Agrobacterium tumefaciens as the causal agent of peach crown gall disease can be controlled by planting resistant cultivars. This study profiles the endophytic bacteria in susceptible and resistant peach cultivars, advancing our understanding of the relationships between endophytic bacterial communities and peach crown gall disease, with potential implications for other complex microbiome-plant-pathogen interactions. The resistant cultivar may defend itself by increasing the diversity and abundance of beneficial endophytic bacteria. The antagonists identified among the genera Streptomyces, Pseudomonas, and Rhizobium may have application potential for biocontrol of crown gall disease in fruit trees.
KEYWORDS: Agrobacterium tumefaciens, crown gall disease, endophytic bacteria, high-throughput sequencing, peach, resistance
ABSTRACT
Crown gall disease caused by Agrobacterium tumefaciens severely impacts the production of peach and other fruit trees. Several peach cultivars are partially resistant to A. tumefaciens, but little is known about the roles of endophytic microbiota in disease resistance. In the present study, the endophytic bacterial communities of resistant and susceptible peach cultivars “Honggengansutao” and “Okinawa” were analyzed using universal 16S rRNA gene amplicon sequencing in parallel with the cultivation and characterization of bacterial isolates. A total of 1,357,088 high-quality sequences representing 3,160 distinct operational taxonomic units (OTUs; Proteobacteria, Actinobacteria, Bacteroidetes, and Firmicutes) and 1,200 isolates of 20 genera and 305 distinct ribotypes were collected from peach roots and twigs. It was found that factors including plant developmental stage, cultivar, and A. tumefaciens invasion strongly influenced the peach endophytic communities. The community diversity of endophytic bacteria and the abundance of culturable bacteria were both higher in the roots of the resistant cultivar, particularly after inoculation. Strikingly, the pathogen antagonists Streptomyces and Pseudomonas in roots and Rhizobium in twigs were most frequently detected in resistant plants. Our results suggest that the higher abundance and diversity of endophytic bacteria and increased proportions of antagonistic bacteria might contribute to the natural defense of the resistant cultivar against A. tumefaciens. This work reveals the relationships between endophytic bacteria and disease resistance in peach plants and provides important information for microbiome-based biocontrol of crown gall disease in fruit trees.
IMPORTANCE Agrobacterium tumefaciens as the causal agent of peach crown gall disease can be controlled by planting resistant cultivars. This study profiles the endophytic bacteria in susceptible and resistant peach cultivars, advancing our understanding of the relationships between endophytic bacterial communities and peach crown gall disease, with potential implications for other complex microbiome-plant-pathogen interactions. The resistant cultivar may defend itself by increasing the diversity and abundance of beneficial endophytic bacteria. The antagonists identified among the genera Streptomyces, Pseudomonas, and Rhizobium may have application potential for biocontrol of crown gall disease in fruit trees.
INTRODUCTION
Agrobacterium tumefaciens, the causal agent of crown gall disease, infects dicotyledonous plants of approximately one hundred botanical families (1). Based on comparative 16S rRNA gene analyses, A. tumefaciens has been formally reclassified as Rhizobium radiobacter (2), which encompasses both pathogenic and nonpathogenic strains. In the present study, we nonetheless refer to the pathogenic strains as A. tumefaciens to distinguish them from nonpathogenic R. radiobacter. The pathogen can survive in soil or plant debris and infects host plants through fresh wounds via chemotactic sensing and motility. By injecting the transfer DNA (T-DNA) derived from a tumor-inducing (Ti) plasmid into the plant genome, A. tumefaciens causes overgrowths of the host, appearing as galls on root collars, roots, and twigs (3). Small, soft, and white lumps first appear a few days after infection, which harden to form woody galls; as a result, the water and nutrient transport by vascular tissues is limited, ultimately stunting the plant growth and causing a yield loss of fruit (4).
Crown gall disease accounts for significant economic losses of peach production in China (5). There are two effective measures to control this disease in orchards, i.e., planting resistant cultivars and introducing biological antagonists. Although peach cultivars “Mr.S.2/5” (6), “Cadaman” (7), “St. Julien 655/2” (8), “Honggengansutao,” and “Xibei13-1” (9) have shown resistance to crown gall disease, the resistance does not appear consistent across geographic locations. The antagonistic bacterium R. radiobacter K84 and its genetically modified strain K1026 can suppress A. tumefaciens through agrocin 84 production (10) and niche competition (11, 12) and have been successfully developed as biocontrol agents. However, universal biocontrol of crown gall disease by these antagonists is challenged by the resistance of many A. tumefaciens strains to K84 (13, 14).
Microorganisms that spend at least part of their life cycle inside plants are called endophytes (15), and their communities may represent an extended phenotype of their hosts (16). Endophytic microbiota are shaped by both the host plant and environmental stimuli and, in turn, may enhance the biotic and abiotic tolerance of their host plants as a multispecies functional unit (17). The abundance and diversity of endophytic microbial communities vary substantially between resistant and susceptible cultivars of some plants (18–21), and the community composition may also be altered by pathogen infection (22–25). Previous studies indicate that endophytic communities can inhibit pathogen invasion and prevent or reduce disease development by outcompeting phytopathogens, producing antimicrobial compounds, or inducing plant resistance (26). Colonization by specific endophytes has been demonstrated to successfully reduce disease incidence and severity in several fruit trees, including citrus (18), grapevine (22), banana (27), and apple (28) trees. In peach tree roots, five endophytic bacteria (Brevundimonas diminuta, Leifsonia shinshuensis, Sphingomonas parapaucimobilis, Brevundimonas vesicularis, and R. radiobacter) isolated from in vitro cultures were found to produce indole-3-acetic acid (IAA; a plant hormone), fix nitrogen, and solubilize phosphate (29). Moreover, endogenous Enterobacter, Pantoea, and Rhizobium isolated from the resistant peach cultivar “Xibei13-1” demonstrate antagonism to A. tumefaciens in vitro and in greenhouse trials (30). Therefore, endophytes with resistance-promoting capabilities are of great scientific and economic importance for fruit trees.
Endophytic bacteria can be characterized by using culture-dependent approaches, which are conducive to physiological and functional analysis (31, 32), or can be analyzed by DNA sequencing, which provides insight into the structure and diversity of endophyte communities (33–36). The combination of isolation, phenotypic testing, and massively parallel sequencing enables more precise dissection of the whole bacterial community (37). Thus, the present study employed both culture-dependent and -independent methods to characterize the bacterial endophyte communities of two peach cultivars, resistant “Honggengansutao” and susceptible “Okinawa,” and focused on the endophyte responses to A. tumefaciens invasion. We aim to provide a better understanding of complex microbiota-plant-pathogen interactions and reveal which endophytic microbiota may contribute to plant resistance to root diseases.
RESULTS
Susceptibility of peach cultivars to crown gall disease.
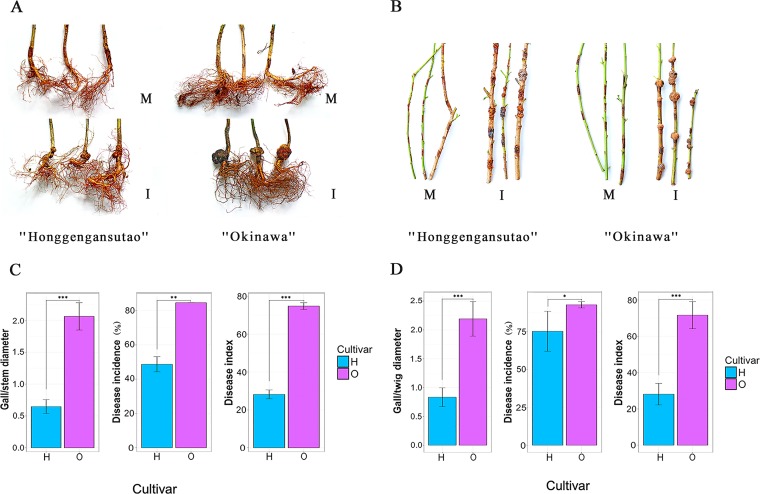
The susceptibility of different peach cultivars to crown gall disease was tested on peach tree roots in the greenhouse and newly grown twigs in the field. Disease onset occurred in both roots and twigs 10 days postinoculation (D10), and crown gall tumors developed rapidly thereafter until D60 (Fig. 1A and B). In root collars, crown gall disease was severe in susceptible cultivar “Okinawa,” as evidenced by larger galls (2.1 versus 0.6 gall/stem diameter ratio) and higher incidence rates (84.6% versus 48.7%) and disease index (74.8 versus 28.2) than the resistant “Honggengansutao” (P ≤ 0.01 in all cases) (Fig. 1C). Similar results were observed in twigs, with average gall/twig diameter ratios of 2.2 versus 0.8, incidence rates of 92.7% versus 75.2%, and disease indexes of 71.6 versus 28.1 in cultivars “Okinawa” and “Honggengansutao,” respectively (P ≤ 0.05 in all cases) (Fig. 1D). No symptoms were observed in uninoculated plants. The results indicated that the resistant cultivar “Honggengansutao” was highly effective in deterring gall development in peach tree roots and twigs.
FIG 1.
Disease occurrence on the peach tree root collars and twigs of resistant cultivar “Honggengansutao” and susceptible cultivar “Okinawa” 60 days after inoculation with A. tumefaciens. Symptomatic development on peach root collars (A) and twigs (B). Disease indices of peach tree root collars (C) and twigs (D). Statistical comparisons between groups were conducted by Student’s t tests. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; M, mock; I, inoculated with A. tumefaciens; H, “Honggengansutao”; O, “Okinawa.”
Endophytic bacterial communities in peach tree roots and twigs.
Tissues from cultivars “Okinawa” and “Honggengansutao” with and without A. tumefaciens inoculation were collected in triplicates from both roots (of greenhouse-grown trees) and twigs (of field-grown trees) at D0, D10, and D60, resulting in 60 samples (see Fig. S1 in the supplemental material). The V5-V7 region of the bacterial 16S rRNA gene, approximately 400 bp in length, was amplified using PCR and sequenced using the Illumina MiSeq platform, generating a total of 1,357,088 high-quality sequences (9,484 to 46,736 sequences per sample) (see Table S1). After clustering using >97% sequence similarity and removing operational taxonomic units (OTUs) of less than 5 counts, 1,842 and 1,318 distinct OTUs were observed in roots and twigs, respectively (Table S1). To describe the endophytic bacterial communities of the root and twig microbiota, a representative sequence of each OTU was assigned to a taxonomic classification by comparison with the Silva database. Negative controls exhibited no specific amplification.
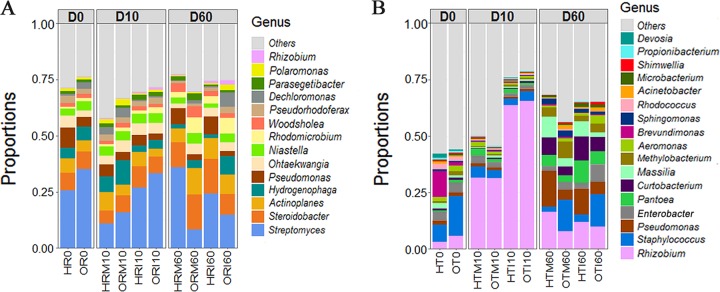
Differences were observed in the community compositions of endophytic bacteria in peach tree roots and twigs. Overall, endophytic assemblages were dominated by Proteobacteria, Actinobacteria, Bacteroidetes, and Firmicutes at the phylum level, accounting for 49.8% to 99.0% of the total bacterial community regardless of compartment, cultivar, treatment, or time of sampling (see Fig. S2). At the genus level, Streptomyces (average abundance of 23.2%) in roots and Rhizobium (average abundance of 24.7%, including A. tumefaciens) in twigs were dominant (Fig. 2). The other top genera were largely different between roots and twigs (Fig. 2); only Pseudomonas and Rhizobium were abundant in both. In comparison to the relatively stable distribution of root endophytes, the community composition of twig endophytes varied notably with time and pathogen inoculation.
FIG 2.
Distributions of endophytic bacteria from roots (A) and twigs (B) across sampling time, cultivar, and treatment. Unidentified genera and genera with a proportion of less than 0.5% are combined in the group “Others.” Genus Rhizobium contains the former genus Agrobacterium. H, “Honggengansutao”; O, “Okinawa”; R, root; T, twig; M, mock; I, inoculated with A. tumefaciens.
Factors affecting the community composition of bacterial endophytes.
Nonmetric multidimensional scaling (NMDS) ordination of the root and twig community data (Fig. 3A and B, respectively) and multiple regression tree analysis (see Fig. S3) indicated that endophyte communities were first structured by sampling time, followed by cultivar and pathogen inoculation. These effects were validated by permutational multivariate analysis of variance (PERMANOVA) (see Table S2), random forest classification (see Table S3), and one-way analysis of similarity (ANOSIM) (see Fig. S4). The susceptible and resistant cultivars also showed different responses to pathogen invasion, displaying similar bacterial communities at D10 but divergent ones at D60 (Fig. 3A and B and S3). In mock-inoculated plants, root endophytic communities of different cultivars diverged with time, while twigs maintained the differentiated endophytic communities. Measures of Shannon diversity also indicated that peach endophytic microbiota changed across sampling time, cultivar, and pathogen inoculation. In roots, of the two cultivars, resistant “Honggengansutao” exhibited significantly higher diversity than “Okinawa,” particularly in the inoculated samples (P ≤ 0.05) (Fig. 3C). In contrast, the endophyte diversity declined in twigs after inoculation; both cultivars showed similar responses to the pathogen inoculation, exhibiting a sharp drop at D10 in the inoculated samples and partial recovery at D60 (Fig. 3D). This indicates that A. tumefaciens infection has effects on the structure and dynamics of endophyte communities in peach tree roots and twigs, which differ in susceptible and resistant cultivars. Fluctuations in inoculated pathogen abundance likely contribute to these changes in community diversity, as shown below in the cultivation assay.
FIG 3.
Distributions of the endophytic microbiota in peach tree roots (A) and twigs (B) within a nonmetric multidimensional scaling (NMDS) ordination. Shannon diversity indices of the microbiota of peach tree roots (C) and twigs (D) based on 16S rRNA sequences. The analysis was conducted based on the Bray-Curtis dissimilarity at OTU level. Statistical comparisons between groups were conducted by one-way ANOVA test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; U, uninoculated; M, mock; I, inoculated with A. tumefaciens; H, “Honggengansutao”; O, “Okinawa.”
Differentially abundant endophytic bacteria in peach cultivars.
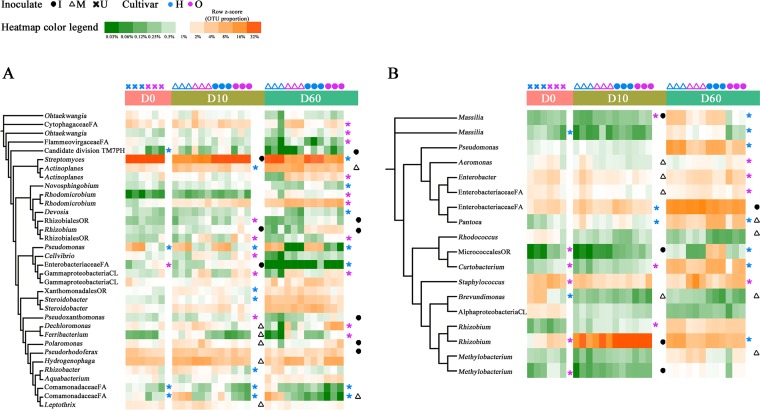
A total of 57 and 34 OTUs were significantly enriched in the roots of “Honggengansutao” and “Okinawa,” respectively (Kruskal-Wallis test, P≤ 0.05) (see Fig. S5A), at one or more time points, but only 5 and 1 were consistently elevated in each cultivar. Pseudomonas sp. (OTU_18r) was found to be closely associated with resistant “Honggengansutao” (Fig. 4A). According to the similarity percentage analysis (SIMPER), OTU_18r contributed 19.8%, 8.9%, and 7.9% to the dissimilarities of root endophytic communities at D0, D10, and D60, respectively (see Table S4). Another major root endophyte of “Honggengansutao,” Streptomyces sp. (OTU_1r), was more abundant in inoculated roots at D10 (30.2% versus 13.4% of mock-inoculated roots, P ≤ 0.05) and was the major differential component of the “Honggengansutao” bacterial community at D60 (30.2% versus 11.6% of “Okinawa” roots, P ≤ 0.05) (Fig. 4A and Table S4). Candidate division OD1 (9/10 OTUs), Planctomycetes (9/9 OTUs), and Chloroflexi (4/7 OTUs) were also more abundant in “Honggengansutao” roots at D10 (Fig. S5A).
FIG 4.
Phylogenetic distribution and heatmaps of the most abundant OTUs (with abundance >0.5%) in the endophytic microbiota of peach tree roots (A) and twigs (B) under different sampling times, cultivars, and treatments. The phylogenetic trees were constructed with 1,000 bootstrap resamplings and annotated using iTOL. Branch lengths are arbitrary. The highest taxonomic resolution of OTUs is labeled. Heatmaps show the relative abundances of OTUs across sample types and replicates. P values are calculated according to the Kruskal-Wallis analysis, and significant differences (P ≤ 0.05) are indicated with asterisks. U, uninoculated; M, mock; I, inoculated with A. tumefaciens; FA, family; PH, phylum; OR, order; CL, class; H, “Honggengansutao”; O, “Okinawa.”
In agreement with the cultivar comparison in roots, more OTUs were enriched in the twigs of the resistant cultivar (65 versus 40; Kruskal-Wallis test, P ≤ 0.05) (Fig. S5B), but the differential OTUs were different from those in roots. More differentially abundant endophytic bacteria emerged with plant growth, as identified in both a Kruskal-Wallis test and SIMPER analysis (see Table S5). Genera Rhizobium/Agrobacterium (OTU_2t), Pseudomonas (OTU_6t), Pantoea (OTU_11t), Curtobacterium (OTU_12t), and Massilia (OTU_22t) were enriched in resistant “Honggengansutao” at D60 (Fig. 4B and Table S5). The abundance of some bacterial endophytes in twigs also responded to A. tumefaciens inoculation (Fig. 4B). Most notably, OTU_2t, which matched the inoculated A. tumefaciens as well as Rhizobium sp., accounted for 70.1% of the total Bray-Curtis dissimilarity and made up 64.7% and 31.4% sequences of the inoculated and mock-inoculated twigs at D10, respectively (Table S5). However, the abundance of Rhizobium had dropped, and the differences between the mock-inoculated and inoculated plants disappeared by D60. Although Bacillus, the well-known antagonist, represented a low proportion of amplicon sequences, it had a higher abundance in the resistant cultivar (P ≤ 0.05) (see Fig. S6).
Cultivation of peach endophytic bacteria.
Endophytic bacteria from both roots and twigs were cultivated, enumerated, isolated, and identified. More colonies were obtained from the resistant cultivar “Honggengansutao” than susceptible “Okinawa” per gram of tissue, especially in roots (3.3 × 104 versus 2.0 × 103) (see Fig. S7). Sixty bacterial isolates of each subset (cultivar, sampling time, treatment, and peach compartment; a total of 1,200) were then selected for further studies.
Based on full-length 16S rRNA sequences, 600 isolates from roots were assigned to 10 genera and 143 unique 16S sequences (ribotypes) (see Fig. S8A and Table S6). Pseudomonas (32.8%) and Rhizobium (18.7%, including A. tumefaciens) were the most frequently cultivated genera, followed by Paenibacillus (15%), Bacillus (13.7%), and Streptomyces (8.7%). The 600 isolates from peach tree twigs were assigned to 15 genera and 162 ribotypes, including Rhizobium (36.2%, including A. tumefaciens), Pantoea (11.7%), Staphylococcus (8.9%), Pseudomonas (5.8%), Bacillus (4.2%), and Enterobacter (3.3%) (Fig. S8B and Table S7). Rhizobium (encompassing the inoculated A. tumefaciens) was strikingly enriched in twigs at D10, accounting for 85.8% and 36.7% of culturable isolates in the inoculated and mock-inoculated plants, respectively.
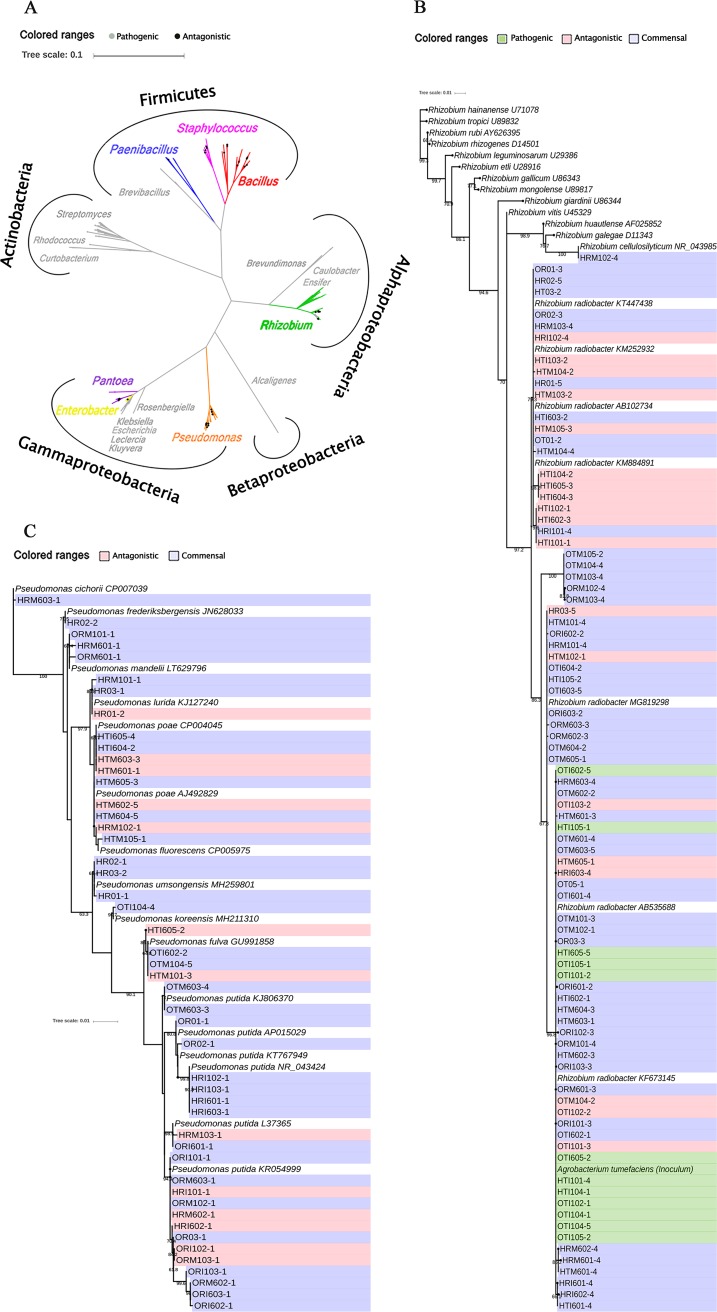
A phylogenetic analysis indicated that the 305 distinct ribotypes (143 from roots and 162 from twigs) were clustered into five branches (Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Actinobacteria, and Firmicutes) and 20 genera (Fig. 5A). Almost all of the Rhizobium isolates (84/85) were closely related to R. radiobacter (including A. tumefaciens) (Fig. 5B). In contrast, Pseudomonas isolates were more diverse, belonging to ten species, with most closely related to P. putida and P. poae (Fig. 5C).
FIG 5.
Phylogenetic analysis of all endophytic isolates (A), Rhizobium isolates (B), and Pseudomonas isolates (C) based on full-length 16S rRNA sequences (1,350 bp). The phylogenetic trees were constructed with 1,000 bootstrap resamplings and annotated using iTOL. Pathogenic Rhizobium strains were identified based on PCR amplification of virulent ipt gene and inoculation assay in sunflower, antagonistic strains were determined by pair culturing method, and the others were defined as commensal. H, “Honggengansutao”; O, “Okinawa”; R, root; T, twig; M, mock; I, inoculated with A. tumefaciens.
Antagonistic/pathogenic characterization of bacterial isolates.
The antagonistic activities of 305 endophytic isolates against A. tumefaciens were tested in vitro (Fig. 5A; Tables S6 and S7). Fifty-four strains, mainly belonging to Rhizobium, Pseudomonas, Bacillus, and Pantoea, showed significant antagonism. These antagonists were mostly isolated from resistant “Honggengansutao” (14/18 in roots and 25/36 in twigs, P ≤ 0.05) (see Table S8). Approximately 50% of the antagonists from “Honggengansutao” were isolated from the mock-inoculated samples, which was higher than that in “Okinawa” (P ≤ 0.05) (Table S8). It suggested that resistant cultivar “Honggengansutao” may possess inherently antagonistic endophytes, even in the absence of A. tumefaciens.
Further analysis of the pathogenicity-related ipt gene by PCR and reinoculation tests in sunflower seedlings (see Fig. S9) indicated that none of the Rhizobium strains from roots was pathogenic, while 12 of the 56 Rhizobium isolates from twigs harbored the pathogenic gene ipt. Among these 12, 8 derived from the inoculated susceptible “Okinawa” and 4 from the inoculated “Honggengansutao” (Fig. 5B).
DISCUSSION
The crown gall disease caused by A. tumefaciens is one of the most important diseases in peach. Continuous plantation leads to the accumulation of A. tumefaciens in soil (5) and makes the disease more serious. Until now, only one biocontrol agent, K84, has been commercialized; however, its application is limited due to its sole efficacy on nopaline strains of A. tumefaciens (38) and inconsistent effects in different environments (39). Other strategies to control crown gall disease are thereby urgently needed. Previous studies indicated that plant endophytes can make up a “second genome” of their host and fulfill important host functions (15, 40). However, few studies on endophytic bacteria have been conducted on peach, and their roles in disease resistance are unknown. In the present study, we focused on the ecological responses of the bacterial endophyte community to A. tumefaciens invasion and characterized the relationships among endophytic microbiota, antagonistic endophytes, and plant resistance to A. tumefaciens. The results not only reveal the composition of microbiota in susceptible and resistant cultivars but also facilitate the development of beneficial endophytes for biocontrol purposes.
High-throughput 16S rRNA gene sequencing gives a detailed picture of microbiota in terms of diversity and composition and may provide clues to microbial functions when coupled with bioinformatics tools. Another solution relies on partnering culture-independent studies with culture-dependent ones, i.e., community analysis and characterization of isolates, where dominant or differential bacteria can be selectively isolated for functional verification in vitro (37, 41, 42). Some culturable strains of Rhizobium, Pseudomonas, Bacillus, and Pantoea are successful biocontrol agents (43, 44) or have high biological control potential against crown gall disease (30, 45–52). In this study, similar genera were found to be strongly associated with pathogen invasion in a resistant peach cultivar, and some strains showed antagonistic activity via in vitro tests (see Fig. S10 in the supplemental material). Streptomyces, a well-known biocontrol agent and the dominant member (23.2%) of the peach tree root community, had no antagonistic activity in the pair culturing test (see Fig. S11). It might contribute to disease suppression through indirect mechanisms, such as systemic acquired resistance and the production of volatile organic compounds (53). However, some important bacteria in the resistant cultivar are relatively unculturable, including prevalent bacterial groups such as Actinoplanes and Massilia as well as seldom characterized and less abundant organisms like candidate phylum OD1, Planctomycetes, and Chloroflexi (Fig. S2). To verify their functions, new cultivation and screening strategies, such as optimization of the culture medium (54) and conditions (55) or multiple in vitro tests involved in different suppressive mechanisms, should be considered.
The microbiota associated with healthy or crown gall diseased trees has been studied previously by Ji et al. (56) using the PCR-denaturing gradient gel electrophoresis (DGGE) technique, with results indicating that the severity of crown gall disease had no effect on the community structure of rhizosphere bacteria. Similarly, Faist et al. (57) reported that the presence/absence of crown gall disease has no effect on the microbial community compositions of rhizosphere soil and grapevine roots and canes. However, our results indicated that the endophytic bacterial community of resistant “Honggengansutao” is higher in density and diversity in roots, contains more antagonists against A. tumefaciens, and has distinct responses to pathogen invasion. These findings endorse the hypothesis that the endophytic community is not made up of random guests in the plant habitat (17, 58). Instead, during community assembly, selective pressure enables the endophytic community to adapt and specialize to host plants; this coevolution and interactions between plants and beneficial microbes make endophytes essential to their hosts (59). For example, the resistant cultivar “Honggengansutao” hosts a sufficient diversity of “protective” endophytes, including Rhizobium, Streptomyces, Pseudomonas, Pantoea, and Bacillus, which can be provoked by pathogen attack and convey protective antagonism against phytopathogens (17). In addition, fewer pathogenic A. tumefaciens harboring the ipt gene were present in the inoculated “Honggengansutao” than in the inoculated “Okinawa” (Fig. 5B). The efficient inhibition of A. tumefaciens by “protective” endophytes might maintain the pathogen population below the threshold required for quorum sensing, restrict the T-DNA transfer from A. tumefaciens to peach, and cause smaller galls (60). Furthermore, it has been reported that salicylic acid (SA)-induced systemic acquired resistance is activated in “Honggengansutao” by pathogen infection (9).
For biocontrol applications, a threshold population level of 105 CFU/g root is required for a significant suppression of pathogens (61, 62). However, the peach endophyte populations are within 10 to 105 CFU/g fresh tissue, which is low to directly suppress pathogens. Considering that a diverse and balanced microbial system would be more conducive to disease resistance (63), this probiotic consortium may enhance disease suppression efficacy via intensified resource competition and interference with the pathogen. For example, communities with high species richness better suppress the pathogen Fusarium oxysporum, while the loss of less abundant bacteria results in a declined production of volatiles that suppress root pathogens (64). Although endophytic bacteria are low in abundance, they might be essential to prevent pathogen establishment and stimulate host immunity (65). These endophytic microbes may also act by other indirect mechanisms, such as plant growth promotion, systemic resistance induction, and better plant interior niche adaptation to contribute to plant health (15, 26).
In the present study, the peach endophytic bacteria mainly belonged to phyla Proteobacteria, Actinobacteria, Bacteroidetes, and Firmicutes, which were dominant during all developmental stages, as previously reported (66). Although both peach tree roots and twigs had Pseudomonas and nonpathogenic Rhizobium as the dominant genera, similarly to sorghum roots and shoots (67), they also harbored tissue-specific endophyte representatives (Fig. 2), i.e., Streptomyces in roots and Rhizobium in twigs. Because 16S rRNA gene amplicon sequences do not distinguish the inoculated pathogenic Agrobacterium from other Rhizobium sp., an enrichment in Rhizobium could be ascribed to the inoculated A. tumefaciens or enrichment in endogenous Rhizobium strains. Molecular detection of the virulent ipt gene indicated that no pathogenic A. tumefaciens was present in uninfected roots and twigs. In infected twigs, approximately half of Rhizobium isolates harbored the ipt gene, suggesting that the enrichment of Rhizobium in inoculated twigs partly resulted from the pathogen infection. This is consistent with New and Kerr’s observation (43) that nonpathogenic Rhizobium was present in healthy trees, while both nonpathogenic and pathogenic Rhizobium isolates were detected in the roots of infected trees.
The dominance of Rhizobium at D10 in twigs might possibly be ascribed to the incisions made during inoculation, which could lead to specific chemotactic movement of Rhizobium toward wound exudates (68, 69). The proportion of Rhizobium dropped with plant age, potentially due to different nutrient supplies (70) or the expansion of other endophytic bacteria (71). However, the proportion of antagonistic Rhizobium was always higher in the resistant cultivar, suggesting that Rhizobium might be responsible in part for the resistance to A. tumefaciens. Whether additional Rhizobium strains beyond K84 can promote peach plant resistance awaits further confirmation by inoculation assays, preferably on sterile seedlings.
Future studies should combine genomics, transcriptomics, metabolomics, and molecular biocontrol mechanism analysis to better characterize antagonists and their mechanisms of action, as performed by Carrion et al. (41). Ultimately, it will be possible to develop plant resistance by promoting specific microbial consortia prior to planting or even to develop customized biocontrol agents for field use, as has been conducted for damping-off disease (72). The development of a gnotobiotic system for peach tree growth will considerably advance this goal.
MATERIALS AND METHODS
Plant materials and A. tumefaciens inoculation.
Peach cultivars “Honggengansutao” and “Okinawa” have been grown in the National Peach Germplasm Repository of China (NPGRC, Zhengzhou, China) for 20 years, and their seeds were collected in 2012 and 2015 for field and greenhouse trials, respectively. All seeds were washed thoroughly, surface sterilized in 0.5% NaClO for 5 min, and rinsed 3 times in sterile deionized water before stratifying at 4°C for 3 months. After germination in autoclaved vermiculite for 1 week at 28°C, seedlings were grown in homogenized soils (0- to 20-cm depth) collected from the field of origin. For the field study, all seedlings were grown in the same experimental field next to NPGRC and treated with the same agronomic practice (no fertilizer or pesticide applied) for 2 years. For the greenhouse trial, seedlings were planted individually in 90-mm plastic pots and grown for 2 months in a greenhouse. Peach trees and seedlings were then subjected to experimental treatments as described below.
A. tumefaciens strain TA-AT-2 (biovar 2), isolated from a peach tree in Tai’an, China, was cultured in yeast extract and beef extract broth (YEB) (9) on a rotary shaker (200 rpm) at 28°C for 20 h, and aliquots used for inoculation were adjusted to a cell density of 109 CFU/ml.
A total of 180 peach plants were grown in the greenhouse experiment (90 per cultivar), including 45 plants of each cultivar inoculated with A. tumefaciens on root collars (I) and 45 mock-inoculated plants used as controls (M). The pathogen inoculation was performed as described by Hao et al. (9). Cuts of 1 cm in length were made into the cambium at the root collar, and 20 μl of either bacterial inoculum (109 CFU/ml) or sterile deionized water was applied to the incision, which was then covered by autoclaved vermiculite.
In the field study, five trees of each cultivar were selected for twig inoculation. Six newly grown twigs of each tree were randomly selected, and each twig was inoculated at five sites (with a 5-cm interval between inoculation sites) with A. tumefaciens suspension. Similar twigs inoculated with sterile deionized water were used as mock controls. At the end of the incubation period (60 days), the gall incidence, maximum diameter of each tumor, and diameters of stems and twigs of each plant (13 plants times 3 replicates per treatment for roots, 5 trees times 6 twigs times 5 sites per treatment for twigs) were measured and used for the calculation of the disease index (5). The data were statistically analyzed using Student’s t tests in R V3.4.3.
Sample collection.
Peach tree roots and twigs were collected from the two cultivars with or without inoculation at three time points (D0, D10, and D60) as shown in Fig. S1 in the supplemental material. Peach tree roots were collected from three randomly selected peach tree seedlings from each treatment group planted in the greenhouse. Roots were surface sterilized using a phosphate buffer wash followed by sonication (30 s at 50 to 60 Hz, 3 times [36]) and homogenized. The roots were dried on sterile filter paper and imprinted on tryptic soy agar (TSA) plates (30). No colonies appeared after incubating the plates at 28°C for 5 days, confirming the effectiveness of the surface sterilization procedure. An aliquot was snap-frozen and stored at −80°C for DNA extraction, and the remainders were stored at 4°C for bacterial isolation.
Similarly, peach tree twigs were collected from trees in orchards at D0, D10, and D60. Twigs from each cultivar were randomly selected at D0, while at D10 and D60, three inoculated or uninoculated twigs of different orientations were collected from each tree by using sterile pruning shears. The leaves were removed, and the twigs were washed 3 times with sterile deionized water, followed by sterilization with 70% ethanol for 30 s and 1% NaOCl for 3 min and 5 sterile deionized water washes. Duplicates of the last rinse (100 μl) were placed on TSA plates at 28°C for 5 days to confirm complete sterilization. Three twigs from each tree were homogenized after discarding segments near inoculation sites (±0.5 cm). A tissue aliquot was snap-frozen for DNA extraction and storage as described above.
DNA extraction and amplicon sequencing.
One-gram samples of frozen root or twig tissue were ground in liquid nitrogen into powder, and genomic DNA was extracted using the FastDNA Spin kit for soil (MP Biomedicals, USA) according to the manufacturer’s instructions. The quality of extracted DNA was checked by 1% agarose gel electrophoresis and spectrophotometry (optical density [OD] at 260/280 nm). DNA samples were stored at −20°C for subsequent analyses.
Using the DNA extracts as the templates, the V5-V7 region of the bacterial 16S rRNA gene spanning ∼400 bp was amplified with primers 799F (5′-AACMGGATTAGATACCCKG-3′ [73]) and 1193R (5′-ACGTCATCCCCACCTTCC-3′ [74]). These primers contained a set of 8-nucleotide barcode sequences unique to each sample. The PCR program was as follows: 95°C for 5 min, 25 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 40 s, and a final extension of 72°C for 10 min. PCRs were performed in triplicates, and the 25-μl mixture contained 2.5 μl of 10× Pyrobest buffer, 2 μl of 2.5 mM deoxynucleoside triphosphates (dNTPs), 1 μl of each primer (10 μM), 0.4 U of Pyrobest DNA polymerase (TaKaRa, Japan), and 15 ng of template DNA. Sterile RNase-free water was used as a negative-control template in each PCR run.
Amplicons with bacterial products of approximately 400 bp were extracted from 2% agarose gels, purified using the AxyPrep DNA gel extraction kit (Axygen Biosciences, USA) according to the manufacturer’s instructions, and quantified using QuantiFluor-ST (Promega, USA). Purified amplicons were pooled in equimolar ratios and subjected to paired-end sequencing (2 × 300) by Allwegene (Beijing, China) using the MiSeq PE300 sequencing platform (Illumina, USA).
Processing of sequencing data.
Sequencing data were processed by the custom pipeline developed by Allwegene (Beijing, China). Raw DNA sequences were filtered based on sequence length and quality, and primer and tag sequences were removed using QIIME software v1.2.1 (75). Sequences that overlapped more than 10 bp were assembled using FLASH v1.2.7 (76), while read pairs which could not be assembled were discarded. Paired-end sequences were clustered into operational taxonomic units (OTUs) at 97% sequence similarity using UCLUST (77), and chimeric sequences were removed using USEARCH v8.0.1623 (78). The taxonomy of these OTUs was assigned by UCLUST using the Silva 119 16S rRNA database (79, 80) as a reference, with assignments made using a confidence threshold of 90%. OTUs identified as plastids (0.003% to 0.03% reads in roots and 6.52% to 33.68% reads in twigs) or mitochondria (0.19% to 1.37% reads in roots and 1.18% to 13.54% reads in twigs) were removed.
Amplicon sequencing data analysis.
OTU tables derived from 16S amplicon sequencing data analyses were analyzed in R v3.4.3 using the phyloSeq (81), Vegan (82), ggplot2 (83), randomForest (84), and mvpart (85) packages. Nonmetric multidimensional scaling (NMDS) ordinations were generated using the metaMD function in Vegan. Multiple regression tree (MRT) analysis and permutational multivariate analysis of variance (PERMANOVA [86]) were used to compare the effects of time, cultivar, and inoculation on the whole bacterial community. The Shannon diversity index (87) was used to account for both the abundance and evenness of present OTUs in each treatment, computed with the phyloSeq package plot_richness function. One-way analysis of similarities (ANOSIM) was used to detect the difference in endophyte assemblages among different time points using anosim in Vegan, while an analysis of variance (ANOVA) was used to test other significant differences among groups. The relative strength of each experimental factor contributing to the patterns in microbial community composition across samples was tested using the function randomForest in the randomForest package in R. Differentially abundant OTUs were identified with similarity percentage (SIMPER) analyses and a Kruskal-Wallis test. Phylogenetic trees of the 16S rRNA sequences (OTU abundance, >0.5%) and alignments between OTUs and isolates were generated by Geneious 11.0.5 (Biomatters, New Zealand) and visualized using the Interactive Tree of Life (iTOL) v4.1.1 (88).
Isolation and identification of bacteria from roots and twigs.
One-gram samples of root or twig tissues were ground in 9 ml of phosphate buffer (pH 7.2) with sterile quartz sand using a sterile mortar and pestle. Serial dilutions were subsequently prepared in sterile deionized water. An aliquot of 100 μl of the suspension was plated on tryptic soy agar (TSA) and incubated at 28°C. The colony numbers and morphologies were counted after 24 to 48 h of growth, and logarithm numbers of CFU per gram (log10 CFU/g) were calculated. Sixty isolates of each subset (time, cultivar, and treatment) (Fig. S1) were randomly selected from both peach tree roots and twigs, confirming that all morphologies were represented, to give the total of 1,200 single colonies for antagonistic assays in vitro.
Individual colonies were cultured separately in tryptic soy broth (TSB) (30) on a rotary shaker (200 rpm) at 28°C overnight. Bacterial suspensions of selected colonies (2 ml) were used for DNA extraction using the genomic DNA extraction kit (Tiangen, China). Universal primers 27f/1492r were employed for the 16S rRNA gene amplification (89), and amplification was confirmed using a 1.2% agarose gel prior to Sanger sequencing by Sango, China. Sequences were evaluated and assembled using DNAStar Lasergene v7.1 (DNAStar, USA). Top hits (all >97% sequence identity) of a BLAST search (http://blast.ncbi.nlm.nih.gov) were used to identify the highest possible taxonomic resolution of isolates to the genus or species level.
Antagonistic assay.
One strain of each ribotype (a group of isolates with identical 16S rRNA sequences) was selected for the antagonistic test. Antagonistic assays were conducted by using the pair culturing method (90). Briefly, 1 ml of the A. tumefaciens cell suspension mixture (108 CFU/ml) of strains ATCC 23308T (biovar 1) and TA-AT-2 (biovar 2) was combined with 20 ml of YEB medium and plated on petri dishes. Peach endophyte isolate cultures were then inoculated on these plates at three places on the petri dishes. After 2 days of incubation at 28°C, the diameter of each inhibition zone was measured. Antagonistic assays were performed in three biological replicates. Noninoculated plates served as controls.
PCR screening for pathogenic genes in Rhizobium isolates and inoculation tests.
Each endophyte isolate which was identified as Rhizobium by sequencing was subjected to further pathogenic analysis. PCR-based screening for pathogenic Rhizobium was performed using ipt 3F/ipt 3R primers and a corresponding PCR amplification protocol, which targets a conserved portion of T-DNA affecting the strain’s pathogenicity (5, 91, 92). PCR products were visualized on a 1.2% agarose gel, and specific amplicons of pathogenic Rhizobium of 247 bp in length were identified. The pathogenicity of the Rhizobium isolates was also confirmed by inoculating sunflower stems with a bacterial suspension and observing the formation of galls (93).
Accession number(s).
The 16S rRNA gene amplicon sequences were deposited in the NCBI Sequence Read Archive (SRA) database under accession numbers SRR6801696 to SRR6801755. The 16S rRNA nucleotide sequences of bacterial isolates were deposited at GenBank under accession numbers MG835926 to MG836230.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the earmarked fund for China Agriculture Research System (CARS-30-3-01) and a grant from the China Scholarship Council (201606350017, to Q.L.). The work conducted by the U.S. Department of Energy Joint Genome Institute, a DOE Office of Science User Facility, is supported by the Office of Science of the U.S. Department of Energy under contract number DE-AC02-05CH11231.
We thank Lirong Wang and Fengge Hao for their assistance in the sample collections.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02931-18.
REFERENCES
- 1.De Cleene M, De Ley J. 1976. The host range of crown gall. Bot Rev 42:389–466. doi: 10.1007/BF02860827. [DOI] [Google Scholar]
- 2.Young JM, Kuykendall LD, Martinez-Romero E, Kerr A, Sawada H. 2001. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int J Syst Evol Microbiol 51:89–103. doi: 10.1099/00207713-51-1-89. [DOI] [PubMed] [Google Scholar]
- 3.Agrios GN. 2005. Plant pathology, 5th ed Academic Press, San Diego, CA. [Google Scholar]
- 4.Kado CI. 2002. Crown gall. The plant health instructor. 10.1094/PHI-I-2002-1118-01. [DOI]
- 5.Li Q, Guo R, Li S, Li S, Wang H. 2015. Determination of tumorigenic Agrobacterium density in soil by real-time PCR assay and its effect on crown gall disease severity. Eur J Plant Pathol 142:25–36. doi: 10.1007/s10658-014-0586-3. [DOI] [Google Scholar]
- 6.Zoina A, Raio A. 1999. Susceptibility of some peach rootstocks to crown gall. J Plant Pathol 81:181–187. [Google Scholar]
- 7.Rhouma A, Boubaker A, Nesme X, Dessaux Y. 2005. Susceptibility of some stone and pome rootstocks to crown gall. Phytopathol Mediterr 44:275–284. [Google Scholar]
- 8.Thomidis T, Exadaktylou E, Tsipouridis C. 2005. Susceptibility of five Prunus rootstocks to Agrobacterium tumefaciens. N Z J Crop Hortic Sci 33:343–345. doi: 10.1080/01140671.2005.9514368. [DOI] [Google Scholar]
- 9.Hao F, Wang L, Cao K, Wang X, Fang W, Zhu G, Chen C. 2015. Systemic acquired resistance induced by Agrobacterium tumefaciens in peach and differential expression of PR1 genes. HortScience 50:666–672. doi: 10.21273/HORTSCI.50.5.666. [DOI] [Google Scholar]
- 10.Kerr A, Htay K. 1974. Biological control of crown gall through bacteriocin production. Physiol Plant Pathol 4:37–48. doi: 10.1016/0048-4059(74)90042-3. [DOI] [Google Scholar]
- 11.Cooksey DA, Moore LW. 1982. Biological control of crown gall with an agrocin mutant of Agrobacterium radiobacter. Phytopathology 72:919–921. doi: 10.1094/Phyto-72-919. [DOI] [Google Scholar]
- 12.Peñalver R, López MM, 1999. Cocolonization of the rhizosphere by pathogenic Agrobacterium strains and nonpathogenic strains K84 and K1026, used for crown gall biocontrol. Appl Environ Microbiol 65:1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alconero R. 1980. Crown gall of peaches from Maryland, South Carolina, and Tennessee and problems with biological control. Plant Dis 64:835–838. doi: 10.1094/PD-64-835. [DOI] [Google Scholar]
- 14.Escobar MA, Dandekar AM. 2003. Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci 8:380–386. doi: 10.1016/S1360-1385(03)00162-6. [DOI] [PubMed] [Google Scholar]
- 15.Hardoim PR, van Overbeek LS, Berg G, Pirttila AM, Compant S, Campisano A, Döring M, Sessitsch A. 2015. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristin A, Miranda H. 2013. The root microbiota—a fingerprint in the soil? Plant Soil 370:671–686. doi: 10.1007/s11104-013-1647-7. [DOI] [Google Scholar]
- 17.Podolich O, Ardanov P, Zaets I, Pirttilä AM, Kozyrovska N. 2015. Reviving of the endophytic bacterial community as a putative mechanism of plant resistance. Plant Soil 388:367–377. doi: 10.1007/s11104-014-2235-1. [DOI] [Google Scholar]
- 18.Araújo WL, Marcon J, Maccheroni W Jr, van Elsas JD, van Vuurde JWL, Azevedo JL. 2002. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol 68:4906–4914. doi: 10.1128/AEM.68.10.4906-4914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graner G, Persson P, Meijer J, Alstrom S. 2003. A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 29:269–276. doi: 10.1016/S0378-1097(03)00449-X. [DOI] [PubMed] [Google Scholar]
- 20.Tan HM, Cao LX, He ZF, Su GJ, Lin B, Zhou SN. 2006. Isolation of endophytic actinomycetes from different cultivars of tomato and their activities against Ralstonia solanacearum in vitro. World J Microbiol Biotechnol 22:1275–1280. doi: 10.1007/s11274-006-9172-y. [DOI] [Google Scholar]
- 21.Upreti R, Thomas P. 2015. Root-associated bacterial endophytes from Ralstonia solanacearum resistant and susceptible tomato cultivars and their pathogen antagonistic effects. Front Microbiol 6:255. doi: 10.3389/fmicb.2015.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulgari D, Casati P, Crepaldi P, Daffonchio D, Quaglino F, Brusetti L, Bianco PA. 2011. Restructuring of endophytic bacterial communities in grapevine yellows-diseased and recovered Vitis vinifera L. plants. Appl Environ Microbiol 77:5018–5022. doi: 10.1128/AEM.00051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douanla-Meli C, Langer E, Mouafo FT. 2013. Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon. Fungal Ecol 6:212–222. doi: 10.1016/j.funeco.2013.01.004. [DOI] [Google Scholar]
- 24.Reiter B, Pfeifer U, Schwab H, Sessitsch A. 2002. Response of endophytic bacterial communities in potato plants to infection with Erwinia carotovora subsp. atroseptica. Appl Environ Microbiol 68:2261–2268. doi: 10.1128/AEM.68.5.2261-2268.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian BY, Cao Y, Zhang KQ. 2015. Metagenomic insights into communities, functions of endophytes, and their associates with infection by root-knot nematode, Meloidogyne incognita, in tomato roots. Sci Rep 5:17087. doi: 10.1038/srep17087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mei C, Flinn BS. 2010. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat Biotechnol 4:81–95. doi: 10.2174/187220810790069523. [DOI] [PubMed] [Google Scholar]
- 27.Nuangmek W, McKenzie EHC, Lumyong S. 2008. Endophytic fungi from wild banana (Musa acuminata Colla) works against anthracnose disease caused by Colletotrichum musae. Res J Microbiol 3:368–374. doi: 10.3923/jm.2008.368.374. [DOI] [Google Scholar]
- 28.Manici LM, Kelderer M, Franke-Whittle IH, Rühmer T, Baab G, Nicoletti F, Caputo F, Topp A, Insam H, Naef A. 2013. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl Soil Ecol 72:207–214. doi: 10.1016/j.apsoil.2013.07.011. [DOI] [Google Scholar]
- 29.Liaqat F, Eltem R. 2016. Identification and characterization of endophytic bacteria isolated from in vitro cultures of peach and pear rootstocks. 3 Biotech 6:120. doi: 10.1007/s13205-016-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Li Q, Zhang Z, Li S. 2017. Screening and identification of peach endophytic bacteria with antagonism against Agrobacterium tumefaciens. Sci Agric Sin 50:3918–3929. doi: 10.3864/j.issn.0578-1752.2017.20.008. [DOI] [Google Scholar]
- 31.Xia Y, Greissworth E, Mucci C, Williams MA, De Bolt S. 2013. Characterization of culturable bacterial endophytes of switchgrass (Panicum virgatum L.) and their capacity to influence plant growth. Glob Change Biol Bioenergy 5:674–682. doi: 10.1111/j.1757-1707.2012.01208.x. [DOI] [Google Scholar]
- 32.de Almeida Lopes KB, Carpentieri-Pipolo V, Oro TH, Stefani Pagliosa E, Degrassi G. 2016. Culturable endophytic bacterial communities associated with field-grown soybean. J Appl Microbiol 120:740–755. doi: 10.1111/jam.13046. [DOI] [PubMed] [Google Scholar]
- 33.Bulgarelli D, Rott M, Schlaeppi K, Ver Loren van Themaat E, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P. 2012. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95. doi: 10.1038/nature11336. [DOI] [PubMed] [Google Scholar]
- 34.Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, Del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL. 2012. Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, Buckler ES, Ley RE. 2013. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci U S A 110:6548–6553. doi: 10.1073/pnas.1302837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards J, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, Eisen JA, Sundaresan V. 2015. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci U S A 112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lebeis SL. 2014. The potential for give and take in plant-microbiome relationships. Front Plant Sci 5:287. doi: 10.3389/fpls.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HM, Wang HX, Ng TB, Li JY. 2003. Purification and characterization of an antibacterial compound produced by Agrobacterium vitis strain E26 with activity against A. tumefaciens. Plant Pathol 52:134–139. doi: 10.1046/j.1365-3059.2003.00807.x. [DOI] [Google Scholar]
- 39.Berg G, Krause R, Mendes R. 2015. Cross-kingdom similarities in microbiome ecology and biocontrol of pathogens. Front Microbiol 6:1311. doi: 10.3389/fmicb.2015.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg G, Grube M, Schloter M, Smalla K. 2014. Unraveling the plant microbiome: looking back and future perspectives. Front Microbiol 5:148. doi: 10.3389/fmicb.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrion VJ, Cordovez V, Tyc O, Etalo DW, de Bruijn I, de Jager VCL, Medema MH, Eberl L, Raaijmakers JM. 2018. Involvement of Burkholderiaceae and sulfurous volatiles in disease-suppressive soils. ISME J 12:2307–2321. doi: 10.1038/s41396-018-0186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckers B, Op De Beeck M, Weyens N, Van Acker R, Van Montagu M, Boerjan W, Vangronsveld J. 2016. Lignin engineering in field-grown poplar trees affects the endosphere bacterial microbiome. Proc Natl Acad Sci U S A 113:2312–2317. doi: 10.1073/pnas.1523264113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.New PB, Kerr A. 1972. Biological control of crown gall: field measurements and glasshouse experiments. J Appl Bacteriol 35:279–287. doi: 10.1111/j.1365-2672.1972.tb03699.x. [DOI] [Google Scholar]
- 44.Jones DA, Ryder MH, Clare BG, Farrand SK, Kerr A. 1988. Construction of a Tra deletion mutant pfpAgK84 to safeguard the biological control of crown gall. Mol Genet Genomics 212:207–214. doi: 10.1007/BF00334686. [DOI] [Google Scholar]
- 45.Gupta AK, Khosla K, Bhardwaj SS, Thakur A, Devi S, Jarial RS, Sharma C, Singh KP, Srivastava DK, Lal R. 2010. Biological control of crown gall on peach and cherry rootstock colt by native Agrobacterium radiobacter isolates. Open Hortic J 3:1–10. doi: 10.2174/1874840601003010001. [DOI] [Google Scholar]
- 46.Khmel IA, Sorokina TA, Lemanova NB, Lipasova VA, Metlitski OZ, Burdeinaya TV, Chernin LS. 1998. Biological control of crown gall in grapevine and raspberry by two Pseudomonas spp. with a wide spectrum of antagonistic activity. Biocontrol Sci Technol 8:45–57. doi: 10.1080/09583159830423. [DOI] [Google Scholar]
- 47.Toklikishvili N, Dandurishvili N, Vainstein A, Tediashvili M, Giorgobiani N, Lurie S, Szegedi E, Glick BR, Chernin L. 2010. Inhibitory effect of ACC deaminase-producing bacteria on crown gall formation in tomato plants infected by Agrobacterium tumefaciens or A. vitis. Plant Pathol 59:1023–1030. doi: 10.1111/j.1365-3059.2010.02326.x. [DOI] [Google Scholar]
- 48.Dandurishvili N, Toklikishvili N, Ovadis M, Eliashvili P, Giorgobiani N, Keshelava R, Tediashvili M, Vainstein A, Khmel I, Szegedi E, Chernin L. 2011. Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumors on tomato plants. J Appl Microbiol 110:341–352. doi: 10.1111/j.1365-2672.2010.04891.x. [DOI] [PubMed] [Google Scholar]
- 49.Hammami I, Rhouma A, Jaouadi B, Rebai A, Nesme X. 2009. Optimization and biochemical characterization of a bacteriocin from a newly isolated Bacillus subtilis strain 14B for biocontrol of Agrobacterium spp. strains. Lett Appl Microbiol 48:253–260. doi: 10.1111/j.1472-765X.2008.02524.x. [DOI] [PubMed] [Google Scholar]
- 50.Tolba IH, Soliman MA. 2013. Efficacy of native antagonistic bacterial isolates in biological control of crown gall disease in Egypt. Ann Agric Sci 58:43–49. doi: 10.1016/j.aoas.2013.01.007. [DOI] [Google Scholar]
- 51.Ben Abdallah D, Frikha-Gargouri O, Tounsi S. 2015. Bacillus amyloliquefaciens strain 32a as a source of lipopeptides for biocontrol of Agrobacterium tumefaciens strains. J Appl Microbiol 119:196–207. doi: 10.1111/jam.12797. [DOI] [PubMed] [Google Scholar]
- 52.Frikha-Gargouri O, Ben Abdallah D, Ghorbel I, Charfeddine I, Jlaiel L, Triki MA, Tounsi S. 2017. Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress Agrobacterium crown gall tumors on tomato plants. Pest Manag Sci 73:568–574. doi: 10.1002/ps.4331. [DOI] [PubMed] [Google Scholar]
- 53.Viaene T, Langendries S, Beirinckx S, Maes M, Goormachtig S. 2016. Streptomyces as a plant’s best friend? FEMS Microbiol Ecol 92:fiw119. doi: 10.1093/femsec/fiw119. [DOI] [PubMed] [Google Scholar]
- 54.Armanhi JSL, de Souza RSC, Damasceno NB, de Araujo LM, Imperial J, Arruda P. 2017. A community-based culture collection for targeting novel plant growth-promoting bacteria from the sugarcane microbiome. Front Plant Sci 8:2191. doi: 10.3389/fpls.2017.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gottschal J, Harder W, Prins RA. 1992. Principles of enrichment, isolation, cultivation, and preservation of bacteria. Prokaryotes 1:149–196. [Google Scholar]
- 56.Ji L, Yang W, Wang Y, Liu HX, Guo JH. 2011. Analysis of the rhizosphere bacterial community collected from different orchards in Shanghai. J Agric 1:55–59. [Google Scholar]
- 57.Faist H, Keller A, Hentschel U, Deeken R. 2016. Grapevine (Vitis vinifera) crown galls host distinct microbiota. Appl Environ Microbiol 82:5542–5552. doi: 10.1128/AEM.01131-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaiero JR, McCall CA, Thompson KA, Day NJ, Best AS, Dunfield KE. 2013. Inside the root microbiome: bacterial root endophytes and plant growth promotion. Am J Bot 100:1738–1750. doi: 10.3732/ajb.1200572. [DOI] [PubMed] [Google Scholar]
- 59.Baltrus DA. 2017. Adaptation, specialization, and coevolution within phytobiomes. Curr Opin Plant Biol 38:109–116. doi: 10.1016/j.pbi.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 60.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raaijmakers JM, Leeman M, van Oorschot MMP, van der Sluis I, Schippers B, Bakker PAHM. 1995. Dose-response relationships in biological control of fusarium wilt of radish by Pseudomonas spp. Phytopathology 85:1075–1081. doi: 10.1094/Phyto-85-1075. [DOI] [Google Scholar]
- 62.Kawaguchi A, Kondo KI, Inoue K. 2012. Biological control of apple crown gall by nonpathogenic Rhizobium vitis strain VAR03-1. J Gen Plant Pathol 78:287–293. doi: 10.1007/s10327-012-0388-4. [DOI] [Google Scholar]
- 63.van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. 2012. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci U S A 109:1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hol WHG, Garbeva P, Hordijk C, Hundscheid MPJ, Gunnewiek PJAK, van Agtmaal M, Kuramae EE, de Boer W. 2015. Non-random species loss in bacterial communities reduces antifungal volatile production. Ecology 96:2042–2048. doi: 10.1890/14-2359.1. [DOI] [PubMed] [Google Scholar]
- 65.Jousset A, Bienhold C, Chatzinotas A, Gallien L, Gobet A, Kurm V, Küsel K, Rillig MC, Rivett DW, Salles JF, van der Heijden MGA, Youssef NH, Zhang X, Wei Z, Hol WHG. 2017. Where less may be more: how the rare biosphere pulls ecosystems strings. ISME J 11:853–862. doi: 10.1038/ismej.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kandel S, Joubert P, Doty S. 2017. Bacterial endophyte colonization and distribution within plants. Microorganisms 5:77. doi: 10.3390/microorganisms5040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maropola MK, Ramond JB, Trindade M. 2015. Impact of metagenomic DNA extraction procedures on the identifiable endophytic bacterial diversity in Sorghum bicolor (L. Moench). J Microbiol Methods 112:104–117. doi: 10.1016/j.mimet.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Currier WW, Strobel GA. 1976. Chemotaxis of Rhizobium spp. to plant root exudates. Plant Physiol 57:820–823. doi: 10.1104/pp.57.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aguilar JM M, Ashby AM, Richards AJM, Loake GJ, Watson MD, Shaw CH. 1988. Chemotaxis of Rhizobium leguminosarum biovar. phaseoli towards flavonoid inducers of the symbiotic nodulation genes. J Gen Microbiol 134:2741–2746. [Google Scholar]
- 70.Yuan J, Chaparro JM, Manter DK, Zhang R, Vivanco JM, Shen Q. 2015. Roots from distinct plant developmental stages are capable of rapidly selecting their own microbiome without the influence of environmental and soil edaphic factors. Soil Biol Biochem 89:206–209. doi: 10.1016/j.soilbio.2015.07.009. [DOI] [Google Scholar]
- 71.Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P. 2013. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 72.Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JHM, Piceno YM, DeSantis TZ, Andersen GL, Bakker PAHM, Raaijmakers JM. 2011. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- 73.Chelius MK, Triplett EW. 2001. The diversity of archaea and bacteria in association with the roots of Zea mays L. Microb Ecol 41:252–263. doi: 10.1007/s002480000087. [DOI] [PubMed] [Google Scholar]
- 74.Bodenhausen N, Horton MW, Bergelson J. 2013. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS One 8:e56329. doi: 10.1371/journal.pone.0056329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 78.Edgar RC. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 79.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McMurdie PJ, Holmes S. 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O`Hara RB, Simpson GL, Solymos P. 2017. vegan: Community Ecology Package version 2.4-5. https://cran.r-project.org/web/packages/vegan/index.html.
- 83.Wickham H, Chang W. 2016. ggplot2: create elegant data visualisations using the grammar of graphics version 2.2.1. https://cran.r-project.org/web/packages/ggplot2/index.html.
- 84.Breiman L. 2001. Random forests. Mach Learn 45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 85.Therneau TM, Atkinson B. 2014. mvpart: multivariate regression trees version 1.6-2. https://cran.r-project.org/web/packages/mvpart/index.html.
- 86.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
- 87.Bowman K, Hutcheson K, Odum E, Shenton L. 1971. Comments on the distribution of indices of diversity. Stat Ecol 3:315–359. [Google Scholar]
- 88.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, NY. [Google Scholar]
- 90.Gao Z, Li Q, Guo R, Li S, Li S, Wang H. 2015. Suppression effect of two peach rhizobacteria Alcaligenes faecalis on crown gall disease caused by Agrobacterium tumefaciens. J Fruit Sci 32:267–273. [Google Scholar]
- 91.Buchmann I, Marner FJ, Schroder G, Waffenschmidt S, Schroder J. 1985. Tumor genes in plants: T-DNA encoded cytokinin biosynthesis. EMBO J 4:853–859. doi: 10.1002/j.1460-2075.1985.tb03710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akiyoshi DE, Klee H, Amasino RM, Nester EW, Gordon MP. 1984. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci U S A 81:5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loper JE, Kado CI. 1979. Host range conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens. J Bacteriol 139:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.