Abstract
Abstract. Classically, the stem/progenitor cells of the pulmonary epithelium are considered to be the basal and mucous cells of the proximal airways, Clara cells in the bronchioles and type II pneumocytes in the alveoli. Recent data suggest that there is a variant of Clara cells, lying in pulmonary neuroendocrine bodies, that meets several stem cell criteria and that type II pneumocytes exist in at least two populations, one of which is more resistant to injury. However, a complete revision of our understanding of pulmonary stem cell biology is underway as a result of the discovery of pulmonary epithelium derived from blood‐borne cells. In addition, the existence in the lung of a ‘universal’ pluripotent cell has long been speculated upon and now some initial evidence has emerged with the identification of a spore‐like cell that can differentiate in vitro to bronchiolar tissue.
INTRODUCTION
The average, normal adult lung contains around 70 m2 of gas diffusion surface. The lining in many areas comprises only a monolayer of cells and, in the case of alveolar type I pneumocytes, can be as thin as 0.1 µm. This epithelial surface is constantly open to potential injury and so, to maintain that protective lining, rapid response mechanisms are in place that lead to epithelial renewal. Key amongst these are the stem and progenitor cells. Although the exact nature of stem cells has been the subject of considerable debate (Morrison 1997; Potten 1997), a generally accepted definition is that they are clonogenic cells that are capable of self‐renewal and multilineage differentiation (Till & McCulloch 1961; Metcalf & Moore 1971; Fuchs & Serge 2000; Weissman 2000; Blau et al. 2001). This stable population of undifferentiated cells gives rise to progenitors that have little or no capacity to self‐renew and that show signs of differentiation. As far as the lung is concerned, research into stem cells has been hindered by the structural and functional complexities of the organ. For several years, the consensus has been that there are various types of stem cells, differing according to their position within the pulmonary tree, and that they often form pools, ready to proliferate in response to injury and effect local repair (Magdeleno et al. 1998; Engelhardt 2001; Emura 2002; Otto 2002). However, the recent finding of blood‐borne cells in the lung and the characterization of a potential universal stem cell, giving rise to intact lung tissue, are challenging some of the long‐held views about the nature of the pulmonary epithelium and its capacity to renew.
DEVELOPMENT AND ORGANIZATION OF PULMONARY EPITHELIUM
The mammalian lung develops as an outgrowth of the embryonic gut. In humans, it originates from a diverticulum of the ventral wall of the primitive oesophagus somewhere between 4 and 5 weeks of gestation. From then on, the endoderm/epithelium undergoes dichotomous branching into the splanchnic mesenchyme that surrounds it. This highly ordered patterning process of repeated bud outgrowth and division of terminal units (Hogan 1999) is known as branching morphogenesis and gives rise to the pulmonary tree and defines the proximal‐distal axis of the lung. The development of the mammalian lung has been divided into four phases and, in humans, the timings of these are; embryonic 0–5 weeks, glandular 5–16 weeks, canalicular 16–26 weeks and saccular 26 weeks to term (Adamson 1991). The primordial lining that forms in the embryonic stage develops into pseudostratified epithelium during the early glandular phase and, as branching progresses, columnar epithelium forms. During the glandular and into the canalicular phase, the initial thick layer of stratified epithelial cells starts to thin and shows gradation, becoming thinner along the length of the tree. Submucosal glands first appear at around 10 weeks in the trachea but not until 16 weeks in the bronchi. Bronchioles appear during the canalicular stage, marking the start of the formation of gas exchange units. A lumen is present at this stage and the epithelium starts to look more cuboidal, an appearance that remains into the final saccular phase where alveolar ducts and air sacs begin to open. The final formation of alveoli takes place post‐natally. Thus, the mature lung has distinct anatomical regions lined by different types of epithelial cells. In the mature lung, the trachea and major bronchi are lined by pseudostratified epithelium. The major phenotypes in the proximal airways are ciliated and mucous secretory (or goblet) cells, with the more infrequent neuroendocrine cells and the less well‐differentiated basal cells lying in a basal position. The bronchioles are also lined by ciliated cells, but possess a separate phenotype known as the Clara cells that are non‐ciliated. The alveoli are lined by flattened squamous (type I pneumocytes) and cuboidal (type II pneumocytes) cells. A further class of epithelial cells that populates the lung is the neuroendocrine cells that first appear around 8 weeks of gestation. These contain biogenic amines, commonly serotonin (Lauweryns et al. 1986), and/or peptides, including bombesin (Wharton et al. 1978) and calcitonin gene‐related peptide (Johnson & Wobken 1987). They are relatively frequent in the developing lung, where they play a major role in airway growth and development, but form > 1% of epithelial cells in adult lung, where they are seen as scattered elements in the epithelium or in innervated epithelial corpuscles, so‐called neuroepithelial bodies (Lauweryns & Cokelaere 1973; Cutz & Orange 1977).
STEM CELL NICHES
Proximal airways
In the trachea and bronchi, the basal and mucous secretory cells are widely believed to be stem cells (Breeze & Wheeldon 1977; Reid & Jones 1979; Kauffman 1980; Donnelly et al. 1982). The basal cells, and the parabasal cells that lie just above them, certainly form a pluripotential reserve cell that, unlike the surrounding epithelium, usually survives injury (Emura 1997). The basal cell appears at around 10 weeks of gestation in the human trachea, is roughly triangular and lies under the columnar epithelial layer, with one edge firmly anchoring the epithelium to the basement membrane. One of the first suggestions that basal cells may be precursors of airway epithelium came with the observation that, in rat lung, they take up [3H]thymidine that is later found in ciliated and goblet cells (Bindreiter et al. 1967). In addition, they accumulate at the sites of injury (Lane & Gordon 1974) and their histological appearance is intermediate, sharing characteristics with ciliated cells and goblet cells (Breeze & Wheeldon 1997). In vitro procedures that involve denuding the trachea have demonstrated the capacity of basal cells to produce all the major cell phenotypes found in the trachea, including basal, ciliated, goblet and granular secretory cells (1988, 1989; Nettesheim et al. 1990; Liu et al. 1994). Evidence that the basal and parabasal cells are stem cells in human lung includes the demonstration, using a proliferation marker (MIB‐1), that they form a disproportionate fraction of the proliferative compartment of the epithelium (Boers et al. 1998).
The evidence that mucous cells are stem cells is not so abundant, but particular interest has been paid to this possibility in view of their potential as targets for gene therapy in cystic fibrosis (Engelhardt et al. 1995; Duan et al. 1998). Bromodeoxyuridine (BrdU) label‐retaining cells (LRCs) are considered to be stem cells (Engelhardt 2001) and such cells have been found in gland ducts of the upper trachea following injury (Borthwick et al. 2001). The same group also showed, by heterotopic tracheal grafting on denuded trachea, that the surface epithelium was reconstituted by cells from the glands. In a model of tracheal epithelial regeneration, secretory, and basal, cells were found to dedifferentiate into a highly proliferative phenotype which gave rise to mucociliary epithelium (Liu et al. 1994).
Bronchioles
Clara cells were first described in 1937 (Clara 1937) and have since been shown to be progenitors of themselves and of ciliated cells in the bronchioles (1976, 1978; Plopper & Dungworth 1987; Boers et al. 1999). However, more recent research has established that a subset of Clara cells fulfils the criteria of adult, niche‐specific stem cells. Pools of stem cells have been discovered that express Clara cell secretory protein (CCSP) but are not typical Clara cells as they are resistant to airway pollutants such as naphthalene (Mahvi et al. 1977; Plopper et al. 1992; Stripp et al. 1995; 2000a, 2000b; Hong et al. 2001; Giangreco et al. 2002). Generally, Clara cells are enriched with cytochrome P‐450 enzymes that would make them vulnerable to naphthelene (Devereux et al. 1981). In addition, these variant CCSP‐expressing (or vCE) cells show multipotent differentiation. The vCE cells are located in discrete pools in neuroepithlial bodies and at the broncho‐alveolar duct junction.
There seems to be some controversy as to whether pulmonary neuroenodrine cells (PNEC) can proliferate. The classical view is that they cannot (Hoyt et al. 1990; Montengua et al. 1992; McDowell et al. 1993; Gosney 1997) and it has been suggested that PNEC populations may be maintained (Linnoila 1982) and become hyperplastic (Sunday and Willett 1992, 1994) from a multipotent progenitor. Such a progenitor has been described in the mouse lung where Clara cell protein and a product of PNECs, calcitonin gene‐related peptide (CGRP) were co‐expressed in most epithelial cells of the distal airways of the murine lung at E13‐15 (Wuenschell et al. 1996), although this evidence is based solely on immunocytochemistry. Certainly, PNECs secrete regulatory factors that play roles in the regulation of epithelial cell renewal, for example gastrin‐releasing peptide (or mammlian bombesin) that is a potent epithelial cell mitogen (Wharton et al. 1978; 1990, 1991; Cutz et al. 1995). A study designed to test whether vCE and/or CGRP‐expressing PNECS have pluripotent capacity for epithelial renewal used a transgenic model with thymidine kinase gene under the direction of the CCSP promoter (Hong et al. 2001). Following temporary ablation of the entire CCSP‐expressing population, hyperplasia of CGRP‐expressing PNECs occurred, but these cells did not repopulate the epithelium, supporting the view that they are not stem cells.
Alveoli
The first evidence that type II pneumocytes (Fig. 1) are stem cells emerged 50 years ago when they were shown to proliferate following injury (Mackin 1954). It was shown that they restore the alveolar epithelium following generalized damage by oxidants, e.g. oxygen and nitrogen dioxide, by giving rise to either new type II cells or the squamous type I pneumocytes, the latter being destroyed following most types of lung injury (Evans et al. 1971; Adamson & Bowden 1974a; Evans et al. 1975; Witschi 1978; Kauffman 1980; Bowden 1981). Injury to type I cells is not the only stimulus for proliferation of type II pneumocytes. They respond in this way when damaged selectively by butylated hydroxytoluene (Adamson et al. 1977) and bleomycin (Adamson & Bowden 1974b, 1979) or as a consequence of inflammatory infiltration, e.g. following particle inhalation (Shami et al. 1986). It has been shown that alveolar type II cell injury and apoptosis may be an important early feature in the pathogenesis of pulmonary fibrosis (Haschek & Witschi 1979; Katzenstein 1985; Maeyama et al. 2001).
Figure 1.
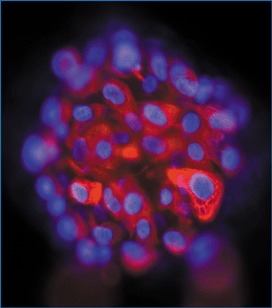
Progenitor cells freshly isolated from the epithelium of human fetal lung (gestational age 12 weeks). The epithelial phenotype is shown by immunostaining for cytokeratin (red). These cells were maintained in vitro and, after two weeks, were found to express surfactant protein C, a specific marker of type II pneumocytes (nuclei counterstained blue with DAPI). Photograph courtesy of Dr HM Romanska.
It has emerged recently that there may be two subpopulations of type II pneumocytes, one of which comes closer to fitting the definition of a stem cell (Reddy et al. 2003). Type II cells isolated from rats following exposure to hyperoxia could be selected according to their immunoreactivity for E‐cadherin into damaged, quiescent cells with low telomerase activity and an E‐cadherin negative population that was undamaged, proliferative and expressed high levels of telomerase activity.
When grown in vitro, alveolar type II cells soon lose their cuboidal shape, becoming flattened, and cease to express phenotypic markers while taking on those characteristic of type I cells (Dobbs et al. 1985; Shannon et al. 1987; Dobbs et al. 1988; Danto et al. 1992). Although, type I cells in vivo appear to be terminally differentiated (Adamson & Bowden 1974a), following differentiation from type II cells in vitro, they have been found to re‐express markers of type II pneumocytes (Leslie et al. 1993).
STEM/PROGENITOR CELLS FROM OUTSIDE THE LUNG
A growing body of evidence has shown that tissues can be repaired by cells acquired via the circulation (for review see Forbes et al. 2002). It has been shown in experimental models that bone marrow‐derived stem cells can engraft in the lung and differentiate into mature epithelial phenotypes (Kotton et al. 2001; Krause et al. 2001) and that this process increases in response to injury (Theise et al. 2002). For the human lung, chimerism has been demonstrated in pulmonary epithelium, including that of the alveoli, following transplantation of haematopoietic stem cells (Kleeberger et al. 2003) or lung (Surratt et al. 2003), although neither study found evidence for engraftment of bone marrow cells specifically. There is some debate as to whether the blood‐borne cells that embed in the lung actually differentiate to lung epithelium or fuse with cells in situ, as has been shown to occur in vitro (Spees et al. 2003). The presence in the lung epithelium of cells recruited from the circulation could provide new therapeutic opportunities for a range of pulmonary diseases by providing means to repair the lung and a novel route for gene therapy.
UNIVERSAL STEM/PROGENITOR CELL
There is some evidence that a pluripotent stem cell exists in the lungs of adult sheep and rats that can generate lung‐like tissue in vitro, specifically of the alveolar (Cortiella et al. 2000) and bronchiolar regions (Vacanti et al. 2001). The isolated cells were extremely small and had a very low demand for oxygen, leading the researchers to term them ‘spore‐like’ (Vacanti et al. 2001). Similar cells were found throughout the body, in each case giving rise in vitro to the tissue from which they were isolated. It is thought that they lie dormant until activated by injury or disease.
REFERENCES
- Adamson IY (1991) Development of lung structure In: Crystal RG, West JB, eds. The Lung: Scientific Foundations. New York: Raven Press Ltd. [Google Scholar]
- Adamson IY, Bowden DH (1974a) The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab. Invest. 30, 35. [PubMed] [Google Scholar]
- Adamson IY, Bowden DH (1974b) The pathogenesis of bleomycin‐induced pulmonary fibrosis in mice. Am. J. Pathol. 77, 185. [PMC free article] [PubMed] [Google Scholar]
- Adamson IY, Bowden DH (1979) Bleomycin‐induced injury and metaplasia of alveolar type 2 cells. Relationship of cellular responses to drug presence in the lung. Am. J. Pathol. 96, 531. [PMC free article] [PubMed] [Google Scholar]
- Adamson IY, Bowden DH, Cote MG, Witschi H (1977) Lung injury induced by butylated hydroxytoluene: cytodynamic and biochemical studies in mice. Lab. Invest. 36, 26. [PubMed] [Google Scholar]
- Bindreiter M, Schuppler J, Stockinger L (1967) Zell proliferation and differenzerung im tracheal epithel der ratte. Exp. Cell Res. 50, 377. [PubMed] [Google Scholar]
- Blau HM, Brezelton TR, Weimann JM (2001) The evolving concept of a stem cell: entity or function? Cell 105, 829. [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB (1998) Number and proliferation of basal and parabasal cells in normal airway epithelium. Am. J. Respir. Crit. Care Med. 157, 2000. [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB (1999) Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 159, 1585. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH (2001) Evidence for stem cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 24, 662. [DOI] [PubMed] [Google Scholar]
- Bowden DH (1981) Alveolar response to injury. Thorax 267, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze RG, Wheeldon EB (1977) The cells of the pulmonary airways. Am. Rev. Respir. Dis. 116, 705. [DOI] [PubMed] [Google Scholar]
- Clara M (1937) Zur Histobiologie des Bronchialepithels. Z. Microsk Anat. Forsch. 41, 321. [Google Scholar]
- Cortiella J, Kojima K, Bonassar LJ, Hendricks G, Vacanti CA, Vanacnti MP (2000) Tissue engineered lung. Tiss. Eng. 6, 661. [Google Scholar]
- Cutz E, Gillan JE, Perrin DG (1995) Pulmonary neuroendocrine cell system: an overview of cell biology and pathology with emphasis of pediatric lung disease In: Askin FB, Langston FC, Bernstein J, eds. Pulmonary Disease: Perspectives in Pediatric Pathology, Vol. 18, p. 32 Basel: Kager. [Google Scholar]
- Cutz E, Orange RP (1977) Mast cells and endocrine (APUD) cells of the lung In: Lichtenstein LM, Austen KF, eds. Asthma: Physiology, Immunopharmacology and Treatment, p. 51 New York: Academic Press. [Google Scholar]
- Danto SL, Zabski SM, Crandell ED (1992) Reactivity of alveolar epithelial cells in primary culture with Type I cell monoclonal antibodies Am. J. Respir. Cell. Mol. Biol. 6, 296–306. [DOI] [PubMed] [Google Scholar]
- Devereux TR, Serabjit‐Singh CJ, Slaughter SR, Wolf CR, Philpot RM, Fouts JR (1981) Identification of cytochrome P‐450 lysozymes in non‐ciliated bronchiolar epithelial (Clara) and alveolar type II cells isolated from rabbit lung. Exp. Lung Res. 2, 221. [DOI] [PubMed] [Google Scholar]
- Dobbs LG, Williams MC, Brandt AE (1985) Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim. Biophys. Acta 846, 155. [DOI] [PubMed] [Google Scholar]
- Dobbs LG, Williams MC, Gonzalez R (1988) Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured but not freshly isolated type II cells. Biochim. Biophys. Acta 970, 146. [DOI] [PubMed] [Google Scholar]
- Donnelly GM, Haack DG, Heird CS (1982) Tracheal epithelium: cell kinetics and differentiation in normal rat tissue. Cell Tiss. Kinet. 15, 119. [DOI] [PubMed] [Google Scholar]
- Duan D, Sehgal A, Yao J, Engelhardt JF (1998) Lef1 transcription factor expression defines airway progenitor cell targets for in utero gene therapy of submucosal glands in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 18, 750. [DOI] [PubMed] [Google Scholar]
- Emura E (1997) Stem cells of the respiratory epithelium and their in vitro cultivation. In vitro Cell Dev . Biol. Anim. 33, 3–14. [DOI] [PubMed] [Google Scholar]
- Emura M (2002) Stem cells of the respiratory tract. Paed. Respir. Rev. 3, 36. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF (2001) Stem cell niches in the mouse airway. Am. J. Respir. Cell Mol. Biol. 24, 649. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L (1995) Progenitor cells of the adult human airway involved in submucosal gland development. Development 121, 2031. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Stephens RJ, Freeman G (1971) Effects of nitrogen dioxide on cell renewal in the rat lung. Arch. Intern. Med. 128, 57. [PubMed] [Google Scholar]
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G (1975) Transformation of alveolar type II cells to type I cells following exposure to nitrogen dioxide. Exp. Mol. Pathol. 22, 145. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Johnson LV, Stephens RJ, Freeman G (1976) Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3 . Lab. Invest. 35, 246. [PubMed] [Google Scholar]
- Evans MJ, Cabral Anderson Lj Freeman G (1978) Role of the Clara cell in renewal of the bronchiolar epithelium. Lab. Invest. 38, 648. [PubMed] [Google Scholar]
- Forbes SJ, Vig P, Poulsom R, Wright NA, Alison MR (2002) Adult stem cell plasticity: new pathways of tissue regeneration become visible. Clin. Sci. (Lond.). 103, 355. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Serge JA (2000) Stem cells: a new lease on life. Cell 100, 143. [DOI] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD, Stripp BR (2002) Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am. J. Pathol. 161, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosney JR (1997) Pulmonary neuroendocrine cell system in pediatric and adult lung disease. Microsc. Res. Techn. 37, 107. [DOI] [PubMed] [Google Scholar]
- Haschek WM, Witschi H (1979) Pulmonary fibrosis – a possible mechanism. Toxicol. Appl. Pharmacol. 51, 475. [DOI] [PubMed] [Google Scholar]
- Hogan BLM (1999) Morphogenesis. Cell 96, 225. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR (2001) Clara cell secretory protein‐expressing cells of the airway neuroepithelial body microenvironment include a label‐retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell Mol. Biol. 24, 671. [DOI] [PubMed] [Google Scholar]
- Hoyt RJ, McNelly NA, Sorokin SP (1990) Dynamics of neuroepithelial body (NEB) formation in developing hamster lung: light microscopic autoradiography after 3H‐thymidine labelling in vivo . Anat. Rec. 227, 340. [DOI] [PubMed] [Google Scholar]
- Inayama Y, Hook GE, Brody AR, Cameron GS, Jetten AM, Gilmore LB, Gray T, Nettesheim P (1988) The differentiation potential of tracheal basal cells. Lab. Invest. 58, 706. [PubMed] [Google Scholar]
- Inayama Y, Hook GE, Brody AR, Jetten AM, Gray T, Mahler J, Nettesheim P (1989) In vitro and in vivo growth and differentiation of clones of tracheal basal cells. Am. J. Pathol. 134, 539. [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Wobken JD (1987) Calcitonin gene‐related immunoreactivity in airway epithelial cells of the human fetus and infant. Cell Tiss. Res. 250, 579. [DOI] [PubMed] [Google Scholar]
- Katzenstein AL (1985) Pathogenesis of ‘fibrosis’ in interstitial pneumonia: an electron microscopic study. Hum. Pathol. 16, 1015. [DOI] [PubMed] [Google Scholar]
- Kauffman SL (1980) Cell proliferation in the mammalian lung. Int. Rev. Pathol. 22 XXX. [PubMed] [Google Scholar]
- Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A (2001) Bone marrow‐derived cells as progenitors of lung alveolar epithelium. Development 128, 5181. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ (2001) Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell 105, 369. [DOI] [PubMed] [Google Scholar]
- Lane BP, Gordon R (1974) Regeneration of rat tracheal epithelium after mechanical injury. Proc. Soc. Exp. Biol. Medical 145, 1139. [DOI] [PubMed] [Google Scholar]
- Lauweryns JM, Cokelaere M (1973) Hypoxia‐sensitive neuro‐epithelial bodies: intrapulmonary secretory neuroreceptors modulated by the CNS. Z. Zellforsch. Milrosk. Anat. 145, 521. [DOI] [PubMed] [Google Scholar]
- Lauweryns JM, Van Ranst L, Verhofstad AAJ (1986) Ultrastructural localization of serotonin in the intrapulmonary neuroepithelial bodies of neonatal rabbits by use of immunoelectron microscopy. Cell Tiss. Res. 243, 455. [DOI] [PubMed] [Google Scholar]
- Leslie CC, McCormack‐Shannon K, Mason RJ, Shannon JM (1993) Proliferation of rat alveolar epithelial cells in low density primary culture. Am. J. Respir. Cell Mol. Biol. 9, 64. [DOI] [PubMed] [Google Scholar]
- Linnoila RL (1982) Effects of diethylnitrosamine on lung neuroendocrine cells. Exp. Lung Res. 3, 225. [DOI] [PubMed] [Google Scholar]
- Liu JY, Nettesheim P, Randell SH (1994) Growth and differentiation of tracheal epithelial progenitor cells. Am. J. Physiol. 266, 296. [DOI] [PubMed] [Google Scholar]
- MacKin CC (1954) The pulmonary alveolar mucoid film and the pneumocytes. Lancet 29, 1099. [DOI] [PubMed] [Google Scholar]
- Maeyama T, Kuwano K, Kawasaki M, Kunitake R, Hagimoto N, Matsuba T, Yoshimi M, Inoshima I, Yoshida K, Hara N (2001) Upregulation of Fas‐signalling molecules in lung epithelial cells from patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 17, 180. [DOI] [PubMed] [Google Scholar]
- Magdeleno SM, Barrish J, Finegold MJ, Demayo FJ (1998) Investigating stem cells in the lung. Adv. Paediatr. 45, 363. [PubMed] [Google Scholar]
- Mahvi D, Bank H, Harley R (1977) Morphology of a naphthalene‐induced bronchiolar lesion. Am. J. Pathol. 86, 559. [PMC free article] [PubMed] [Google Scholar]
- McDowell EM, Sorokin SP, Hoyt RF (1993) Patterns of proliferation and differentiation during fetal development. Anat. Rec. 236, 11. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Moore MAS (1971) Haematopoietic Cells. Amsterdam: North Holland. [Google Scholar]
- Montengua LM, Springall DR, Gaer J, Winter RJD, Zhoa Y, McBride JT, Taylor KM, Barer G, Polak JM (1992) CGRP‐immunoreactive endocrine cell proliferation in normal and hypoxic rat lung studied by immunocytochemical detection of incorporation of 5′‐bromodeoxyuridine. Cell Tiss. Res. 268, 9. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, Anderson DJ (1997) Regulatory mechanisms in stem cell biology. Cell 88, 287. [DOI] [PubMed] [Google Scholar]
- Nettesheim P, Jetten AM, Inayama Y, Brody AR, George MA, Gilmore LB, Gray T, Hook GE (1990) Pathways of differentiation of airway epithelial cells. Environ. Health. Perspect. 85, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto WR (2002) Lung epithelial stem cells. J. Pathol. 197, 527. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Dungworth DL (1987) Structure, function, cell injury and cell renewal of bronchiolar and alveolar epithelium In: Mcdowell EM, ed. Lung Carcinoma, p. 29 London: Churchill Livingstone. [Google Scholar]
- Plopper CG, Suverkropp C, Morin D, Nishio S, Buckpitt A (1992) Relationship of cytochrome P‐450 activity to Clara cell cytotoxicity. I. Histopathologic comparison of the respiratory tract of mice, rats and hamsters after parenteral administration of naphthalene. J. Pharmacol. Exp. Ther. 26, 353. [PubMed] [Google Scholar]
- Potten CS (1997), ed. Stem Cells. Cambridge: Academic Press. [Google Scholar]
- Reddy R, Buckley S, Doerken M, Barsky Y, Weinberg K, Anderson KD, Warburton D, Driscoll B (2003) Isolation of a putative progenitor subpopulation of alveolar epihtelial type 2 cells. Am. J. Physiol. Lung Cell Mol. Physiol. XX, XXX. Available online at: 10.1152/ajplung.00159.2003. [DOI] [PubMed] [Google Scholar]
- Reid L, Jones R (1979) Bronchial mucosal cells. Fed. Proc. 38, 191. [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Power JHT, Stripp BR (2000a) Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am. J. Pathol. 156, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Hong KU, Giangreco A, Mago GW, Guron C, Morimoto Y, Stripp BR (2000b) Conditional Clara cell ablation reveals a self‐renewing progenitor function of pulmonary neuroendocrine cells. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L1256. [DOI] [PubMed] [Google Scholar]
- Shami SG, Evans MJ, Martinez LA (1986) Type II cell proliferation related to migration of inflammatory cells into the lung. Exp. Mol. Pathol. 44, 344. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Mason RJ, Jennings SD (1987) Functional differentiation of alveolar type II cells in vitro: effects of cell shape, cell–matrix interactions and cell–cell interactions. Biochim. Biophys. Acta 931, 143. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ (2003) Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc. Natl Acad. Sci. USA 100, 2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripp BR, Maxson K, Mera R, Singh G (1995) Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am. J. Physiol. 269, L791. [DOI] [PubMed] [Google Scholar]
- Sunday ME, Willett CG (1992) Induction and spontaneous regression of intense pulmonary neuroendocrine cell differentiation in a model of preneoplastic lung injury. Cancer Res. 52, 2677S. [PubMed] [Google Scholar]
- Sunday ME, Hua J, Dai HB, Nusrat A, Torday JS (1990) Bombesin increases fetal lung growth and maturation in utero and in organ cultures. Am. J. Respir. Cell Mol. Biol. 3, 199. [DOI] [PubMed] [Google Scholar]
- Sunday ME, Choi N, Spindel ER, Chin WW, Mark E (1991) Gastrin‐releasing peptide gene expression in small cell and large cell undifferentiated lung carcinomas. Hum. Pathol. 22, 1030. [DOI] [PubMed] [Google Scholar]
- Sunday ME, Willett CG, Patidar K, Graham SA (1994) Modulation of oncogene and tumor suppressor gene expression in a hamster model of chronic lung injury with varying degrees of pulmonary neuroendocrine cell hyperplasia. Lab. Invest. 70, 875. [PubMed] [Google Scholar]
- Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badve S, Saxena R, Krause DS (2002) Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp. Haematol. 30, 1333. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 14, 1419. [PubMed] [Google Scholar]
- Vacanti MP, Roy A, Cortiella J, Bonassar L, Vacanti C (2001) Identification and initial characterization of spore‐like cells in adult mammals. J. Cell. Biochem. 80, 455. [PubMed] [Google Scholar]
- Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157. [DOI] [PubMed] [Google Scholar]
- Wharton J, Polak JM, Bloom SR, Ghatei MA, Solcia E, Brown MR, Pearse AGE (1978) Bombesin‐like immunoreactivity in the lung. Nature 273, 769. [DOI] [PubMed] [Google Scholar]
- Witschi H (1978) Proliferation of type II alveolar cells: a review of common responses in toxic lung injury. Toxicology 5, 267. [DOI] [PubMed] [Google Scholar]
- Wuenschell CW, Sunday ME, Singh G, Minoo P, Slavkin HD, Warburton D (1996) Embryonic mouse lung epithelial progenitor cells co‐express immunohistochemial markers of diverse mature cell lineages. J. Histochem. Cytochem. 44, 113. [DOI] [PubMed] [Google Scholar]


