calvarial specimens implanted with and without morcellized fragments of rectus abdominis muscle and harvested on day 90. Error bars represent SD of measurements of mineralized bone plus osteoid volumes (**P < 0.05 vs. 100 μg hTGF‐β2 specimens without morcellized fragments of autogenous rectus abdominis muscle).
Results
hTGF‐β2 combined with collagenous matrix as carrier induces bone differentiation in the rectus abdominis muscle of Papio ursinus
Newly generated tissues by 5 and 25 μg hTGF‐β2 implanted in the rectus abdominis muscle had grown to form large corticalized spherical ossicles (Fig. 2a). Juxtaposed ossicles as per the heterotopic implantation scheme (Fig. 1a) had grown towards each other and had coalesced, extending across the rectus abdominis muscle (Fig. 3a). Cut surfaces showed mineralization of external cortices and were macroscopically brownish‐red in colour, indicating induction of haematopoietic bone marrow. Undecalcified sections cut at 5 μm showed corticalization with newly formed mineralized bone (2, 3). Implantation of insoluble collagenous bone matrix reconstituted with 5 and 25 μg of hTGF‐β2 in the rectus abdominis muscle induced differentiation of endochondral bone (2, 3).
Figure 2.
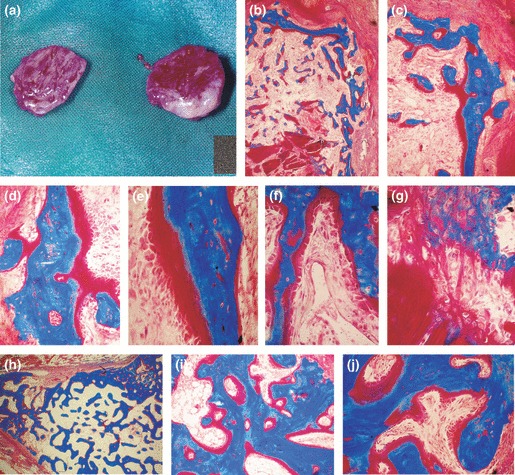
Induction of bone formation by 5 μg recombinant hTGF‐β2 reconstituted with insoluble collagenous bone matrix and implanted in the rectus abdominis muscle of adult baboons Papio ursinus. (a) Induction of large corticalized ossicles by 5 μg hTGF‐β2 harvested on day 30. (b, c) Corticalized mineralized bone covered by osteoid seams induced by 5 μg hTGF‐β2 in the rectus abdominis muscle. (d–f) High power views showing mineralized bone in blue surfaced by large osteoid seams populated by contiguous osteoblasts facing invading capillaries (f). (g) Induction of bone formation within dissolving collagenous matrix as carrier, induction of osteoblastic‐like cells and mineralization in blue of the newly formed bone matrix. (h–j) Mineralized newly formed bone surfaced by osteoid seams 90 days after implantation of 5 μg hTGF‐β2. (j) Thick osteoid seams covered by contiguous osteoblasts. Undecalcified sections cut at 5 μm and stained free‐floating with Goldner’s trichrome. (b) original magnification ×25; (c) original magnification ×75 (d–g) original magnification ×125, 175, 125 and 175 respectively; (h–j) original magnification ×15, 75, 125 respectively.
Figure 3.
Induction and morphogenesis of large mineralized and corticalized ossicles by 25 μg hTGF‐β2 implanted in the rectus abdominis muscle. (a) Induction of large heterotopic ossicles on day 30. Mineralization of the newly formed bone in blue surrounding scattered remnants of the collagenous matrix. Chondrogenesis on day 30 after implantation of 25 μg hTGF‐β2 (inset in a). (b) Detail of mineralized bone covered by osteoid seams at the zone of fusion of the coalesced ossicles. (c inset) Detail of newly formed trabecula covered by osteoid seams populated by contiguous osteoblasts in contact with invading capillaries. (d–g) Details of the induction of bone formation with newly formed mineralized bone in blue covered by osteoid seams. (h–j) Mineralized bone surfaced by large osteoid seams in ossicles generated by 25 μg hTGF‐β2 on day 90. Chondrogenesis on day 90 with vascular invasion and chondrolysis (inset in h). Undecalcified sections cut at 5 μm and stained free‐floating with Goldner’s trichrome (a) original magnification ×2.7; (b) original magnification ×25; (c) ×275; (d–g) original magnification ×75, 175, 125, 200, 275 respectively; (h–j) original magnification ×45, 60, 125 respectively.
Specimens of 5 μg hTGF‐β2 on day 30 showed corticalization by mineralized bone covered by osteoid seams (Fig. 2b,c). Mineralized bone trabeculae surrounded scattered remnants of collagenous matrix with prominent capillary sprouting in close contact with newly deposited large osteoid seams populated by contiguous osteoblasts (Fig. 2d–f). Induction of bone formation initiated in contact with the carrier matrix by differentiating osteoblasts secreting bone matrix rapidly undergoing mineralization (Fig. 2g). On day 90, specimens of 5 μg hTGF‐β2 induced corticalized ossicles with newly formed and mineralized trabeculae were covered by large osteoid seams populated by contiguous osteoblasts (Fig. 2h–j).
Specimens of 25 μg hTGF‐β2 showed induction of large coalesced ossicles (Fig. 3a,b) with mineralized trabeculae of newly formed bone covered by osteoid seams at both time periods (Fig. 3). Subjacent to mineralized cortex of newly formed bone, there was vascular invasion between trabeculae surfaced by osteoid seams in contact with invading capillaries (Fig. 3c). In close proximity to the collagenous matrix remnants, islands of endochondral ossification could be identified on day 30 (Fig. 3a inset). Newly formed trabeculae were embedded into a highly vascular matrix controlling de novo induction of bone formation (Fig. 3c). Initiation of bone formation was within remnants of the collagenous matrix carrier (Fig. 3e,g). The supporting fibrovascular matrix showed prominent angiogenesis and capillary invasion, almost surfacing osteoblastic‐like cells atop newly secreted osteoid seams (Fig. 3c,g).
Addition of minced fragments of temporalis muscle to 5 and 25 μg hTGF‐β2 implants did not alter the bone induction cascade as evaluated by morphological and histomorphometrical analyses (6, 2, 3). On day 90, remodelling of newly formed ossicles with induction of haematopoietic bone marrow resulted in volumetric reduction of harvested ossicles. Remnants of hypertrophic chondrocytes with vascular invasion, chondrolysis and differentiating osteoblastic‐like cells were still present on day 90 in two specimens of 25 μg hTGF‐β2 (Fig. 3h inset).
Morphology of calvarial regeneration by 100 and 250 μg of hTGF‐β2 osteogenic devices with and without morcellized fragments of rectus abdominis muscle
Doses of 100 and 250 μg hTGF‐β2, combined with insoluble collagenous bone matrix induced limited bone formation localized at interfacial regions of calvarial defects only (Fig. 4). On day 30, addition of morcellized fragments of rectus abdominis muscle did not result in greater induction of bone compared to untreated hTGF‐β2 osteogenic devices (6, 4, 5). On day 90, specimens of 100 and 250 μg hTGF‐β2 yielded scattered islands of newly formed bone across treated defects. Mineralized bone matrix with scattered remnants of collagenous bone matrix as carrier surfaced by osteoid seams (Fig. 4i,j).
Figure 4.
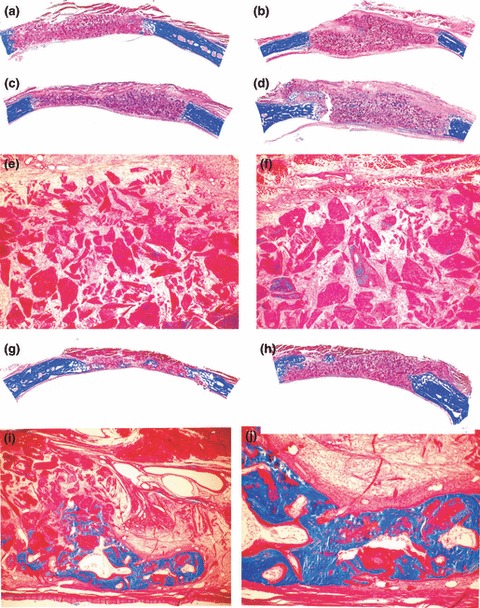
Morphology of calvarial repair and induction of bone formation by hTGF‐β2 osteogenic devices without the addition of minced fragments of autogenous rectus abdominis muscle and harvested on day 30 and 90 after implantation. Lack of bone induction in calvarial defects harvested on day 30 after implantation of 100 (a, c) and 250 (b, d) μg hTGF‐β2. Note greater surface area of the implanted osteogenic devices in specimens of 250 μg hTGF‐β2 (b, d). (e, f) Higher power views of (c) and (d) showing scattered remnants of collagenous matrix as carrier with fibrovascular invasion but lack of bone differentiation. (g, h) Calvarial specimens harvested on day 90 after implantation of 100 (g) and 250 (h) μg hTGF‐β2 showing scattered islands of newly formed and mineralized bone in blue across the treated defects. (i, j) High power views of (g) showing mineralized bone in blue covered by osteoid seams and scattered remnants of the collagenous matrix as carrier. Undecalcified sections cut at 5 μm stained free‐floating with Goldner’s trichrome. (a, b, c, d, g, h) original magnification ×2.7; (e, f) original magnification ×37; (i, j) original magnification ×75 and 25 respectively.
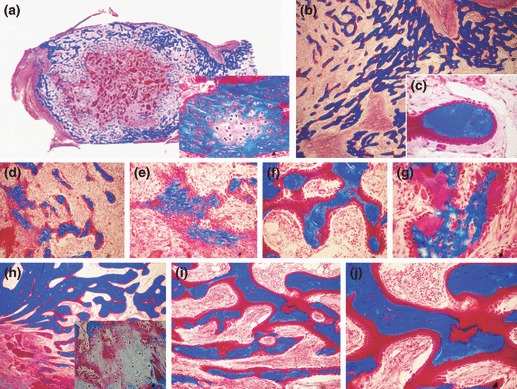
Figure 5.
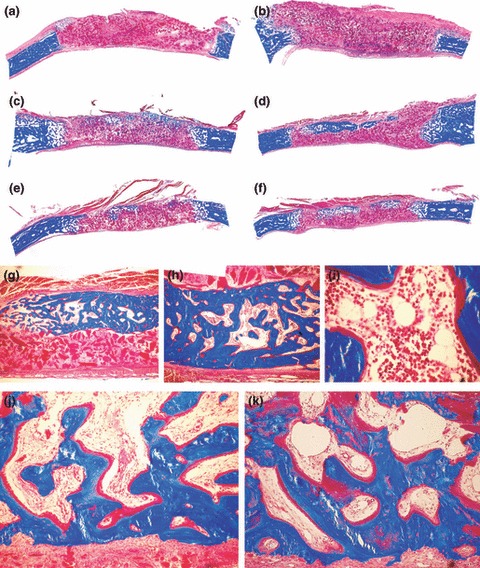
Morphology of calvarial regeneration and induction of bone formation in calvarial defects implanted with 100 and 250 μg hTGF‐β2 with the addition of morcellized fragments of autogenous rectus abdominis muscle and harvested on day 30 (a, b) and 90 (c–k) after implantation. (a, b) Lack of bone differentiation in calvarial defects implanted with 100 (a) and 250 (b) μg hTGF‐β2 showing minimal bone formation at the severed calvarial margins. (c, e, d, f) Reconstitution of the hTGF‐β2 osteogenic device with autogenous fragments of minced rectus abdominis muscle partially restores the biological activity of the hTGF‐β2 protein, resulting in the induction of large islands of mineralized bone on day 90 after implantation of 100 (c, e) and 250 μg (d, f) hTGF‐β2. (g–i) Higher power views of newly formed mineralized bone covered by osteoid seams with detail (i) of the induction of hematopoietic marrow on day 90. (j, k) Detail of mineralized trabecular bone in blue covered by osteoid seams populated by contiguous osteoblasts after the addition of morcellized fragments of autogenous rectus abdominis muscle on day 90. Undecalcified sections cut at 5 μm stained free‐floating with Goldner’s trichrome. (a–f) original magnification ×2.7; (g–i) original magnification ×25, 57, 175 respectively; (j, k) original magnification ×75.
Morcellized fragments of autologous rectus abdominis muscle with responding stem cells enhance the bone induction cascade of the hTGF‐β2 osteogenic devices
On day 90, addition of morcellized fragments of rectus abdominis muscle resulted in significantly greater induction of bone formation compared to TGF‐β2‐treated specimens without addition of minced fragments of autologous rectus abdominis muscle (6, 5). Addition of rectus abdominis muscle cells to 250 μg hTGF‐β2 calvarial specimens resulted in induction of newly formed blocks of mineralized bone across treated calvarial defects surfaced by osteoid seams, facing newly generated haematopoietic marrow (Fig. 5g–i). Restoration of bone induction cascade by morcellized fragments of rectus abdominis muscle resulted in induction of bone in sub‐pericranial location, thus in proximity to the temporalis muscle (Fig. 5), particularly when calvarial defects were implanted with 250 μg hTGF‐β2 (Fig. 5d,f). Blocks of newly induced and mineralized bone were not in a continuum across treated defects but were separated by fibrovascular tissue with scattered collagenous matrix particles. In one calvarial specimen of 250 μg hTGF‐β2, there was induction of a large island of cartilage at the periphery of newly formed and mineralized bone (not shown).
Morphometric analyses of heterotopic specimens induced by hTGF‐β2 in the rectus abdominis muscle
Volume fractions of tissue components in hTGF‐β2 heterotopic specimens harvested on day 30 and 90 are presented in Fig. 6a. Addition of minced fragments of temporalis muscle did not alter induction of bone formation as measured by morphometric analyses of mineralized bone and osteoid volumes at both time periods (Fig. 6a). On day 90, implantation of 25 μg hTGF‐β2 combined with insoluble collagenous matrix as carrier, induced a 3‐fold increase in mineralized bone and osteoid volumes when compared to ossicles induced by 5 μg hTGF‐β2 (*P < 0.05, Fig. 6a).
Morphometric analyses of calvarial specimens: effect of hTGF‐β2 and of morcellized rectus abdominis muscle
Volume fractions of tissue components in orthotopic specimens treated with 100 and 250 μg hTGF‐β2 combined with insoluble collagenous matrix are presented in Fig. 6b. Addition of morcellized fragments of rectus abdominis muscle significantly increased induction of newly formed mineralized bone and osteoid volumes on day 90 (**P < 0.05, Fig. 6b). On days 30 and 90, addition of morcellized fragments of autogenous rectus abdominis muscle to 250 μg hTGF‐β2 showed higher mineralized bone and osteoid volumes compared to untreated hTGF‐β2 osteogenic devices; however, these differences were not significant (Fig. 6b).
Northern blot analyses of heterotopic induced tissues
Intensity of expression of OP‐1 (osteogenic protein‐1/bone morphogenetic protein‐7), BMP‐3 and type IV collagen were normalized as percentages against γ‐actin intensity levels (in densitometric units). Relative densitometric units of evaluated mRNAs are presented in Fig. 7. Tissue specimens generated by 25 μg hTGF‐β2 induced higher expression of OP‐1 mRNA but not BMP‐3 on day 30; conversely on day 90, tissue specimens of 5 μg hTGF‐β2 induced higher expression of OP‐1 mRNA and equivalent expression of BMP‐3 mRNAs relative to mRNA levels induced by 25 μg hTGF‐β2 on day 90 (Fig. 7). Collagen type IV mRNAs were highly expressed at both time periods and by both doses of the hTGF‐β2 osteogenic devices correlating with the robust osteogenesis together with prominent vascular invasion with capillary sprouting, as observed morphologically at both time periods (2, 3).
Figure 7.
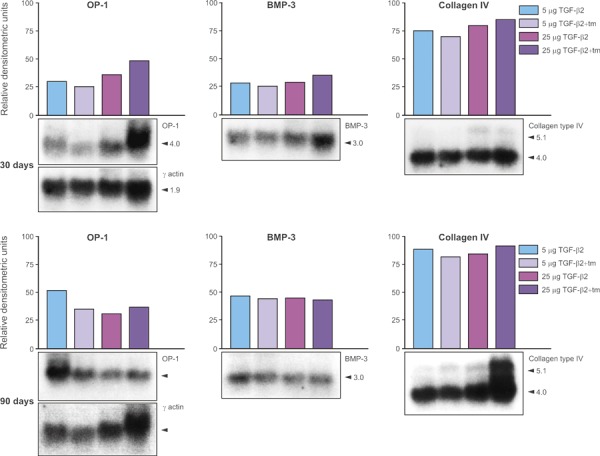
Northern analyses of mRNA expression of osteogenic protein‐1 (OP‐1), bone morphogenetic protein‐3 (BMP‐3) and collagen type IV in tissue generated by 5 and 25 μg hTGF‐β2 reconstituted with collagenous matrix as carrier and harvested on day 30 and 90 after heterotopic implantation in the rectus abdominis muscle. Samples of 100/200 mg from duplicate specimens from the rectus abdominis on day 30 and 90 were pooled and the RNA extracted. Northern blot hybridizations were carried out with radioactively labelled probes to the indicated markers (shown in panels below each histogram), and the signals were quantified relative to γ‐actin blots by densitometric analysis. mRNA levels are expressed as relative densitometric units.
Discussion
The apparent redundancy of molecular signals initiating induction of bone formation remains largely uncharacterized. We have shown that mammalian TGF‐β isoforms are determinants of induction of endochondral bone formation in non‐human primate Papio ursinus (this study; 8, 10, 13). This appears to be specific to primates. Heterotopic implantation of the hTGF‐β1 isoform in rodents induces granulation tissue only without any evidence of endochondral bone formation (11). Several other studies in rodents and lagomorphs have confirmed that heterotopic implantation of naturally derived or recombinant hTGF‐β isoforms results in induction of granulation tissue and of a fibrogenic response without cartilage or bone formation (11, 31, 32, 33, 34). Binary application of TGF‐β1 with basic fibroblast growth factor, induces persistent fibrosis in mice (35). Mammalian TGF‐β1 and β2 isoforms are implicated in cutaneous scarring in rats due to increased monocyte and macrophage infiltration with fibronectin and collagens type I and III deposition (36).
The exact mechanism by which TGF‐β signalling results in induction of endochondral bone formation in non‐human primates remains to be characterized. Current research does not as yet provide evidence that results in the non‐human primate Papio ursinus are predictive of biological activity of mammalian TGF‐β isoforms in Homo sapiens, although DNA homologies between primates are certainly higher than homologies between rodents and primates. In a number of systematic studies in the different microenvironments of heterotopic intramuscular and orthotopic craniofacial sites including the rectus abdominis muscle, the calvarium, the mandible and mandibular periodontal furcation defects, respectively, we have shown that primate tissues and microenvironments respond remarkably differently when compared to rodents, lagomorphs and canine tissues, at identical doses of the various osteogenic proteins of the TGF‐β superfamily (9, 13). In Papio ursinus and possibly thus by extension to Homo sapiens, mammalian TGF‐β isoforms do induce rapid and substantial endochondral bone formation in heterotopic sites of the rectus abdominis muscle (8, 9, 10). Mechanistically, it is of importance to elucidate why the mammalian TGF‐β isoforms have such different and critically important effects in Papio ursinus compared to rodents and lagomorphs; more importantly, however, the question is, can we extrapolate results from Papio ursinus to Homo sapiens? Although genes of chimpanzees and humans are 99% identical, regulation of human genes may be different. A recent study comparing genomes of humans, chimpanzees and rhesus macaques found that most differences were in non‐coding regulatory sequences, which control gene expression (37). Differences in gene regulation between primates during development and in response to morphogens may result in differences in sensitivity and responsiveness to TGF‐β superfamily members.
Although structurally similar, BMPs/OPs and TGF‐β receptors bind in dramatically different ways, mediating graded and switch‐like assembly mechanisms that may have co‐evolved with branch‐specific groups of cytoplasmic effectors (38). Intensity of expression of the two morphogenetic proteins studied, OP‐1 and BMP‐3, did not vary significantly according to doses of implanted hTGF‐β2 or to time periods evaluated; similarly, mRNA expression of type IV collagen is highly and equally expressed at both time periods with no correlation with the μg doses of implanted hTGF‐β2 in the rectus abdominis muscle. Induction of large osteoid seams at both time periods by both μg doses of the recombinant morphogen may explain similar expression patterns of OP‐1 and BMP‐3. Prominent vascular invasion in heterotopic constructs generated by both doses of the hTGF‐β2 isoform may also explain the similar if not equal expression patterns of collagen type IV, pre‐dating the prominent angiogenesis within de novo‐generated corticalized constructs.
In parallel experiments, we have shown that consistent and rather limited induction of bone formation in orthotopic calvarial defects implanted with hTGF‐β3 osteogenic devices is due to the influence of Smad‐6 and ‐7 down‐stream antagonists of the TGF‐β signalling pathway (10). RT‐PCR analyses of newly formed ossicles generated by the hTGF‐β3 isoform have shown robust expression of Smad‐6 and ‐7 in orthotopic calvarial sites with limited expression in heterotopic rectus abdominis ones (10). Our morphological and molecular studies have suggested that Smad‐6 and ‐7 over‐expression in hTGF‐β3‐treated calvarial defects may be due to vascular endothelial tissue of arachnoids expressing signalling proteins, which modulate expression of inhibitory Smads in pre‐osteoblastic and osteoblastic cell lines, thus controlling induction of bone in the primate calvarium (10).
The acid test of the phenomenon of ‘bone: formation by autoinduction’ (39) is de novo generation of heterotopic bone after extraskeletal implantation of osteogenic molecular signals of the TGF‐β superfamily (13) combined with complementary substrata; a molecular signal labelled as osteoinductive must thus be endowed with the striking prerogative of initiating endochondral bone formation in heterotopic extraskeletal sites of animal models (9, 10, 23, 39). Conclusively, in bioassay for bone induction in Papio ursinus, the mammalian TGF‐β isoforms do initiate induction of bone formation (6, 7, 8, 9, 10, 11). TGF‐β isoforms may induce expression of BMP/OP‐related gene products, ultimately resulting in induction of bone formation. Molecular analyses of tissues generated by mammalian TGF‐β isoforms shows expression of BMP‐3 and OP‐1 as evaluated by Northern blot analyses and RT‐PCR (this study; 8, 10). This underlies the importance of BMPs/OP pathways in induction of de novo heterotopic bone formation.
Our working hypothesis is that TGF‐β signalling induces endochondral bone differentiation by regulating Noggin expression and, therefore, BMP/OP activity (38, 40). If these molecular and cellular scenarios are correct, addition of Noggin together with a mammalian TGF‐β isoform would inhibit osteogenic activity of expressed and secreted proteins resulting in limited and/or no bone formation by induction. Alternatively, although unlikely, mammalian TGF‐β isoforms initiate induction of endochondral bone formation via a novel non‐BMP/OP pathway and in primates only.
Pericytes are ubiquitous sub‐endothelial cells; they are elongated cells with primary and secondary processes encircling the capillary at right angles and are embedded in and sharing a common basement membrane with overlying endothelial cells (41, 42). Current evidence indicates that pericytes may be competent for generation of a variety of mesenchymal tissues including skeletal muscle (43, 44) and osteoblasts (45). This suggests the existence of perivascular progenitor stem cells and a potential perivascular stem cell niche (42). The presence of such a niche in adult rectus abdominis muscles is additionally supported by recent identification of myoendothelial cells in human skeletal muscle (20). Both pericytes and myoendothelial cells may respond to osteogenic proteins of the TGF‐β superfamily differentiating and expanding into osteoblastic cells initiating induction of bone formation as a secondary response (9, 46, 47). Muscle‐derived stem cells including myoendothelial cells possess high myogenic (48) and osteogenic (20) capacity which depends on induction of specific microenvironments (19, 48) providing promising approaches for treatment of musculoskeletal disorders.
Soluble and insoluble signals modulating responding stem cells is the basic tissue engineering paradigm for tissue induction and morphogenesis (9, 23). Apparent redundancy of molecular signals also, including TGF‐β isoforms initiating induction of endochondral bone formation, in primate species needs further clarification. A concerted effort should now be devoted to evaluate recombinant hTGF‐β proteins both singly or in synergistic binary applications with recombinant human osteogenic proteins to morphologically and mechanistically initiate induction of bone formation in clinical contexts.
Acknowledgements
This study was supported by the South African Medical Research Council, the University of the Witwatersrand, Johannesburg, the National Research Foundation and ad hoc grants of the Bone Research Laboratory. We thank Genzyme Corporation for supplying the recombinant hTGF‐β2, Thato Matsaba for the Northern blot analyses, Louise Renton and June Teare for the undecalcified sections of the newly formed ossicles, the Central Animal Services of the University for the help with primate experimentation.
References
- 1. Cabiling DS, Kim E, Yan D, Jacob S, Nah HD, Kirschner RE (2007) Differential effects of TGF‐beta isoforms on murine fetal dural cells and calvarial osteoblasts. Plast. Reconstr. Surg. 120, 614–624. [DOI] [PubMed] [Google Scholar]
- 2. Jenkins G (2008) The role of proteases in transforming growth factor‐β activation. Int. J. Biochem. Cell Biol. 40, 1068–1078. [DOI] [PubMed] [Google Scholar]
- 3. Massagué J (2000) How cells read TGF‐beta signals. Nat. Rev. Mol. Cell Biol. 1, 169–178. [DOI] [PubMed] [Google Scholar]
- 4. Guo X, Wang XF (2009) Signaling cross‐talk between TGF‐beta/BMP and other pathways. Cell Res. 19, 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balooch G, Balooch M, Nalla RK, Schilling S, Filvaroff EH, Marshall GW et al. (2005) TGF‐β regulates the mechanical properties and composition of bone matrix. Proc. Natl. Acad. Sci. USA 102, 18813–18818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ripamonti U, Duneas N, Van Den Heever B, Bosch C, Crooks J (1997) Recombinant transforming growth factor‐β1 induces endochondral bone in the baboon and synergizes with recombinant osteogenic protein‐1 (bone morphogenetic protein‐7) to initiate rapid bone formation. J. Bone Miner. Res. 2, 1584–1595. [DOI] [PubMed] [Google Scholar]
- 7. Duneas N, Crooks J, Ripamonti U (1998) Transforming growth factor‐β1: induction of bone morphogenetic protein gene expression during endochondral bone formation in the baboon, and synergistic interaction with osteogenic protein‐1 (BMP‐7). Growth Factors 15, 259–277. [DOI] [PubMed] [Google Scholar]
- 8. Ripamonti U, Crooks J, Matsaba T, Tasker J (2000) Induction of endochondral bone formation by recombinant human transforming growth factor‐β2 in the baboon (Papio ursinus). Growth Factors 17, 269–285. [DOI] [PubMed] [Google Scholar]
- 9. Ripamonti U (2006) Soluble osteogenic molecular signals and the induction of bone formation. Biomaterials 27, 807–822. [DOI] [PubMed] [Google Scholar]
- 10. Ripamonti U, Ramoshebi LN, Teare J, Renton L, Ferretti C (2008) The induction of endochondral bone formation by transforming growth factor‐β3: experimental studies in the non‐human primate Papio ursinus . J. Cell. Mol. Med. 12, 1029–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM et al. (1986) Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro . Proc. Natl. Acad. Sci. USA 83, 4167–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sena K, Sumner DR, Virdi AS (2010) Effect of recombinant human transforming growth factor‐β2 dose on bone formation in rat femur titanium implant model. J. Biomed. Mater. Res. A 92, 1210–1217. [DOI] [PubMed] [Google Scholar]
- 13. Ripamonti U (2003) Osteogenic proteins of the TGF‐β superfamily In: Henry HL, Norman AW, eds. Encyclopedia of Hormones, pp. 80–86. Boston: Academic Press. [Google Scholar]
- 14. Kishigami S, Mishina Y (2005) BMP signalling and early embryonic patterning. Cytokine Growth Factor Rev. 16, 265–278. [DOI] [PubMed] [Google Scholar]
- 15. Hogan BLM (1996) Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10, 1580–1594. [DOI] [PubMed] [Google Scholar]
- 16. Ripamonti U, Petit J‐C, Teare J (2009) Cementogenesis and the induction of periodontal tissue regeneration by the osteogenic proteins of the transforming growth factor‐β superfamily. J. Periodontal Res. 44, 141–152. [DOI] [PubMed] [Google Scholar]
- 17. Thomadakis G, Ramoshebi LN, Crooks J, Ripamonti U (1999) Immunolocalization of bone morphogenetic protein2 and ‐3 and osteogenic protein‐1 during murine tooth root morphogenesis and in other craniofacial structures. Eur. J. Oral Sci. 107, 368–377. [DOI] [PubMed] [Google Scholar]
- 18. Lensch MW, Daheron L, Schlaeger TM (2006) Pluripotent stem cells and their niches. Stem Cell Rev. 2, 185–201. [DOI] [PubMed] [Google Scholar]
- 19. Ripamonti U, Crooks J, Khoali L, Roden L (2009) The induction of bone formation by coral‐derived calcium carbonate/hydroxyapatite constructs. Biomaterials 30, 1428–1439. [DOI] [PubMed] [Google Scholar]
- 20. Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A et al. (2007) Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat. Biotechnol. 25, 1025–1034. [DOI] [PubMed] [Google Scholar]
- 21. Sampath TK, Reddi AH (1981) Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc. Natl. Acad. Sci. USA 78, 7599–7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sampath TK, Reddi AH (1983) Homology of bone‐inductive proteins from human, monkey, bovine and rat extracellular matrix. Proc. Natl. Acad. Sci. USA 80, 6591–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reddi AH (2000) Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 6, 351–359. [DOI] [PubMed] [Google Scholar]
- 24. Ripamonti U, Reddi AH (1995) Bone morphogenetic proteins: applications in plastic and reconstructive surgery. Adv. Plast. Reconstr. Surg. 11, 47–73. [Google Scholar]
- 25. Schnitzler CM, Ripamonti U, Mesquita JM (1993) Histomorphometry of iliac crest trabecular bone in adult male baboons in captivity. Calcif. Tissue Int. 52, 447–454. [DOI] [PubMed] [Google Scholar]
- 26. Ripamonti U (1991) Bone induction in nonhuman primates. An experimental study on the baboon (Papio ursinus). Clin. Orthop. Relat. Res. 269, 284–294. [PubMed] [Google Scholar]
- 27. Public Service Department (1990) National Code for Animal Use in Research, Education, Diagnosis and Testing of Drugs and Related Substances in South Africa. Pretoria, South Africa: Public Service Department. [Google Scholar]
- 28. Ripamonti U, Ferretti C, Teare J, Blann L (2009) The transforming growth factor‐β isoforms and the induction of bone formation: implications for reconstructive craniofacial surgery. J. Craniofac. Surg. 20, 1544–1555 [DOI] [PubMed] [Google Scholar]
- 29. Parfitt AM (1983). Bone Histomorphometry: Techniques and Interpretation. Stereologic basis of bone histomorphometry; theory of quantitative microscopy and reconstruction of the third dimension In: Recker RR, ed. Boca Raton, pp. 53–87. Florida: CRC Press. [Google Scholar]
- 30. Ripamonti U, Parak R, Petit J‐C (2009) Induction of cementogenesis and periodontal ligament regeneration by recombinant human transforming growth factor‐β3 in Matrigel with rectus abdominis responding cells. J. Periodontal Res. 44, 81–87. [DOI] [PubMed] [Google Scholar]
- 31. Saadeh PB, Mehrara BJ, Steinbrech DS, Dudziak ME, Spector JA, Greenwald JA et al. (1999) Transforming growth factor‐β1 modulates the expression of vascular endothelial growth factor by osteoblasts. Am. J. Physiol. 277, 628–637. [DOI] [PubMed] [Google Scholar]
- 32. Sampath TK, Muthukumaran N, Reddi AH (1987) Isolation of osteogenin, an extracellular matrix‐associated bone inductive protein, by heparin affinity chromatography. Proc. Natl. Acad. Sci. USA 84, 7109–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hammonds GR, Schwall R, Dudley A, Berkemeier L, Lai C, Lee J et al. (1991) Bone‐ inducing activity of mature BMP‐2b produced from a hybrid BMP‐2a/2b precursor. Mol. Endocrinol. 5, 149–155. [DOI] [PubMed] [Google Scholar]
- 34. Matsaba T, Ramoshebi LN, Crooks J, Ripamonti U (2001) Transforming growth factor‐ß1 supports the rapid morphogenesis of heterotopic endochondral bone initiated by human osteogenic protein‐1 via the synergistic upregulation of molecular markers. Growth Factors 19, 73–86. [DOI] [PubMed] [Google Scholar]
- 35. Shinozaki M, Kawara S, Hayashi N, Kakinuma T, Igarashi A, Takehara K et al. (1997) Induction of subcutaneous tissue fibrosis in newborn mice by transforming growth factor β1– Simultaneous application with basic fibroblast growth factor causes persistent fibrosis. Biochem. Biophys. Res. Commun. 240, 292–297. [PubMed] [Google Scholar]
- 36. Shah M, Foreman DM, Ferguson MWJ (1995) Neutralization of TGF‐β1 and TGF‐β2 or exogenous addition of TGF‐β3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 108, 985–1002. [DOI] [PubMed] [Google Scholar]
- 37. Haygood R, Fedrigo O, Hanson B, Yokoyama K‐D, Wray GA (2007) Promoter regions of many neural‐ and nutrition‐related gene have experienced positive selection during human evolution. Nat. Genet. 39, 1140–1144. [DOI] [PubMed] [Google Scholar]
- 38. Groppe J, Greenwald J, Wiater E, Rodriguez‐Leon J, Economides AN, Kwiatkowski W et al. (2003) Structural basis of BMP signalling inhibition by noggin, a novel twelve‐membered cystine knot protein. J. Bone Joint Surg. 85‐A, 52–58. [DOI] [PubMed] [Google Scholar]
- 39. Urist MR (1965) Bone: formation by autoinduction. Science 150, 893–899. [DOI] [PubMed] [Google Scholar]
- 40. Gazzerro E, Canji V, Canalis E (1998) Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J. Clin. Invest. 102, 2106–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rouget C (1879) Sur la contractilité des capillairs sanguins. C. R. Acad. Sci. 88, 916–918. [Google Scholar]
- 42. Kovacic JC, Boehm M (2009) Resident vascular progenitor cells: an emerging role for non‐terminally differentiated vessel‐resident cells in vascular biology. Stem Cell Res. 2, 2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L et al. (2007) Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9, 255–267. [DOI] [PubMed] [Google Scholar]
- 44. Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T et al. (2007) Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 15, 867–877. [DOI] [PubMed] [Google Scholar]
- 45. Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE et al. (1998) Vascular pericytes express osteogenic potential in vitro and in vivo . J. Bone Miner. Res. 13, 828–838. [DOI] [PubMed] [Google Scholar]
- 46. Ripamonti U, Richter PW, Nilen RWN, Renton L (2008) The induction of bone formation by smart biphasic hydroxyapatite tricalcium phosphate biomimetic matrices in the non‐human primate Papio ursinus . J. Cell. Mol. Med. 12, 2609–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ripamonti U (2008c) Biomimetism, biomimetic matrices and the induction of bone formation. J. Cell. Mol. Med. 13, 2953–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burdick J, Vunjak‐Novakovic G (2009) Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. 15, 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]


