Human fungal infections are a growing problem associated with increased morbidity and mortality. Moreover, a growing number of antifungal-resistant fungal isolates have been reported over the past decade. Thus, the need for novel antifungal agents is imperative. In this study, we show that an endophytic bacterium, Burkholderia gladioli, isolated from the medicinal plant Lycoris aurea, is able to abundantly secrete a compound, toxoflavin, which has a strong fungicidal activity not only against plant fungal pathogens but also against human fungal pathogens Aspergillus fumigatus and Candida albicans, Cryptococcus neoformans, and the model filamentous fungus Aspergillus nidulans. More importantly, toxoflavin also displays an efficacious inhibitory effect against azole antifungal-resistant mutants of A. fumigatus. Consequently, our findings provide a promising approach to abundantly produce toxoflavin, which has novel broad-spectrum antifungal activity, especially against those currently problematic drug-resistant isolates.
KEYWORDS: Aspergillus, Burkholderia gladioli, antifungal, endophytic bacterium, fungal pathogens, toxoflavin
ABSTRACT
Fungal infections not only cause extensive agricultural damage but also result in serious diseases in the immunodeficient populations of human beings. Moreover, the increasing emergence of drug resistance has led to a decrease in the efficacy of current antifungals. Thus, screening of new antifungal agents is imperative in the fight against antifungal drug resistance. In this study, we show that an endophytic bacterium, Burkholderia gladioli HDXY-02, isolated from the medicinal plant Lycoris aurea, showed broad-spectrum antifungal activity against plant and human fungal pathogens. An antifungal ability assay indicated that the bioactive component was produced from strain HDXY-02 having an extracellular secreted component with a molecular weight lower than 1,000 Da. In addition, we found that this new antifungal could be produced effectively by liquid fermentation of HDXY-02. Furthermore, the purified component contributing to the antifungal activity was identified to be toxoflavin, a yellow compound possessing a pyrimido[5,4-e][1,2,4]triazine ring. In vitro bioactivity studies demonstrated that purified toxoflavin from B. gladioli HDXY-02 cultures had a significant antifungal activity against the human fungal pathogen Aspergillus fumigatus, resulting in abolished germination of conidia. More importantly, the growth inhibition by toxoflavin was observed in both wild-type and drug-resistant mutants (cyp51A and non-cyp51A) of A. fumigatus. Finally, an optimized protocol for the large-scale production of toxoflavin (1,533 mg/liter) has been developed. Taken together, our findings provide a promising biosynthetic resource for producing a new antifungal reagent, toxoflavin, from isolates of the endophytic bacterium B. gladioli.
IMPORTANCE Human fungal infections are a growing problem associated with increased morbidity and mortality. Moreover, a growing number of antifungal-resistant fungal isolates have been reported over the past decade. Thus, the need for novel antifungal agents is imperative. In this study, we show that an endophytic bacterium, Burkholderia gladioli, isolated from the medicinal plant Lycoris aurea, is able to abundantly secrete a compound, toxoflavin, which has a strong fungicidal activity not only against plant fungal pathogens but also against human fungal pathogens Aspergillus fumigatus and Candida albicans, Cryptococcus neoformans, and the model filamentous fungus Aspergillus nidulans. More importantly, toxoflavin also displays an efficacious inhibitory effect against azole antifungal-resistant mutants of A. fumigatus. Consequently, our findings provide a promising approach to abundantly produce toxoflavin, which has novel broad-spectrum antifungal activity, especially against those currently problematic drug-resistant isolates.
INTRODUCTION
The incidence of invasive fungal infections (IFIs) increases with each passing year; immunocompromised patients are at a higher risk of IFIs due to their substantial morbidity and mortality (1, 2). However, the currently available antifungal agents used in clinical practice are limited; echinocandins, polyenes, azoles, and flucytosine are antifungal drugs currently available for the treatment of IFIs (3). Particularly, azole antifungals are most extensively used for the treatment of infections; expectedly, azole-resistant clinical isolates of Aspergillus fumigatus have been isolated from patients worldwide (4–7). The increase in incidence and the occurrence of drug resistance pose a challenge to the limited antifungal drugs currently available in the clinic. Consequently, it is imperative to develop new antifungal agents.
In recent years, a series of new natural compounds with effective bioactivity and fewer side effects have been found, thus providing a new direction for antimicrobial drug discovery (8, 9). For example, natural compounds with the antifungal activity against biofilms produced by Candida species have been verified (10). Moreover, the natural compounds berberine and alkaloids, both with antimicrobial abilities, have been isolated from medicinal plants and used in clinics (11). Since a previous study identified that paclitaxel, isolated from Taxus brevifolia, was produced by an endophyte (12), endophytic microbes have been explored for the production of antibiotics and other bioactive drugs or materials (13, 14).
As a natural member of the plant microecosystem, endophytes, especially the medicinal plant endophytes, can produce rich and diverse natural products, including terpenoids, alkaloids, steroids, anthraquinones, cyclopeptides, and flavonoids, and some of these compounds have been shown to have antimicrobial activities (15, 16). Lycoris plants are of important medicinal value since they can accumulate alkaloids with pharmacological activities, including anticancer and antiviral effects (17, 18). Antimicrobial activities of alkaloids from Lycoris species have also been discovered. For example, alkaloids, including lycorine, amarbellisine, hippeastrine, and pancracine, have antifungal activities against Candida albicans or Staphylococcus aureus (11).
While considering Lycoris plants as a potential source of endophytes with the ability to produce novel natural products, there is a lack of information about the antifungal abilities of endophytic bacteria in Lycoris plants. Therefore, the aim of this study was to screen endophytic bacteria with antagonistic effects against fungal pathogens from Lycoris radiata (L′Her.) Herb., Lycoris aurea (L′Hér.) Herb. and Lycoris chinensis Traub. Finally, as expected, we found that Burkholderia gladioli HDXY-02 isolated from L. aurea has strong antagonistic activities against all tested fungal pathogens. The antibiotic produced by HDXY-02 that contributes to its antifungal activity was toxoflavin (PKF118-310), which possesses the pyrimido[5,4-e][1,2,4]triazine ring (also known as xanthothricin) and has considerable antibiotic activity (19–21) and antitumor properties (22–24). More importantly, the result showed that purified toxoflavin from B. gladioli HDXY-02 fermentation broth has a remarkable antifungal activity against all tested species, including azole drug-susceptible and -resistant A. fumigatus species. In addition, a high-yield production scheme for toxoflavin from B. gladioli HDXY-02 was also developed in this study.
RESULTS
Isolation of strain HDXY-02 having broad-spectrum antifungal activities.
To screen the antifungal activities of endophytic bacteria isolated from Lycoris plants (L. aurea, L. radiata, and L. chinensis), 110 morphologically distinct endophytic bacteria were tested for their antagonistic activities against selected fungal pathogens. Thirty endophytic bacteria (Fig. 1A) were found to give a clear zone of growth inhibition against the test fungi using a dual-culture assay as described in Materials and Methods. Among purified isolates, strain HDXY-02, isolated from L. aurea, exhibited the highest antifungal activity against tested pathogens, including Magnaporthe oryzae, Rhizoctonia solani, Fusarium graminearum, and A. fumigatus (Fig. 1B). To explore whether the antifungal ability of strain HDXY-02 was due to an extracellular secreted substance, further experimentation was carried out, as demonstrated in the schematic illustration in Fig. 1C. The result showed that an extracellular secretion of strain HDXY-02 with a molecular weight lower than 1,000 Da was responsible for the strong growth inhibition of A. fumigatus (Fig. 1D). Consequently, strain HDXY-02 was used throughout the rest of the study.
FIG 1.
Antagonistic activities of endophytic bacteria isolated from Lycoris plants. (A) Endophytic bacteria with antifungal abilities, including strain HDXY-02. (B) Dual-culture assay of strain HDXY-02 against M. oryzae (a), R. solani (b), F. graminearum (c), and A. fumigatus (d). Arrows indicate where strain HDXY-02 was spotted. (C) Schematic diagram of the experimental setup. Semipermeable membranes (MWCO, 1 and 3.5 kDa) were placed on the solid medium. Then, either an overnight culture of strain HDXY-02 or sterilized LB broth was added onto the membrane and cultured for another 16 h. Finally, the membrane was taken away and A. fumigatus was spotted onto the solid medium and cultured at 37°C for 24 h. (D) Inhibitory verification of extracellular secretions of strain HDXY-02 against A. fumigatus Af293 with a molecular weight smaller than 1,000 Da.
Growth profiles of strain HDXY-02.
Strain HDXY-02 could grow at all temperatures tested (16, 25, 30, and 37°C), with optimum growth at 30°C (see Fig. S1 in the supplemental material). The growth of HDXY-02 on Luria-Bertani (LB) agar plates was suppressed by kanamycin (25 µg/ml) and chloramphenicol (34 µg/ml). Resistance was observed for tetracycline (50 µg/ml), streptomycin (25 µg/ml), and ampicillin (100 µg/ml).
Species identification for strain HDXY-02 as B. gladioli.
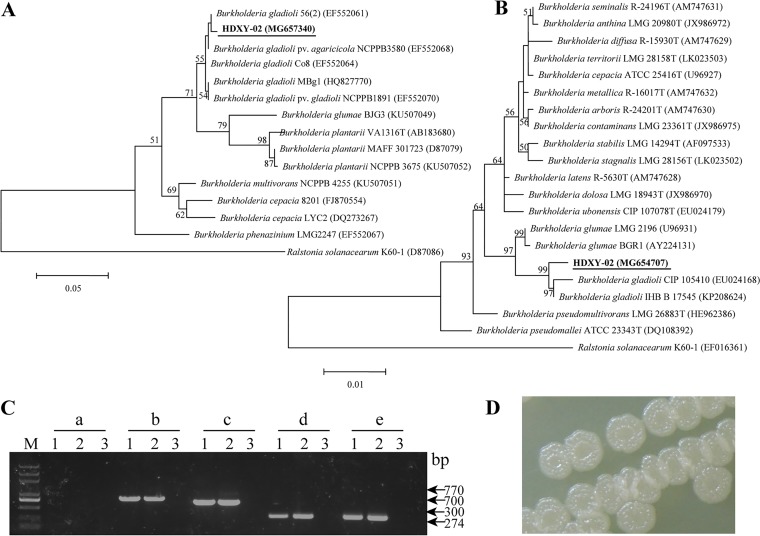
Cells of strain HDXY-02 were Gram-negative rod-shaped bacterial cells of size 1.0 to 3.0 μm by 0.5 to 1.0 μm. Partial 16S rRNA gene and 16S-23S spacer region sequences of strain HDXY-02 showed high similarities ranging from 99 to 100% to the sequences of other B. gladioli strains. Phylogenetic analyses demonstrated that HDXY-02 was closely related to other B. gladioli strains (Fig. 2A and B).
FIG 2.
Species identification for strain HDXY-02 as B. gladioli. (A) Phylogenetic tree based on 16S-23S rRNA gene spacer sequences of Burkholderia species. (B) Phylogenetic tree based on partial 16S rRNA gene of Burkholderia species; Ralstonia solanacearum K60-1 was used as an outgroup. GenBank accession numbers are indicated in brackets. Bootstrap values of >50% are shown. (C) Analytical PCR with five pairs of primers, Bglu-F/toxA-R2 (a), Bgla-F/toxA-R2 (b), LP1/LP4 (c), GLA-F/GLA-R (d), and PG1/PG2 (e), in strain HDXY-02 (lanes 1 and 2) and water blank (lanes 3). M, DNA marker. The sizes of PCR products are indicated on the right. (D) Colony morphology of B. gladioli HDXY-02.
As shown in Fig. 2C, PCR amplification with the primers Bglu-F/toxA-R2 (Burkholderia glumae-specific) yielded no products when performed with strain HDXY-02 as a template, while a product of approximately 770 bp was shown using Bgla-F/toxA-R2 (B. gladioli-specific) as primers (25). Moreover, PCR products of the expected size (the sequences were verified by sequencing) for strain HDXY-02 were amplified with the B. gladioli-specific primer pairs GLA-F/GLA-R (26), LP1/LP4 (27), and PG1/PG2 (28). B. gladioli HDXY-02 showed a wrinkled colony phenotype at 30°C (Fig. 2D).
Based on the morphological, biochemical (Table 1), and phylogenetic analyses, strain HDXY-02 was identified as B. gladioli.
TABLE 1.
Physiological and biochemical characteristics of strain HDXY-02
| Characteristic | Finding in strain HDXY-02a |
|---|---|
| Gelaune liquefaction | + |
| Arginine dihydrolase production | − |
| Hydrolysis of starch | − |
| Indole production | − |
| Acidified glucose | − |
| Utilization of: | |
| d-Glucose | + |
| d-Mannose | + |
| N-Acetylglucosamine | + |
| d-Mannitol | + |
| Malate | + |
| Gluconate | + |
+, positive or can be used; −, negative or cannot be used.
Purification and characterization of toxoflavin from B. gladioli HDXY-02.
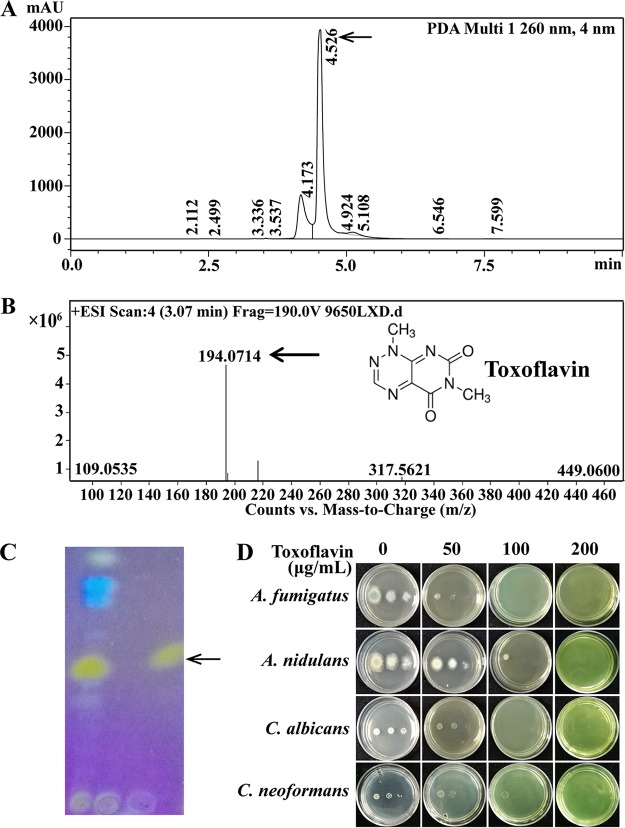
The chloroform and dichloromethane extracts of B. gladioli HDXY-02 showed high activities against the tested fungi. As a result, dichloromethane was chosen as the suitable solvent. After column chromatography, fractions with antifungal abilities were collected. The results of high-pressure liquid chromatography (HPLC) and thin-layer chromatography (TLC) also showed that strain HDXY-02 could produce the yellow pigment toxoflavin, which was visible to the naked eye and more vivid under UV light illumination (Fig. 3A and C). Analysis by high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) confirmed the production of the azapteridine toxoflavin (m/z 194.0714, [M+H]+) by B. gladioli HDXY-02 (Fig. 3B). After crystallization from 1-propanol, toxoflavin with 95% purity (HPLC) was obtained.
FIG 3.
Structural analysis and characterization of the main active antimicrobial ingredient produced by strain HDXY-02. (A) HPLC analysis of extraction separated from strain HDXY-02 culture broth. (B) Identification of toxoflavin with MALDI-Q-TOF-MS. (C) TLC analysis under UV light at 365 nm. (D) Antimicrobial assay. Serial dilutions of 1 μl of 10-fold conidia (107 to 105) of A. fumigatus, A. nidulans, C. albicans, and C. neoformans were spotted onto solid medium containing various toxoflavin concentrations (0 to 200 μg/ml).
The inhibitory action of purified toxoflavin against human fungal pathogens A. fumigatus, C. albicans, Cryptococcus neoformans, and the fungal model organism Aspergillus nidulans was further tested and presented in Fig. 3D, which showed that purified toxoflavin from B. gladioli HDXY-02 cultures had an effective inhibitory action against all test strains. The MICs and minimum fungicidal concentrations (MFCs) are shown in Table 2. The MICs of toxoflavin against M. oryzae, R. solani, and F. graminearum were determined to be between 128 and 256 μg/ml, which was comparable to the findings of an antifungal activity assay of B. gladioli HDXY-02 studied by dual-culture assay. Notably, the MIC and MFC values for toxoflavin against A. fumigatus were 64 and 128 μg/ml, respectively, in the aforementioned assay. Subsequently, we wondered whether the purified toxoflavin had any antifungal activities against azole antifungal-resistant isolates. Two azole-resistant mutants, WX01:AfCox10243Q and WX03:AfCyp51AF219L (29), were selected. Similar to that of the wild-type A. fumigatus A1160, a comparable inhibitory activity (MIC = 64 μg/ml) of toxoflavin against these two resistant mutants was observed (Table 2). These data suggest that toxoflavin might have a fungal-inhibition mechanism different from that of traditional azole antifungals.
TABLE 2.
MICs and MFCs of toxoflavin isolated from the culture filtrate of strain HDXY-02 against several fungi
| Species | Strain | Concn (μg/ml) |
|
|---|---|---|---|
| MIC | MFC | ||
| M. oryzae | HN13-2-1-1 | 128 | 128 |
| R. solani | RS01 | 128 | 256 |
| F. graminearum | 2021 | 256 | >256 |
| A. fumigatus | Af293 (FGSC A1100) | 64 | 128 |
| A. fumigatus | FGSC A1160 | 64 | 128 |
| A. fumigatus | AfCox10243Q (WX01) | 64 | 128 |
| A. fumigatus | AfCyp51AF219L (WX03) | 64 | 128 |
| A. nidulans | LB01 (FGSC A118) | 128 | 256 |
| C. albicans | ATCC 10231 | 64 | 256 |
| C. neoformans | H99 (ATCC 208821) | 128 | 128 |
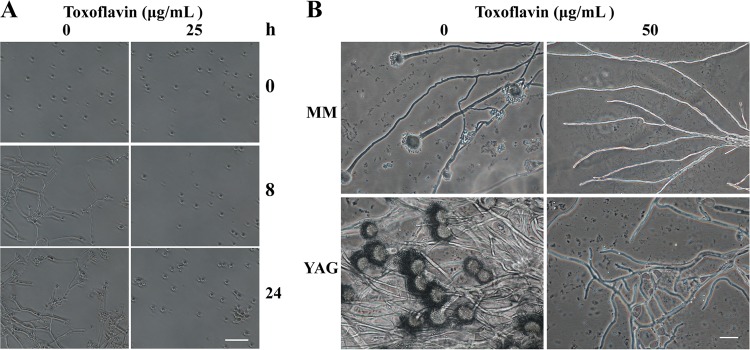
To characterize the effects of toxoflavin on A. fumigatus Af293, a microscopy study was carried out. Spores of A. fumigatus Af293 began to germinate in minimal medium (MM) broth without toxoflavin after incubation for 8 h at 37°C, and the percentage of germination was approximately 100%. In comparison, spores incubated in MM broth with toxoflavin (25 μg/ml) were unable to germinate after culture for 24 h (Fig. 4A). Further microscopic observations indicated that no detectable conidiogenous structure of A. fumigatus Af293 existed when grown on MM or yeast extract agar glucose (YAG) solid plates suspended with purified toxoflavin (50 μg/ml) after culture for 24 h (Fig. 4B).
FIG 4.
Antifungal activity of toxoflavin on A. fumigatus Af293. (A) Time course of spore germination of Af293. Scale bar, 50 μm. (B) Hyphal microscopic observations. Scale bar, 20 μm.
Optimization of medium components for toxoflavin production.
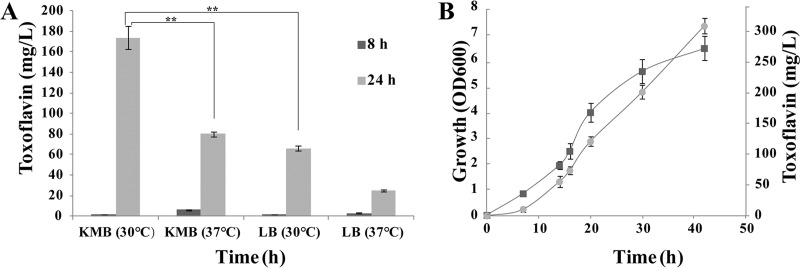
LB and King’s B medium (KMB) have been routinely used for toxoflavin production of Burkholderia spp. (30–32). Thus, LB medium and KMB was chosen for further testing. As shown in Fig. 5A, toxoflavin production by B. gladioli HDXY-02 was observed to be much higher in the KMB broth (170 mg/liter) than in LB broth after 24 h of cultivation at 30°C. Therefore, KMB was selected for further medium optimization. The result suggests that toxoflavin production correlates with the growth curve of the bacterium HDXY-02 (Fig. 5B).
FIG 5.
Production of toxoflavin under different fermentation conditions. (A) Toxoflavin production of strain HDXY-02 in KMB and LB medium broth at different temperatures. (B) Time course of growth and toxoflavin production about strain HDXY-02. Squares, growth; circles, toxoflavin production.
Twelve medium components, including glucose, glycerol, peptone, MgSO4, NaCl, aspartic acid, phenylalanine, tyrosine, MnCl2, CaCl2, FeSO4, and K2HPO4, were observed to have a significant influence on toxoflavin production by B. gladioli HDXY-02 (Fig. S2). These 12 components were further tested with the Plackett-Burman design (PBD) to observe their significance on toxoflavin production (Table S1). As shown in a Pareto chart (Fig. S3), glucose, peptone, and phenylalanine had significant impacts on toxoflavin yield. The effects of glucose, peptone, and phenylalanine were (+) 193.4, 269.0, and 181.0, respectively, suggesting that glucose, peptone, and phenylalanine were the most significant factors (P < 0.05) affecting toxoflavin yield by B. gladioli HDXY-02 (Table 3).
TABLE 3.
Statistical analysis of Plackett-Burman design for each factor on toxoflavin productiona
| Parameter | Effect | Coefficient | SE | t | P |
|---|---|---|---|---|---|
| Constant | 737.7 | 30.6 | 24.08 | 0.000* | |
| Glucose | 193.4 | 96.7 | 30.6 | 3.16 | 0.016* |
| Glycerol | –74.4 | –37.2 | 30.6 | –1.21 | 0.264 |
| Peptone | 269.0 | 134.5 | 30.6 | 4.39 | 0.003* |
| NaCl | 24.0 | 12.0 | 30.6 | 0.39 | 0.707 |
| MgSO4 | 44.2 | 22.1 | 30.6 | 0.72 | 0.494 |
| Tyrosine | –70.4 | –35.2 | 30.6 | –1.15 | 0.288 |
| Aspartic acid | –10.4 | –5.2 | 30.6 | –0.17 | 0.870 |
| Phenylalanine | 181.0 | 90.5 | 30.6 | 2.95 | 0.021* |
| MnCl2 | –14.8 | –7.4 | 30.6 | –0.24 | 0.816 |
| CaCl2 | –103.4 | –51.7 | 30.6 | –1.69 | 0.135 |
| FeSO4 | –12.8 | –6.4 | 30.6 | –0.21 | 0.840 |
| K2HPO4 | 100.2 | 50.1 | 30.6 | 1.64 | 0.146 |
*, R2 = 87.06%; R2 (adjusted) = 64.88%.
In this work, peptone, glucose, and phenylalanine were further optimized using the Box-Behnken design (BBD). Three levels for glucose, peptone, and phenylalanine are presented in Table S2. The experimental responses and the predicted response of 15 runs are shown in Table 4. The main effect plot and interaction plot (Fig. S4 and S5) of toxoflavin production are presented. Response surface curves are shown in Fig. S6. These results show that glucose, the quadratic terms of glucose, peptone, and the quadratic terms of phenylalanine had significant (P < 0.05) effects on toxoflavin production using a t test and P value (Table 5). The regression model is as follows:
where Y, X1, X2, and X3 are the predicted yields of toxoflavin (mg/liter), glucose (g/liter), peptone (g/liter), and phenylalanine (g/liter), respectively.
TABLE 4.
Experimental design of Box-Behnken design for coded variables and predicted results
| Trial | Coded level |
Real level (g/liter) |
Toxoflavin production (mg/liter) |
|||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | X1 | X2 | X3 | Actual | Predicted | |
| 1 | –1 | 0 | –1 | 20 | 18 | 0.25 | 1,019 | 1,033.5 |
| 2 | –1 | –1 | 0 | 20 | 15 | 0.5 | 1,073 | 1,010 |
| 3 | –1 | 1 | 0 | 20 | 21 | 0.5 | 1,244 | 1,268 |
| 4 | 0 | 1 | –1 | 25 | 21 | 0.25 | 1,320 | 1,281.5 |
| 5 | 0 | –1 | –1 | 25 | 15 | 0.25 | 950 | 998.5 |
| 6 | 0 | –1 | 1 | 25 | 15 | 0.75 | 916 | 954.5 |
| 7 | 1 | 1 | 0 | 30 | 21 | 0.5 | 1,002 | 1,065 |
| 8 | 0 | 0 | 0 | 25 | 18 | 0.5 | 1,412 | 1,452 |
| 9 | 1 | 0 | 1 | 30 | 18 | 0.75 | 776 | 761.5 |
| 10 | 0 | 0 | 0 | 25 | 18 | 0.5 | 1,572 | 1,452 |
| 11 | –1 | 0 | 1 | 20 | 18 | 0.75 | 837 | 861.5 |
| 12 | 1 | 0 | –1 | 30 | 18 | 0.25 | 765 | 740.5 |
| 13 | 0 | 0 | 0 | 25 | 18 | 0.5 | 1,372 | 1,452 |
| 14 | 0 | 1 | 1 | 25 | 21 | 0.75 | 1,223 | 1,174.5 |
| 15 | 1 | –1 | 0 | 30 | 15 | 0.5 | 844 | 820 |
TABLE 5.
Regression analysis and significance test of variables for toxoflavin productiona
| Term | Effect | Coefficient | SE | t | P |
|---|---|---|---|---|---|
| Constant | 1,452 | 52.1 | 27.85 | 0.000* | |
| x1 | –196.5 | –98.3 | 31.9 | –3.08 | 0.028* |
| x2 | 251.5 | 125.8 | 31.9 | 3.94 | 0.011* |
| x3 | –75.5 | –37.7 | 31.9 | –1.18 | 0.290 |
| x12 | –664.2 | –332.1 | 47.0 | –7.07 | 0.001* |
| x22 | –158.2 | –79.1 | 47.0 | –1.68 | 0.153 |
| x32 | –541.3 | –270.6 | 47.0 | –5.76 | 0.002* |
| x1 x2 | –6.5 | –3.3 | 45.2 | –0.07 | 0.945 |
| x1 x3 | 96.5 | 48.3 | 45.2 | 1.07 | 0.334 |
| x2 x3 | –31.5 | –15.7 | 45.2 | –0.35 | 0.741 |
*, R2 = 95.48%; R2 (adjusted) = 87.33%.
Based on the predicted medium optimization model, a validation experiment was executed twice in triplicate with the suggested values of glucose (24.24 g/liter), peptone (20.45 g/liter), and phenylalanine (0.472 g/liter). The experimental results were consistent with the predicted toxoflavin production, and we attained a maximal yield of 1,533 mg/liter in 48 h, which was a nearly 4-fold improvement compared to the results obtained with KMB.
DISCUSSION
In the present study, a promising endophytic bacterium, B. gladioli HDXY-02, has been isolated from the medicinal plant L. aurea, producing a high yield of toxoflavin with a strong antifungal ability against all test fungal pathogens, especially for azole-resistant human fungal pathogens.
Azoles are currently the most prescribed drugs clinically in the treatment of aspergillosis (33). However, the emergence of azole-resistant A. fumigatus strains is now threatening the efficacy of azole drugs in the management of Aspergillus diseases (34, 35). For the antifungal efficacy, the currently recognized MIC is 10 μg/ml. Thus, compounds producing MICs lower than this are considered highly efficacious, whereas those producing values in the range of 10 < MIC ≤ 100 μg/ml are considered moderately efficacious (36, 37). According to this study, toxoflavin isolated from B. gladioli HDXY-02 could be classified as moderately efficacious against A. fumigatus (MIC, 64 μg/ml) (Table 2). In the present study, we found that toxoflavin has comparable inhibitory activities against tested azole-resistant isolates using both azole target 14-α sterol demethylase cyp51A and non-cyp51A gene mutations, with an MIC equal to that of the wild type (Table 2). In addition, our findings indicate that toxoflavin also has an effective antifungal ability against an encapsulated dangerous fungal organism, Cryptococcus neoformans, which is able to cause a systemic infection passing the blood-brain barrier (38, 39).
Previous studies have reported toxoflavin has growth-inhibiting abilities against the bacteria Escherichia coli, Bacillus subtilis, and Proteus vulgaris (19) and against the fungal species C. albicans and Saccharomyces cerevisiae (21). However, there was no detailed quantification assay for the antifungal activity of toxoflavin, especially against human fungal azole drug-resistant species. Therefore, findings in this study deepen our knowledge for the new biological antifungal function of toxoflavin. In addition, microscopic observations indicated that toxoflavin could inhibit the conidial germination and the development of conidiophore in A. fumigatus (Fig. 4). These results suggest that toxoflavin may be a potential new antifungal against azole-resistant A. fumigatus.
In addition to its antimicrobial potential, it has been shown that toxoflavin acts as a small molecule inhibitor of signaling pathways and targets involved in cancer development and treatment (24, 40–42). It was also suggested as a potential promising chemotherapeutic drug candidate for different cancers (22, 24, 43, 44). Although the clinical development of toxoflavin might be limited by its potential toxicity (19), a number of toxoflavin analogues have been prepared due to their effective biological activity and extensive use (21, 45). Moreover, some of the compounds exhibited improved inhibitory activities against a variety of bacterial and fungal strains (21).
As an active small molecule with effective activities, toxoflavin was produced by various attempts. There are several chemical synthesis-derived methods for toxoflavin production (46–48). However, these schemes are technically complicated, not very efficient, and time-intensive. Nevertheless, the chemical reagents used for the chemical synthesis processes, phosphorus oxychloride (POCl3) (49), formaldehyde, and released toxic hydrochloric acid (HCl), are all very harmful to human health. There are only a few factories able to produce toxoflavin, and thus toxoflavin has become very expensive. Developing a highly efficient and safe method to produce toxoflavin is therefore both urgent and necessary.
In fact, toxoflavin was known to be produced by B. gladioli (32), B. glumae (50, 51), Pseudomonas protegens (52), and Streptomyces spp. (53). Unfortunately, the amount of toxoflavin produced by these microorganisms was too low to be applied (20). However, in the present study, the production of toxoflavin by B. gladioli HDXY-02 resulted in yields of up to approximately 170 mg/liter after culture for 24 h in the related KMB, demonstrating more efficient production than that of other previously isolated species (about 20 mg/liter) (20). Moreover, using the optimized medium, we have obtained toxoflavin yields as high as 1,533 mg/liter after culture for 48 h. Thus, the findings presented here suggest that B. gladioli HDXY-02, with its excellent performance in toxoflavin biosynthesis, might be a promising starting strain for use in further engineering improvements in order to increase the production of toxoflavin.
MATERIALS AND METHODS
Isolation of endophytic bacteria having antifungal activity from Lycoris plants.
Healthy Lycoris plants (L. aurea, L. radiata, and L. chinensis) were collected from the Institute of Botany, Jiangsu Province and Chinese Academy of Sciences, Nanjing, China. Different tissues (leaves, bulbs, roots, and flowers) were disinfected using 70% (vol/vol) ethanol for 1 min and 1% (vol/vol) sodium hypochlorite for 20 min. After being rinsed with sterile distilled water more than five times, the tissues were ground to homogenates. Then, serial dilutions of the homogenates were plated on nutrient agar plates (Solarbio, Beijing, China), followed by incubation for 3 days at 30°C. Colonies were then picked and purified for further antifungal tests. The efficacies of the bacterial isolates in fungal growth inhibition were screened using dual-culture assay. Agar plugs 5 mm in diameter or spores (2 µl of 107 spores/ml) of pathogenic fungi were inoculated onto potato dextrose agar (PDA) plates (in the center). Bacterial cultures (10 µl of 107 cells/ml) were spotted onto the sterilized filter papers equidistant from the center. Further, the plates were incubated at 28 or 30°C for 3 to 7 days and observed for growth. Pathogen cultures grown in the absence of bacterial isolates were used as controls. Sizes of clear zones of the isolates were recorded, and colonies with clear zones surrounding them were selected.
To further validate the antifungal activity of strain HDXY-02, an experimental procedure was carried out as depicted in Fig. 1C. The sterilized semipermeable membranes with molecular weight cutoffs (MWCO) of 1 and 3.5 kDa were cut out and placed on YAG plates. Then, either 50 μl of the overnight culture of strain HDXY-02 or sterilized LB broth was added onto the membrane, followed by culture for another 16 h. Finally, the membrane was taken away, and A. fumigatus spores (2 µl of 107 spores/ml) were spotted onto YAG plates and then cultured at 37°C for 24 h.
Strains and culture conditions.
Strain HDXY-02 isolated from L. aurea has been deposited at the China General Microbiological Culture Collection Center (CGMCC; accession no. CGMCC 14054). LB medium (1% tryptone, 0.5% yeast extract, 1% NaCl) and King’s B medium (KMB; 2% peptone, 1.5% glycerol [vol/vol], 0.15% K2HPO4·3H2O, 0.15% MgSO4·7H2O [pH 7.2]) were used for the culture of strain HDXY-02. For solid media, 2% agar was added with antibiotics as required.
The growth curves were analyzed at different temperatures. Overnight cultures of strain HDXY-02 were inoculated in 50 ml of KMB broth (with an initial optical density at 600 nm [OD600] of 0.05) and incubated with shaking at 200 rpm for 48 h at 16, 25, 30, or 37°C. The OD600 was measured after incubation for 2, 5, 6, 8, 9, 10, 11, 24, 25, 26, 29, 31, and 48 h by using a DU 800 UV/VIS spectrophotometer (Beckman Coulter, Inc., Brea, CA).
C. albicans, C. neoformans, A. nidulans, and A. fumigatus strains were grown at 37°C. The media used for Aspergillus were minimal medium (MM; 1% glucose, nitrate salts, and trace elements, adjusted to a final pH of 6.5) and YAG (2% glucose, 0.5% yeast extract, and trace elements). For solidification, 2% agar was added. C. albicans and C. neoformans strains were grown on the yeast extract peptone dextrose medium (YEPD; 1% yeast extract, 2% peptone, and 2% dextrose, adjusted to a final pH of 5.6, plus 2% agar). The plant pathogens Magnaporthe oryzae, Rhizoctonia solani, and Fusarium graminearum used in the screening steps were grown at 28°C on the PDA medium (potato extract, 2% glucose, 2% agar, pH unadjusted). Potato dextrose broth (PDB; potato extract, 2% glucose, pH natural) was used for antifungal susceptibility testing of toxoflavin against plant pathogens and cultured at 28°C.
Strain identification for HDXY-02.
Genomic DNA was extracted using a TIANamp Bacteria DNA kit (catalog no. DP302; Tiangen Biotech, Beijing, China) according to the manufacturer’s protocols. 16S rRNA gene and 16S-23S spacer sequences of strain HDXY-02 were sequenced with the primers 16S-27F/16S-1492R and L1/L2 (Table 6). The 16S rRNA gene and 16S-23S spacer sequences were deposited in GenBank under accession numbers MG654707 and MG657340, respectively. The phylogenetic reconstruction was based on an approximately maximum-likelihood algorithm using the software MEGA v6.0 (54). To determine whether strain HDXY-02 belonged to Burkholderia gladioli, PCR amplification was performed with species-specific primers (Table 6). The primer pairs Bglu-F/toxA-R2 (B. glumae specific) and Bgla-F/toxA-R2 (B. gladioli specific) yielded 519- and 770-bp fragments, respectively (25). GLA-F/GLA-R (26), LP1/LP4 (27), and PG1/PG2 (28) are B. gladioli-specific primer pairs. PCR amplification was performed with a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA).
TABLE 6.
Sequences of primers used in species identification of B. gladioli HDXY-02
| Primer | Sequence (5′–3′) |
|---|---|
| 16S-27F | AGAGTTTGATCCTGGCTCAG |
| 16S-1492R | TACGGYTACCTTGTTACGACTT |
| L1 | AGTCGTAACAAGGTAGCCGT |
| L2 | GTGCCAAGGCATCCACC |
| Bgla-F | CGCATGAAAGTAAAAAATAA |
| Bglu-F | GCCCATTCCGAGGGAACATA |
| toxA-R2 | CGAATCCGCGTAATTGAAGA |
| LP1 | GGGGGGTCCATTGCG |
| LP4 | AGAAGCTCGCGCCACG |
| GLA-F | CGAGCTAATACCGCGAAA |
| GLA-R | AGACTCGAGTCAACTGA |
| PG1 | TTCAATGACAAACGTTCGGG |
| PG2 | GCTTTCGCTTGACAGGCC |
Sugar utilization, catalase activity, oxygen requirement, indole production, citrate utilization, oxidase activity, and VP tests were performed as described previously (55).
Identification and purification of toxoflavin produced by the HDXY-02 strain.
B. gladioli HDXY-02 was inoculated with 2% inoculum size in KMB broth with shaking at 200 rpm for 48 h at 30°C. After centrifugation at 10,000 × g, the cell-free supernatant was extracted using an equal volume of dichloromethane three times. The resulting dichloromethane extract of the supernatant was evaporated using a rotary evaporator (BÜCHI R-210) and then resuspended with methanol. The extract was chromatographed on a silica gel column (200 to 300 mesh; Qingdao, China) and eluted with dichloromethane with increasing concentrations of methanol (90:10 → 80:20 → 60:40 → 50:50 → 30:70 → 0:100 [vol/vol]). Fractions with antifungal abilities were then combined, evaporated, and resuspended in methanol for further study. The solution was filtered through a 0.22-μm-pore-size filter before HR-ESI-MS analysis was performed on an Agilent 1260 UPLC DAD 6530 QTOF mass spectrometer.
For the TLC analysis, extracts dissolved in methanol (1 to 4 μl) were spotted on Silica Gel G TLC plates (Merck, Darmstadt, Germany) and developed with chloroform/methanol (95:5, vol/vol), and then the plates were observed under UV light at 365 nm. Toxoflavin (98% purity) purchased from Sigma-Aldrich (St. Louis, MO) was used as the standard. Toxoflavin was further purified by recrystallization from 1-propanol. The purity of toxoflavin was analyzed using HPLC (LC-20A; Shimadzu, Japan) with a reversed-phase Shimadzu InertSustain C18 column (5 μm, 4.6 mm by 250 mm) and a Shimadzu SPD-M20A detector. The mobile phase used was a 20% acetonitrile-water solution; the flow rate was 0.6 ml/min. The column was maintained at 35°C, and detection was performed at 260 nm. The injection volume was 10 μl.
Antimicrobial bioassays of purified toxoflavin from strain HDXY-02.
Antifungal susceptibility testing of toxoflavin against strains (Table 2) was performed according to the Clinical and Laboratory Standards Institute (CLSI) standardized protocols M38-A2 (56) and M27-A3 (57). Inoculum suspensions were diluted in RPMI 1640 medium (Sangon Biotech, Shanghai, China) or PDB, and the final inoculum was between 0.4 × 104 and 5 × 104 CFU/ml in each well. Toxoflavin (dissolved in H2O) was serially diluted, with final concentrations ranging from 8 to 256 μg/ml. The total volume in each well was 200 μl. The 96-well microtiter plates were incubated at 35°C for 48 h for human pathogens or 28°C for 72 h for plant pathogens. Growth (toxoflavin-free) and fungus-free controls were included. The MICs were defined as the lowest concentrations that resulted in no discernible growth. The MFC assays were carried out as follows. A 0.1-ml aliquot of culture was taken from a well that did not show any sign of growth and then spread onto YAG, PDA, or YEPD plates, respectively. After incubation for 48 h at 35°C for human pathogens or at 28°C for plant pathogens, fungal CFU were counted. The MFCs were defined as the lowest toxoflavin concentrations that killed 99% of the inoculum.
Effect of toxoflavin on spore germination of A. fumigatus.
Spores of A. fumigatus Af293 were collected and suspended in MM broth (0.5 × 104 and 1 × 104 CFU/ml) with toxoflavin (25 μg/ml) and inoculated onto precleaned glass coverslips. The glass coverslips with spores were grown at 37°C and observed at each time point (0, 8, and 24 h); 100 spores were counted, and the percentage of germination was determined in triplicate. For hyphal microscopic observations, A. fumigatus was inoculated onto YAG and MM solid plates with toxoflavin (50 μg/ml) for 24 h. The prepared slides were performed using a Nikon Eclipse 80i microscope, and images were captured using a Nikon DS-Fi1 digital camera (×40).
Quantification of bacterial growth and toxoflavin production.
To quantify bacterial growth, 1 ml of the overnight culture was inoculated into 250-ml Erlenmeyer flasks containing 50 ml of LB medium or KMB. After incubation at 30°C for different periods (7, 8, 14, 16, 20, 24, 30, and 42 h), the levels of grown bacteria were determined by measuring the absorbance of the bacterial culture at 600 nm. Toxoflavin production was quantified according to a previously published method (31), with some modifications. Briefly, 1 ml of the culture was centrifuged at 10,000 × g for 1 min, and then the cell-free supernatant was extracted three times, each time with 1 ml of chloroform. The chloroform extract was evaporated, and the residue was dissolved in 1 ml of 80% methanol. The absorbance was measured at 393 nm to determine the relative amount of toxoflavin.
Optimization of media and statistical analyses.
The overnight culture (1 ml) was harvested by centrifugation at 10,000 × g for 1 min; pellets were washed with sterile saline (0.85%) and then inoculated into 250-ml Erlenmeyer flasks containing 50 ml of fermentation medium based on KMB with different compositions (Table S1). Cultivations were conducted at 200 rpm for 48 h at 30°C.
The effects of carbon sources, nitrogen sources, aromatic amino acids, nitrates, inorganic salts, and metal ions were examined for toxoflavin production by using a one-variable-at-a-time (OVAT) approach. Based on the results of OVAT analysis, 12 variables (k = 12) were selected for further analysis using PBD to identify the key variables. Each variable was tested at high (+) and low (−) concentrations, as described in Table S1. The detailed PBD design is represented in Table 7.
TABLE 7.
Matrix of the PBD and results for toxoflavin production by B. gladioli HDXY-02
| No. | Variable levels |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | x5 | x6 | x7 | x8 | x9 | x10 | x11 | x12 | Toxoflavin concn (mg/liter) | |
| 1 | 1 | –1 | 1 | 1 | –1 | –1 | –1 | –1 | 1 | –1 | 1 | –1 | 918 |
| 2 | 1 | 1 | –1 | 1 | 1 | –1 | –1 | –1 | –1 | 1 | –1 | 1 | 498 |
| 3 | –1 | 1 | 1 | –1 | 1 | 1 | –1 | –1 | –1 | –1 | 1 | –1 | 679 |
| 4 | –1 | –1 | 1 | 1 | –1 | 1 | 1 | –1 | –1 | –1 | –1 | 1 | 838 |
| 5 | 1 | –1 | –1 | 1 | 1 | –1 | 1 | 1 | –1 | –1 | –1 | –1 | 1,019 |
| 6 | 1 | 1 | –1 | –1 | 1 | 1 | –1 | 1 | 1 | –1 | –1 | –1 | 765 |
| 7 | 1 | 1 | 1 | –1 | –1 | 1 | 1 | –1 | 1 | 1 | –1 | –1 | 620 |
| 8 | 1 | 1 | 1 | 1 | –1 | –1 | 1 | 1 | –1 | 1 | 1 | –1 | 950 |
| 9 | –1 | 1 | 1 | 1 | 1 | –1 | –1 | 1 | 1 | –1 | 1 | 1 | 1,016 |
| 10 | 1 | –1 | 1 | 1 | 1 | 1 | –1 | –1 | 1 | 1 | –1 | 1 | 1,002 |
| 11 | –1 | 1 | –1 | 1 | 1 | 1 | 1 | –1 | –1 | 1 | 1 | –1 | 312 |
| 12 | 1 | –1 | 1 | –1 | 1 | 1 | 1 | 1 | –1 | –1 | 1 | 1 | 976 |
| 13 | –1 | 1 | –1 | 1 | –1 | 1 | 1 | 1 | 1 | –1 | –1 | 1 | 572 |
| 14 | –1 | –1 | 1 | –1 | 1 | –1 | 1 | 1 | 1 | 1 | –1 | –1 | 837 |
| 15 | –1 | –1 | –1 | 1 | –1 | 1 | –1 | 1 | 1 | 1 | 1 | –1 | 372 |
| 16 | –1 | –1 | –1 | –1 | 1 | –1 | 1 | –1 | 1 | 1 | 1 | 1 | 494 |
| 17 | 1 | –1 | –1 | –1 | –1 | 1 | –1 | 1 | –1 | 1 | 1 | 1 | 889 |
| 18 | 1 | 1 | –1 | –1 | –1 | –1 | 1 | –1 | 1 | –1 | 1 | 1 | 707 |
| 19 | –1 | 1 | 1 | –1 | –1 | –1 | –1 | 1 | –1 | 1 | –1 | 1 | 886 |
| 20 | –1 | –1 | –1 | –1 | –1 | –1 | –1 | –1 | –1 | –1 | –1 | –1 | 404 |
Three optimal variables from PBD were selected for further medium optimization using BBD. A P value of <0.05 was considered to represent a significant effect on toxoflavin production. The design matrix of a three-factor three-level BBD with 15 experimental trials is given in Table 4. The variables were used to fit a model by a response equation:
where Y is the predicted response (toxoflavin production), β0 is the model constant; x1, x2, and x3 are the independent variables; β1, β2, and β3 are the linear coefficients; β12, β13, and β23 are the interaction coefficients; and β11, β22, and β33 are the quadratic coefficients. Minitab 17.0 (MINITAB, PA) was used to generate the design and analyze the effects and statistical analysis of variables.
Supplementary Material
ACKNOWLEDGMENTS
This study was financially supported by the National Natural Science Foundation of China (grants 81330035 and 31770086 to L. Lu; grant 31572151 to R. Wang), the Jiangsu Provincial Public Institutions Program for Research Conditions and Building Capacity (BM2015019), the Program for Jiangsu Excellent Scientific and Technological Innovation Team (17CXTD00014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
We thank Huaigu Chen and Yongfeng Liu for providing test strains of plant pathogens.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00106-19.
REFERENCES
- 1.Miceli MH, Churay T, Braun T, Kauffman CA, Couriel DR. 2017. Risk factors and outcomes of invasive fungal infections in allogeneic hematopoietic cell transplant recipients. Mycopathologia 182:495–504. doi: 10.1007/s11046-017-0115-y. [DOI] [PubMed] [Google Scholar]
- 2.Ostrosky-Zeichner L, Al-Obaidi M. 2017. Invasive fungal infections in the intensive care unit. Infect Dis Clin North Am 31:475–487. doi: 10.1016/j.idc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Pound MW, Townsend ML, Dimondi V, Wilson D, Drew RH. 2011. Overview of treatment options for invasive fungal infections. Med Mycol 49:561–580. doi: 10.3109/13693786.2011.560197. [DOI] [PubMed] [Google Scholar]
- 4.Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 5.Fuhren J, Voskuil WS, Boel CH, Haas PJ, Hagen F, Meis JF, Kusters JG. 2015. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J Antimicrob Chemother 70:2894–2898. doi: 10.1093/jac/dkv177. [DOI] [PubMed] [Google Scholar]
- 6.Rivero-Menendez O, Alastruey-Izquierdo A, Mellado E, Cuenca-Estrella M. 2016. Triazole resistance in Aspergillus spp.: a worldwide problem? JoF 2:21. doi: 10.3390/jof2030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonçalves SS, Souza AC, Chowdhary A, Meis JF, Colombo AL. 2016. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses 59:198–219. doi: 10.1111/myc.12469. [DOI] [PubMed] [Google Scholar]
- 8.Roemer T, Xu D, Singh SB, Parish CA, Harris G, Wang H, Davies JE, Bills GF. 2011. Confronting the challenges of natural product based-antifungal discovery. Chem Biol 18:148–164. doi: 10.1016/j.chembiol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Di Santo R. 2010. Natural products as antifungal agents against clinically relevant pathogens. Nat Prod Rep 27:1084–1098. doi: 10.1039/b914961a. [DOI] [PubMed] [Google Scholar]
- 10.Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ. 2013. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 62:10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 11.Evidente A, Andolfi A, Abou-Donia AH, Touema SM, Hammoda HM, Shawky E, Motta A. 2004. (–)-Amarbellisine, a lycorine-type alkaloid from Amaryllis belladonna L. growing in Egypt. Phytochemistry 65:2113–2118. doi: 10.1016/j.phytochem.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Stierle A, Strobel G, Stierle D. 1993. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 13.Strobel GA. 2003. Endophytes as sources of bioactive products. Microbes Infect 5:535–544. doi: 10.1016/S1286-4579(03)00073-X. [DOI] [PubMed] [Google Scholar]
- 14.Strobel G, Daisy B, Castillo U, Harper J. 2004. Natural products from endophytic microorganisms. J Nat Prod 67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HW, Song YC, Tan RX. 2006. Biology and chemistry of endophytes. Nat Prod Rep 23:753–771. doi: 10.1039/b609472b. [DOI] [PubMed] [Google Scholar]
- 16.Qin JC, Zhang YM, Gao JM, Bai MS, Yang SX, Laatsch H, Zhang AL. 2009. Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorg Med Chem Lett 19:1572–1574. doi: 10.1016/j.bmcl.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Lamoral-Theys D, Decaestecker C, Mathieu V, Dubois J, Kornienko A, Kiss R, Evidente A, Pottier L. 2010. Lycorine and its derivatives for anticancer drug design. Mini Rev Med Chem 10:41–50. doi: 10.2174/138955710791112604. [DOI] [PubMed] [Google Scholar]
- 18.Li SY, Chen C, Zhang HQ, Guo HY, Wang H, Wang L, Zhang X, Hua SN, Yu J, Xiao PG, Li RS, Tan XH. 2005. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res 67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latuasan HE, Berends W. 1961. On the origin of the toxicity of toxoflavin. Biochim Biophys Acta 52:502–508. doi: 10.1016/0006-3002(61)90408-5. [DOI] [PubMed] [Google Scholar]
- 20.Levenberg B, Linton SN. 1966. On the biosynthesis of toxoflavin, an azapteridine antibiotic produced by Pseudomonas cocovenenans. J Biol Chem 241:846–852. [PubMed] [Google Scholar]
- 21.Nagamatsu T, Yamasaki H, Hirota T, Yamato M, Kido Y, Shibata M, Yoneda F. 1993. Syntheses of 3-substituted 1-methyl-6-phenylpyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-diones (6-phenyl analogs of toxoflavin) and their 4-oxides, and evaluation of antimicrobial activity of toxoflavins and their analogs. Chem Pharm Bull (Tokyo) 41:362–368. doi: 10.1248/cpb.41.362. [DOI] [PubMed] [Google Scholar]
- 22.Wei W, Chua MS, Grepper S, So S. 2010. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int J Cancer 126:2426–2436. doi: 10.1002/ijc.24810. [DOI] [PubMed] [Google Scholar]
- 23.Raoof A, Depledge P, Hamilton NM, Hamilton NS, Hitchin JR, Hopkins GV, Jordan AM, Maguire LA, McGonagle AE, Mould DP, Rushbrooke M, Small HF, Smith KM, Thomson GJ, Turlais F, Waddell ID, Waszkowycz B, Watson AJ, Ogilvie DJ. 2013. Toxoflavins and deazaflavins as the first reported selective small molecule inhibitors of tyrosyl-DNA phosphodiesterase II. J Med Chem 56:6352–6370. doi: 10.1021/jm400568p. [DOI] [PubMed] [Google Scholar]
- 24.Franci G, Sarno F, Nebbioso A, Altucci L. 2017. Identification and characterization of PKF118-310 as a KDM4A inhibitor. Epigenetics 12:198–205. doi: 10.1080/15592294.2016.1249089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YA, Chao CS, Jung CH. 2013. Combination of a simple differential medium and toxA-specific PCR for isolation and identification of phytopathogenic Burkholderia gladioli. Eur J Plant Pathol 136:523–533. doi: 10.1007/s10658-013-0184-9. [DOI] [Google Scholar]
- 26.Furuya N, Ura H, Iiyama K, Matsumoto M, Takeshita M, Takanami Y. 2002. Specific oligonucleotide primers based on sequences of the 16S-23S rDNA spacer region for the detection of Burkholderia gladioli by PCR. J Gen Plant Pathol 68:220–224. doi: 10.1007/PL00013080. [DOI] [Google Scholar]
- 27.Whitby PW, Pope LC, Carter KB, Lipuma JJ, Stull TL. 2000. Species-specific PCR as a tool for the identification of Burkholderia gladioli. J Clin Microbiol 38:282–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clode FE, Kaufmann ME, Malnick H, Pitt TL. 1999. Evaluation of three oligonucleotide primer sets in PCR for the identification of Burkholderia cepacia and their differentiation from Burkholderia gladioli. J Clin Pathol 52:173–176. doi: 10.1136/jcp.52.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei XL, Chen PY, Gao RS, Li YQ, Zhang AX, Liu FF, Lu L. 2017. Screening and characterization of a non-cyp51A mutation in an Aspergillus fumigatus cox10 strain conferring azole resistance. Antimicrob Agents Chemother 61:e02101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki F, Sawada H, Azegami K, Tsuchiya K. 2004. Molecular characterization of the tox operon involved in toxoflavin biosynthesis of Burkholderia glumae. J Gen Plant Pathol 70:97–107. doi: 10.1007/s10327-003-0096-1. [DOI] [Google Scholar]
- 31.Chen R, Barphagha IK, Karki HS, Ham JH. 2012. Dissection of quorum-sensing genes in Burkholderia glumae reveals non-canonical regulation and the new regulatory gene tofM for toxoflavin production. PLoS One 7:e52150. doi: 10.1371/journal.pone.0052150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Park J, Kim S, Park I, Seo YS. 2016. Differential regulation of toxoflavin production and its role in the enhanced virulence of Burkholderia gladioli. Mol Plant Pathol 17:65–76. doi: 10.1111/mpp.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 21-22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Hagiwara D, Watanabe A, Kamei K, Goldman GH. 2016. Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus fungi. Front Microbiol 7:1382. doi: 10.3389/fmicb.2016.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awouafack MD, McGaw LJ, Gottfried S, Mbouangouere R, Tane P, Spiteller M, Eloff JN. 2013. Antimicrobial activity and cytotoxicity of the ethanol extract, fractions and eight compounds isolated from Eriosema robustum (Fabaceae). BMC Complement Altern Med 13:289. doi: 10.1186/1472-6882-13-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuete V. 2010. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med 76:1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 38.Charlier C, Chrétien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. 2005. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol 166:421–432. doi: 10.1016/S0002-9440(10)62265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stie J, Fox D. 2012. Blood-brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin. Microbiology 158:240–258. doi: 10.1099/mic.0.051524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. 2004. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 5:91–102. doi: 10.1016/S1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 41.Barker N, Clevers H. 2006. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov 5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 42.Choi G, Lee J, Ji JY, Woo J, Kang NS, Cho SY, Kim HR, Ha JD, Han SY. 2013. Discovery of a potent small molecule SIRT1/2 inhibitor with anticancer effects. Int J Oncol 43:1205–1211. doi: 10.3892/ijo.2013.2035. [DOI] [PubMed] [Google Scholar]
- 43.Leow PC, Tian Q, Ong ZY, Yang Z, Ee PL. 2010. Antitumor activity of natural compounds, curcumin and PKF118-310, as Wnt/β-catenin antagonists against human osteosarcoma cells. Invest New Drugs 28:766–782. doi: 10.1007/s10637-009-9311-z. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Font E, Felipe-Abrio I, Calabuig-Fariñas S, Ramos R, Terrasa J, Vögler O, Alemany R, Martín-Broto J, Obrador-Hevia A. 2017. Disruption of TCF/β-catenin binding impairs Wnt signalling and induces apoptosis in soft tissue sarcoma cells. Mol Cancer Ther 16:1166–1176. doi: 10.1158/1535-7163.MCT-16-0585. [DOI] [PubMed] [Google Scholar]
- 45.Turbiak AJ, Showalter HDH. 2009. A novel synthesis of N1-(substituted)- pyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-diones. Tetrahedron Lett 50:1996–1997. doi: 10.1016/j.tetlet.2009.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daves GD, Robins RK, Cheng CC. 1961. The total synthesis of toxoflavin. J Am Chem Soc 83:3904–3905. doi: 10.1021/ja01479a041. [DOI] [Google Scholar]
- 47.Black TH. 1987. An improved, large-scale synthesis of xanthothricin and reumycin. J Heterocyclic Chem 24:1373–1375. doi: 10.1002/jhet.5570240529. [DOI] [Google Scholar]
- 48.Mao YJ, Tian W, Huang ZW, An J. 2014. Convenient synthesis of toxoflavin that targets β-catenin/Tcf4 signaling activities. J Heterocyclic Chem 51:594–597. doi: 10.1002/jhet.1111. [DOI] [Google Scholar]
- 49.Quistad GB, Zhang N, Sparks SE, Casida JE. 2000. Phosphoacetylcholinesterase: toxicity of phosphorus oxychloride to mammals and insects that can be attributed to selective phosphorylation of acetylcholinesterase by phosphorodichloridic acid. Chem Res Toxicol 13:652–657. doi: 10.1021/tx000028o. [DOI] [PubMed] [Google Scholar]
- 50.Jeong Y, Kim J, Kim S, Kang Y, Nagamatsu T, Hwang I. 2003. Toxoflavin produced by Burkholderia glumae causing rice grain rot is responsible for inducing bacterial wilt in many field crops. Plant Dis 87:890–895. doi: 10.1094/PDIS.2003.87.8.890. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Kim JG, Kang Y, Jang JY, Jog GJ, Lim JY, Kim S, Suga H, Nagamatsu T, Hwang I. 2004. Quorum sensing and the LysR-type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae. Mol Microbiol 54:921–934. doi: 10.1111/j.1365-2958.2004.04338.x. [DOI] [PubMed] [Google Scholar]
- 52.Philmus B, Shaffer BT, Kidarsa TA, Yan Q, Raaijmakers JM, Begley TP, Loper JE. 2015. Investigations into the biosynthesis, regulation, and self-resistance of toxoflavin in Pseudomonas protegens Pf-5. Chembiochem 16:1782–1790. doi: 10.1002/cbic.201500247. [DOI] [PubMed] [Google Scholar]
- 53.Machlowitz RA, Fisher WP, Mckay BS, Tytell AA, Charney J. 1954. Xanthothricin, a new antibiotic. Antibiot Chemother (Northfield) 4:259–261. [PubMed] [Google Scholar]
- 54.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leboffe MJ, Pierce BE. 2008. Microbiology: laboratory theory and application. Morton Publishing Company, Englewood, CO. [Google Scholar]
- 56.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 57.Clinical and Laboratory Standards Institute 2008. Reference methods for broth dilution antifungal susceptibility testing of yeasts; approved standard, document M27-A3. Clinical and Laboratory Standard Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.