Abstract
Objectives
miR‐375 is one of the highly expressed microRNAs (miRNAs) found in pancreatic islets of both humans and mice. In this study, we investigated functions of miRNA miR‐375 in porcine pancreatic stem cells (PSC).
Materials and methods
We transfected mimic and inhibitor of miR‐375 in PSCs to measure functional roles of the microRNA and its effects on cell cycle proliferation and cell differentiation were determined. Luciferase assays were also performed to reveal the target gene of miR‐375.
Results
Overexpression of miR‐375 suppressed proliferation, promoted apoptosis and inhibited differentiation into islet‐like cells. PDK1 was identified as being a target of miR‐375. Furthermore, we found that overexpression of miR‐375 inhibited activation of the PDK1‐AKT signalling pathway.
Conclusion
miR‐375 directly targeted PDK1 in porcine PSCs, suppressing cell proliferation and differentiation into islet‐like cells.
Introduction
According to the statistics from the International Diabetes Federation (IDF), there were 381.8 million diabetic patients worldwide in 2013, while the number will reach 591.9 million by 2035 1, 2, 3. The success of islet transplantation suggests that diabetes can be potentially cured by the replacement of deficient beta cells 4. However, a shortage of suitable islets for transplantation remains to be a common problem. Porcine pancreatic stem cell (PSC) is currently viewed as one of the most promising alternative sources for diabetes treatment because of the highly conserved insulin structure and similar physiological glucose levels between pigs and humans 5, 6, 7. However, the shortage of quantity and the lack of mechanistic understanding of PSC proliferation and differentiation have severely hindered the clinical applications of porcine PSC for diabetes treatment.
microRNAs (miRNAs) are a type of short (21‐ to 23‐nt long), non‐coding RNAs that bind to the 3′‐untranslated regions (3′‐UTRs) of target genes and generally function as negative regulators of gene transcription. miRNAs control the expression of many genes 7 and are involved in a variety of crucial biological processes, which include development, differentiation, apoptosis, cell proliferation and diseases 8, 9, 10, 11. Recently, a number of studies have demonstrated that miRNAs can regulate the development of pancreatic cells 12, 13, 14, 15.
miR‐375, which was first cloned from a pancreatic β‐cell line, Min6, is highly conserved throughout evolution 16. Recent studies have shown that miR‐375 is expressed in pancreatic islets and is required for normal glucose homeostasis 17, 18, 19. Furthermore, in pancreatic islets, miR‐375 plays an important role in the complex regulatory network of pancreatic function and is linked to diabetes 20, 21, 22, 23. These studies raise the interesting possibility of applying miR‐375 to treatment of diabetes.
In adult pancreas, PDK1 expresses in pancreatic islets and pancreatic ducts. It has been reported that miR‐375 directly regulates Pdk1 mRNA expression and reduces its protein levels, resulting in lower glucose stimulation and consequently triggering insulin gene expression and DNA synthesis in β cells 24. In addition, PDK1 is closely linked to the regulation of cell proliferation 24, 25, 26, including that of pancreatic carcinoma cells 24, 25, 26. Despite the studies that link miR‐375 to pancreatic development, little is known about the specific function and the mechanism of miR‐375 in porcine PSCs.
In this study, we studied the effect of miR‐375 on porcine PSC proliferation, apoptosis, and differentiation to insulin‐secreting cells. We further explored the potential mechanism for miR‐375 in the regulation of porcine PSC function. Our study has led to new discoveries that can be potentially used for the future application of porcine PSCs to diabetes treatment.
Materials and methods
Cells culture
Two‐month‐old foetal porcine pancreases were obtained from the Yaoan abattoir in Yangling Hi‐tech area for developing porcine PSCs. Porcine PSCs were stored and obtained as described previously 6. Cells were passaged with 0.25% (w/v) trypsin (Invitrogen, Carlsbad, CA, USA) when reaching 70–80% confluency. The culture medium (low‐glucose DMEM; Invitrogen), containing 15% FBS, 0.1 mm β‐mercaptoethanol (Sigma, St. Louis, MO, USA), 2 mm glutamine (Invitrogen) and 100 mg/ml penicillin/streptomycin, was replaced every 2–3 days.
Cells transfection
Negative control (N.C) miRNAs: 5′‐CAGUACUUUUGUGUAGUACAA‐3′, the mimic (100 nmol/l): 5′‐UCACGCGAGCCGAACGAACAAA‐3′ and the inhibitor (2′‐O‐Me modified antisense oligo nucleotides; 200 nmol/l): 5′‐UUUGUUCGUUCGGCUCGCGUGA‐3′ of miR‐375 were delivered into porcine PSCs using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Forty‐eight hours after transfection, cells were lysed for Western blot or fixed for immunofluorescent staining, and total RNA was isolated for real‐time quantitative PCR (qRT‐PCR) analysis.
Prediction of miR‐375 targeted genes
miR‐375 target genes were predicted using the TargetScan algorithm from TargetScan (Version 6.0; http://www.targetscan.org/).
Immunofluorescence staining
Cells were permeabilized with 0.1% Triton X‐100 for 10 min, blocked with 10% goat serum in PBS at room temperature for 1 h, and incubated with the primary antibodies overnight at 4 °C. The primary antibodies include PDX1 (1:200; Abcam, Cambridge, MA, USA), ki67 (1:200; Millipore, Billenca, MA, CA, USA), C‐Myc (1:200; Chemicon, Temecula, CA, USA), PCNA (1:200; Millipore), C‐peptide (1:200; Abcam) and Insulin (1:200; Chemicon). After three washes with PBS, the cells were incubated with the secondary antibodies at room temperature for 1 h, followed by three washes in the same buffer. They were then incubated with Hoechst33342 (Sigma) at room temperature for 5 min. Images were captured and analysed with a Leica fluorescent microscope. The percentage of PDK1 and ki67 positive staining was calculated by counting the cells under the fluorescent microscope.
BrdU incorporation assay
The proliferation ability of porcine PSCs was evaluated by BrdU incorporation assay. First, after transfection, porcine PSCs were treated with 30 mg/ml BrdU (Sigma) for 6 h and then subjected to BrdU immunostaining. Specifically, cells were fixed in 4% paraformaldehyde (PFA) for 15 min at room temperature and washed three times for 10 min with PBS (pH 7.4) containing 0.1% Triton X‐100. The cells were then washed for three times with PBS (pH 7.4) alone. Anti‐BrdU (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) dissolved in 0.1 m PBS (pH 7.4) containing 5% goat serum was added, and the cells were incubated overnight at 4 °C. Cells were washed in PBS (pH 7.4) three times and then incubated with the secondary antibody (FITC; Millipore 1:500) for 1 h at room temperature. After three washes, cells were visualized under a Leica fluorescent microscope and analysed for BrdU uptake. The number of BrdU‐positive cells was counted under the fluorescent microscope.
CCK‐8 assay
For CCK‐8 (Beyotime) assay, porcine PSCs were seeded on 96‐well plate. Seventy‐two hours after transfection, cells were incubated in CCK‐8 solution for 2 h at 37 °C. The amount of formazan dye was measured by examining the absorbance at 450 nm with a microplate reader.
qRT‐PCR
Quantitative reverse transcriptase‐polymerase chain reaction (QRT‐PCR) was set up in 25 μl reaction mixtures containing 12.5 μl 1× SYBR@ PremixExTaq™ (TaKaRa Biotech Co. Ltd, Dalian, Liaoning, China), 0.5 μl sense primer, 0.5 μl antisense primer, 11 μl distilled water and 0.5 μl template. Reaction conditions were as follows: 95 °C for 30 s, followed by 45 cycles at 95 °C for 5 s and 58 °C for 20 s. The expression levels of mRNAs were normalized to β‐actin in each well. Expression was quantified as ratio of mRNA levels in untreated porcine PSC to those in transferred porcine PSC. qRT‐PCR primers are shown in Table 1.
Table 1.
Primer sequences used for qRT‐PCR
| Primers | Forward | Reverse | Tm (°C) |
|---|---|---|---|
| c‐Myc | 5′‐ctggtgggcgagatcatca‐3′ | 5′‐cactgccatgaatgatgttcc3′ | 54 |
| PCNA | 5′‐agtggagaacttggaaatggaa‐3′ | 5′‐agtggagaacttggaaatggaa‐3′ | 58 |
| Cyclin A | 5′‐tggctgtgaactacattga‐3′ | 5′‐acaaactctgctacttctgg‐3′ | 58 |
| Cyclin D1 | 5′‐tgaactacctggaccgct‐3′ | 5′‐caggttccacttgagttgt‐3′ | 58 |
| Insulin | 5′‐aagcgtggcatcgtggag‐3′ | 5′‐tcaggactttattgggtttgg‐3′ | 58 |
| NeuroD1 | 5′‐agctcccatgtcttccacgta‐3′ | 5′‐gaagttgccgttgatgctga‐3′ | 58 |
| Nkx 6.1 | 5′‐agacccactttttccggaca‐3′ | 5′‐ccaacgaataggccaaacga‐3′ | 58 |
| miR‐375 | 5′‐acactccagctgggtttgttcgttcggctc‐3′ | 5′‐ctcaactggtgtcgtggagtcggcaattcagttgagtcacgcga‐3′ | 58 |
| Pdk1 | 5′‐ggaacagcgcagtacgtttct‐3′ | 5′‐ctcgtttccagctcggaatgg‐3′ | 58 |
| Pdx1 | 5′‐gagcccgaggagaacaagc‐3′ | 5′‐tgacagccagctccaccc‐3′ | 58 |
| Mafa | 5′‐ttcagcaaggaggaggtcat‐3′ | 5′‐acaggtcccgctctttgg‐3′ | 58 |
| Ngn3 | 5′‐gcgagttggcactgagca‐3′ | 5′‐aagctgtggtccgctatgc‐3′ | 58 |
| 18S | 5′‐gtggtgttgaggaaagcagaca‐3′ | 5′‐tgatcacacgttccacctcatc‐3′ | 58 |
| β‐actin | 5′‐gcggcatccacgaaactac‐3′ | 5′‐tgatctccttctgcatcctgtc‐3′ | 58 |
Flow cytometry analysis
For cell cycle analysis, cells were seeded on 6‐well plates (2 × 105 cells/well) in growth media and allowed to attach overnight. The cells were transfected with the miR‐375 mimic, inhibitor or control and incubated for 72 h. The cells were then washed with PBS, stained with propidium iodide by following the protocol attached with the cell cycle staining kit (MultiSciences Biotech, Hangzhou, China) and then analysed using a Beckman Coulter flow cytometer (Beckman Coulter, Brea, CA, USA). The percentages of cells in the G1, S, and G2‐M phases were determined.
Luciferase reporter assay
A 2367 bp fragment from the 3′‐UTR of pdk1 containing the predicted miR‐375 binding site was cloned into the NotI and XhoI sites of psiCHECK‐2 (Promega, Madison, WI, USA) vector. The 3′‐UTR of pdk1 containing the miR‐375 binding site was amplified using the following primers: forward, 5′‐ACCCAACCACACAAAGAACAAAA‐3′ and reverse, 5′‐TTTTGTTCTTTGTGTGGTTGGGT‐3′. 293T cells were seeded on 48‐well plates and co‐transfected with the luciferase reporter vector and the miR‐375 mimic, inhibitor or NC as described above. The cells were harvested 48 h after transfection, and luciferase activity was measured using the dual‐luciferase assay system (Vigorous).
Evaluation of apoptosis
Cellular apoptosis was detected using flow cytometric analysis of Annexin V staining. Annexin V–FITC versus PI assay was performed as described by kit protocol. Briefly, adherent cells were harvested and suspended in the Annexin‐binding buffer (~2000 cells/ml). Cells were thereafter incubated with Annexin V–FITC and PI for 15 min at room temperature and immediately analysed with flow cytometry. The data are presented as bi‐parametric dot plots showing Annexin V–FITC green fluorescence versus PI red fluorescence.
Western blot analysis
Seventy‐two hours after transfection with miR‐375 mimic, inhibitor or NC, cells were subjected to Western blot analysis. The primary antibodies used for Western blot analysis are anti‐PDK1 antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA), anti‐Akt antibody (1:1000; Cell Signaling Technology), anti‐phosphorylated Akt antibody (Ser473; 1:1,000; Cell Signaling Technology), anti Bcl2 antibody (1:1000; SB), anti P53 antibody (1:1000; SB), anti‐C‐Myc antibody(1:1000; Chemicon), anti‐PCNA antibody (1:1000; Millipore) and anti‐β‐actin antibody (1:1,000; Cell Signaling Technology).
Generation of islet‐like clusters from porcine PSC
We used a two‐step protocol to obtain islet‐like cells as described previously 6. First, porcine PSCs were cultured in RPMI 1640 medium supplemented with 1% BSA, 100 mm nicotinamide (NIC; Sigma), 10 ng/ml exendin‐4 (Sigma), 1 mm sodium pyruvate (Invitrogen), 2 mm glutamine (Invitrogen), 1 mm β‐mercaptoethanol (Sigma), 100 mg/ml penicillin/streptomycin, 20 ng/ml EGF (Millipore) and 20 ng/ml bFGF (Millipore) (Media I) for 2 days. After transfected with the miR‐375 mimic or the control, cells were then transferred into ultra‐low attachment plates and cultured in the same medium for 2 days. Next, the inducing conditioned media was supplemented with 25 μmol/l ZnAc, 1×ITS, 10 nmol/l Exendin4 and 4 nmol/l Betacellulin (Media II). The clusters were cultured in Media II on ultra‐low attachment plates for another 6 days. The clusters were then collected for the experiments discussed in the following sections.
Glucose stimulation and insulin content analysis
After 10 days of induction, porcine PSC‐derived islet clusters were plated on six‐well plates in triplicate, with approximately 200 clusters/well, and cultured overnight to ensure that clusters were attached to the plates. Clusters were subsequently washed three times in glucose‐free RPMI 1640 medium and preincubated in low (2 mm) glucose RPMI 1640 medium for 2 h to remove residual insulin. Clusters were washed twice in PBS and incubated in low‐glucose RPMI 1640 medium for 30 min, and the resulting supernatant was collected. Clusters were again washed twice in PBS and incubated in high‐glucose RPMI1640 medium for 30 min, and the supernatant was collected. Finally, clusters were incubated in RPMI 1640 containing 2 mm glucose and 30 mm KCl (depolarization challenge) for 30 min, and then the supernatant was collected. The insulin content release by islet‐like clusters was detected by radioimmunoassay (RIA).
Statistical analysis
Each experiment was repeated for at least three times. Data are presented as mean ± SDs of the mean and were analysed by two‐tailed independent‐sample Student's t test or one‐way ANOVA, as appropriate. P values <0.05 were considered statistically significant (*P < 0.05; **P < 0.01).
Results
miR‐375 suppresses porcine PSC proliferation
To explore whether miR‐375 played a role in proliferation of porcine PSCs, we transfected the miR‐375 mimic, inhibitor or NC, respectively, into porcine PSCs (n = 3) and assessed the impact on proliferation using BrdU labelling. Cells with the miR‐375 mimic showed a lower percentage of BrdU‐positive cells than the control group. In contrast, cells treated with the miR‐375 inhibitor exhibited a higher percentage of BrdU‐positive cells (Fig. 1a,b). We also measured cell growth using the CCK‐8‐based colorimetric assay, which showed that the miR‐375 mimic inhibited proliferation of PSCs when compared with the control group, while miR‐375 had an opposite effect on PSCs proliferation (Fig. 1c). Next, we examined the expression of cell proliferation markers in PSCs treated with the miR‐375mimic, inhibitor or NC. As shown in Fig. 1d, the mRNA expression of PCNA, C‐Myc, Cyclin A and Cyclin D was reduced in cells transfected with the miR‐375 mimic, while the cells treated with the miR‐375 inhibitor increased the expression of these genes. Consistent with the pattern of mRNA expression, the PCNA and C‐Myc proteins were also down‐regulated in porcine PSCs treated with the miR‐375 mimic (Fig. 1e,f). To further validate the effect of miR‐375 on cell proliferation, we examined the changes in cell‐cycle progression and found that the percentage of S‐phase cells decreased after miR‐375 mimic treatment, whereas the miR‐375 inhibitor increased the ratio of S‐phase cells (Fig. 1,h). These results demonstrated that miR‐375 acts as inhibitor of porcine PSC proliferation.
Figure 1.
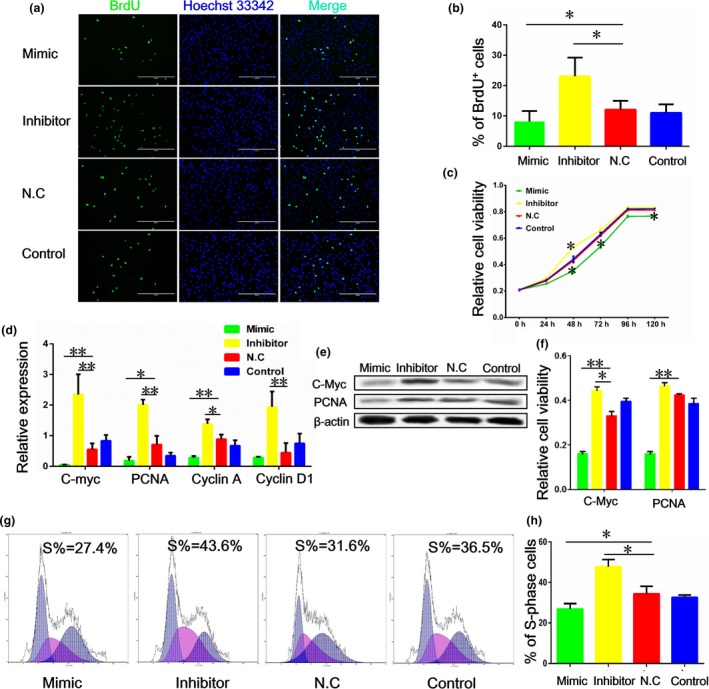
miR‐375 suppresses porcine PSC proliferation. Cells were transfected with mimic, inhibitor and nonsense oligomer (NC group) of miR‐375. Non‐transfected cells were used as the blank control (control group). (a) BrdU incorporation assay of porcine pancreatic stem cells (PSCs) after treatment with the mimic and inhibitor of miR375, representative images of BrdU staining are shown. (b) Quantification and statistical analysis for (a). (c) Cell viability as measured with CCK‐8 assay. (d) The mRNA levels of cell proliferation markers including PCNA, C‐Myc, Cyclin A and Cyclin D1, as detected with qRT‐PCR. (e) Western blot analysis of C‐Myc and PCNA protein. (f) Quantification of results from (e). Image J (V1.48d) gradation analysis was carried out. (g) Cell cycle analysis. (h) Quantification of S‐phase cells as described in (g). Values are mean ± SD of triplicate experiments. *P < 0.05; **P < 0.01.
Effect of miR‐375 on apoptosis of porcine PSCs
Latreille 27 reported that the level of circulating miR‐375 is elevated in human T1D, while the level is decreased in many types of cancer cells 28, 29, 30. T1D is generally characterized by death of islet β cells, while cancer cells often share similar features to stem cells. These results led us to hypothesize that miR‐375 might have a role in apoptosis of porcine PSCs. To test this hypothesis, we examined the percentage of apoptotic cells after various treatments using flow cytometry analysis (n = 3). We found that the rate of apoptosis was elevated after miR‐375 mimic treatment, while the rate was reduced with miR‐375 inhibitor treatment (Fig. 2a,b). Furthermore, the expression of P53, an apoptosis‐inducing gene, was up‐regulated, while Bcl2, an anti‐apoptosis gene, was down‐regulated in cells treated with the miR‐375 mimic. In contrast, miR‐375 inhibitor caused an opposite effect on P53 and Bcl2 expression (Fig. 2c,d). These results suggest that miR‐375 can promote apoptosis of porcine PSCs, likely by targeting P53 and Bcl2.
Figure 2.
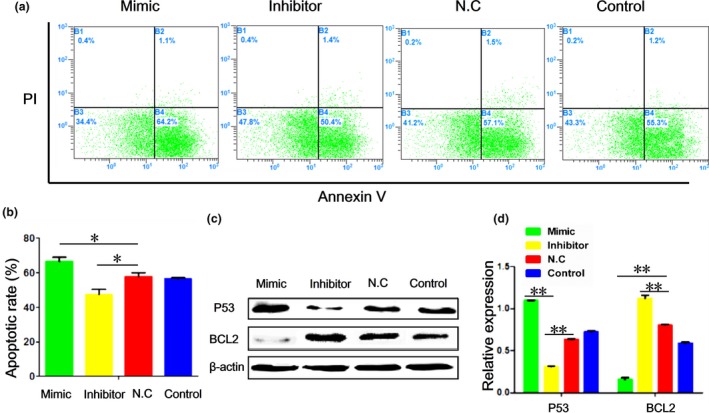
miR‐375 promotes apoptosis of porcine PSC s. (a) Rates of apoptosis in cells stained with Annexin V/propidium iodide (PI) and determined with flow cytometry. (b) Quantification of the results from (a). (c) Western blotting of P53 and Bcl2 proteins. (d) Quantification of results from (c). Image J (V1.48d) gradation analysis was carried out. Values are mean ± SD of triplicate experiments. *P < 0.05; **P < 0.01.
miR‐375 suppresses porcine PSC differentiation
We induced porcine PSCs to differentiate into insulin‐secreting cells using a step‐wise protocol established previously by us, with minor modifications 6. Experiments with DTZ staining showed that the number of islet‐like clusters differentiated from porcine PSCs (brownish red) was reduced in cells treated with the miR‐375 mimic (Fig. 3a). We also detected fewer cells that co‐expressed insulin and C‐peptide in clusters transfected with the mimic than the control miRNA (Fig. 3b). Insulin secretion, as determined by the use of RIA, also decreased in cells with the miR‐375mimic (Fig. 3c). Furthermore, two crucial transcription factors associated with β‐cell differentiation, Nkx6.1 and NeuroD1, were down‐regulated in cells transfected with the miR‐375 mimic in comparison with those transfected with the control miRNA (Fig. 3d). Thus, miR‐375 inhibits porcine PSC differentiation into insulin‐secreting cells.
Figure 3.
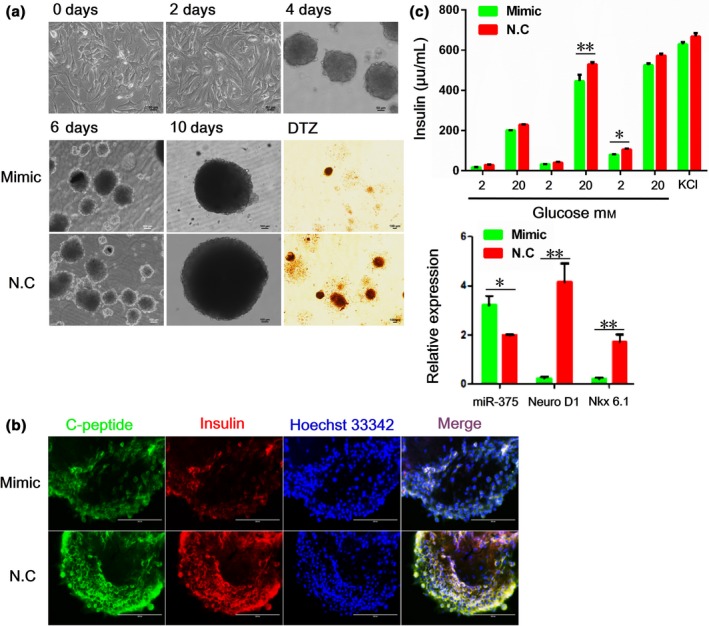
miR‐375 suppresses porcine PSC differentiation. (a) Top: Phenotypes of islet‐like clusters derived from porcine PSCs on days 0, 2 (adherent culture) and 4 (suspension culture) after induction of differentiation, scale bar, 50 μm. Bottom: Phenotypes of insulin‐secreting clusters on days 6 and 10 after induction of differentiation of porcine pancreatic stem cells (PSCs). Scale bar, 100 μm. (b) Immunofluorescence of insulin and C‐peptidein insulin‐secreting clusters. Scale bar, 200 μm. (c) Insulin release of insulin‐secreting clusters in response to glucose stimulation as measured by radioimunoassay. (d) The mRNA levels of miR‐375, NeuroD1 and Nkx6.1 in insulin‐secreting clusters assessed with qRT‐ PCR. Values are mean ± SD of triplicate experiments. *P < 0.05; **P < 0.01.
miR‐375 targets PDK1 post‐transcriptionally
Bioinformatics analysis of target gene prediction, including the use of PicTar, TargetScan and miRanda, led us to predict PDK1 as one of the potential targets of miR‐375. The predicted binding of miR‐375 to PDK1 3′UTR is shown in Fig. 4a. We examined the relative expression of miR‐375 and PDK1 in porcine PSCs. As shown in Fig. 4b, miR‐375 expression was elevated when cells were transfected with the miR‐375 mimic, but was reduced when cells were treated with the miR‐375 inhibitor. However, Pdk1 expression was unaltered by miR‐375 overexpression at the transcriptional level. Instead, the PDK1 protein level was significantly reduced following transfection of cells with the miR‐375 mimic, but was elevated after treatment with the miR‐375 inhibitor (Fig. 4c,d).
Figure 4.
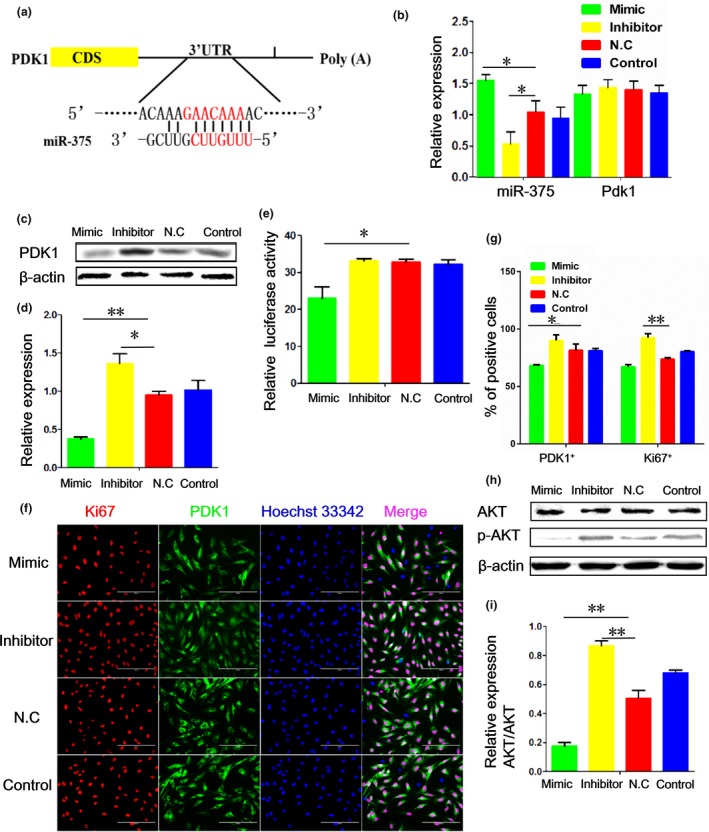
miR‐375 targets PDK 1 by binding to 3′‐ UTR of pdk1 transcript. (a) A putative binding site of miR‐375 to PDK1 3′‐UTR predicted by TargetScan. (b) The mRNA levels of miR‐375 and Pdk1 in porcine pancreatic stem cells (PSCs) as detected by qRT‐PCR. (c) Western blotting of PDK1. (d) Quantification of the results from (c) with Image J (V1.48d) gradation analysis. (e) Analysis of dual‐luciferase activity after overexpression of Pdk1 3′UTR and the miR‐375 mimic or inhibitor in porcine PSCs. (f) Immunofluorescence of Ki67 and PDK1 in porcine PSCs with various treatments. (g) Quantification of the results from (f). (h) Western blotting of AKT and p‐AKT after transfection of porcine PSCs with the miR‐375 mimic and inhibitor. (i) Quantification of the results from (h) with Image J (V1.48d) gradation analysis. Values are mean ± SD of triplicate experiments. *P < 0.05; **P < 0.01.
To investigate whether PDK1 acted as the direct target gene of miR‐375, we generated a dual‐luciferase reporter system containing the miR‐375 target site of PDK1 3′UTR and delivered it into porcine PSCs together with the miR‐375 mimic, inhibitor or control miRNA, respectively. As shown in Fig. 4e, the relative luciferase activity was reduced in cells co‐transfected with the miR‐375 mimic. In contrast, little change was observed in cells con‐transfected with the miR‐375 inhibitor. Thus, miR‐375 inhibits the expression of PDK1 by directly targeting the 3′UTR of the Pdk1 transcript.
To investigate whether PDK1 plays a role in the regulation of porcine PSC proliferation and apoptosis, we first assessed the subcellular localization of PDK1. Immunofluorescence of PDK1 showed significant co‐localization of PDK1 in cytoplasm with Ki67 in the nucleus. Also, the percentages of Ki67‐positive and PDK1‐positive cells were significantly elevated after transfection with the miR‐375 inhibitor, while the miR‐375 mimic had an opposite effect (Fig. 4f,g).
Effect of PDK1 on proliferation and apoptosis
To directly assess the function of PDK1, we treated the porcine PSCs with a specific PDK1 inhibitor (OSU‐03012). We found that the G1 population of porcine PSCs was elevated from 19% in control cells to 23.7% in OSU‐03012‐treated cells, while the S‐phase fraction was reduced from 68.3% to 49.2% (Fig. 5a). In addition, the apoptotic rate after OSU‐03012 treatment was elevated to 74.8%, compared with 61.2% in the control group (Fig. 5b). Next, we examined the expression of cell proliferation and differentiation markers in PSCs treated with OSU‐03012. As shown in Fig. 5c,d, the mRNA expression of PCNA and Cyclin D1 was reduced in cells after OSU‐03012 treatment, and treatment with it decreased the expression of Ngn3 and Nkx6.1 as well. Consistent with the pattern of mRNA expression, the C‐Myc proteins were also down‐regulated in porcine PSCs treated with the miR‐375 mimic (Fig. 5e). Furthermore, OSU‐03012 decreased the protein levels of PCNA, and BCL2, but increased the level of p53 in porcine PSCs (Fig. 5e,f).
Figure 5.
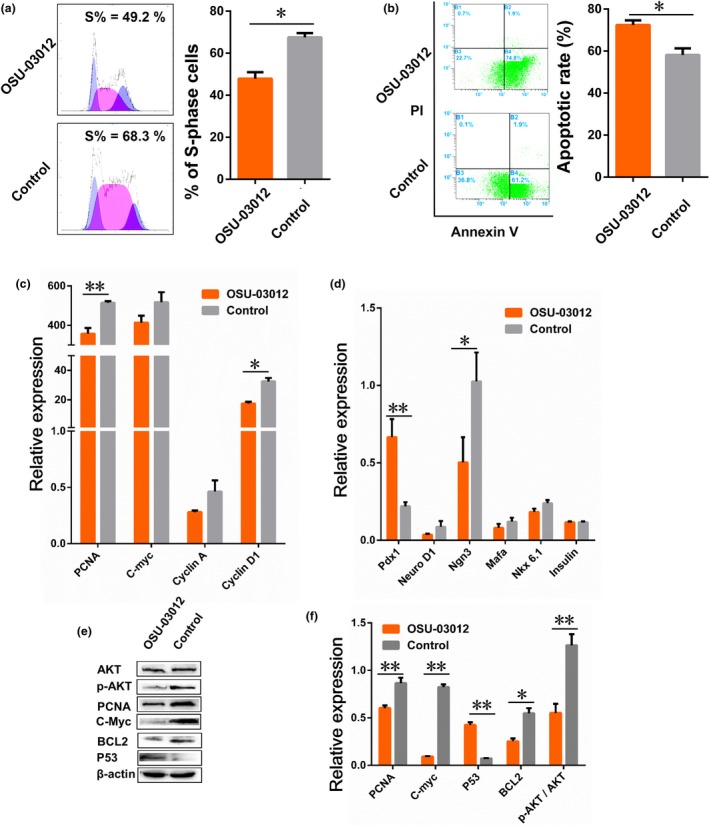
Effect of PDK 1 on apoptosis and proliferation of porcine PSC s. (a, b) The changes in cells cycle and apoptosis measured with flow cytometer. After treated with OSU‐03012 for 24 h. (c, d) The mRNA levels of cell proliferation and differentiation markers, including C‐Myc, Cyclin A, Cyclin D1, Pdx1, NeuroD1, Mafa Nkx6.1 and insulin, as detected with qRT‐PCR. (e) Western blotting of AKT, p‐AKT, PCNA, C‐Myc, BCL2 and P53 proteins. (f) Quantification of the results from (e) using Image J (V1.48d) gradation analysis. Values are mean ± SD of triplicate experiments. *P < 0.05; **P < 0.01.
As a downstream signalling molecule of PI3K, PDK1 plays a central role in many signal transduction pathways and activates more than 20 substrates including AKT, PKC and S6 kinase 5. It has recently been reported that miR‐375 regulates cell survival and growth through the PDK1/Akt pathway 24, 26. We, therefore, assessed the expression of total and phosphorylated Akt in cells with various treatments. We found that the level of phosphorylated Akt was markedly reduced in porcine PSCs transfected with the miR‐375 mimic, but was substantially elevated after the miR‐375 inhibitor treatment. In contrast, the levels of total Akt were largely unaltered. Thus, miR‐375 inhibits porcine PSCs proliferation and survival by targeting PDK1 and subsequently the Akt pathway (Fig. 4h,i). As expected, Akt phosphorylation at Ser473 was completely inhibited by OSU‐03012, while the total levels of Akt protein remained unchanged (Fig. 5e,f). Data represent mean ± SD, and error bars indicate SD. Similar results were obtained in at least three independent experiments.
Discussion
Diabetes mellitus is a metabolic disease characterized by insulin resistance, relative insulin deficiency and elevated blood glucose. The usage of porcine islet cells derived from PSCs is currently viewed as one of the most promising alternatives for human islet transplantation for diabetes treatment. To obtain a stable cell line for the mechanistic study of pancreatic cells development, we previously isolated and established immortalized porcine pancreatic mesenchymal stem cells (iPMSCs) 5, 31. The islet‐derived iPMSCs share characteristics of typical PSCs and have unlimited proliferation potential. These cells can secrete insulin and C‐peptide in vitro and reverse hyperglycaemia in the mouse diabetic model. To optimize the proliferation and differentiation capacity of PSCs, we investigated the effects of molecules such as the GSK3 inhibitor‐BIO, Conophylline and Wnt3a 5, 31.
A growing number of reports have shown that non‐coding RNAs play a critical role in the regulation of cell growth and differentiation 32, 33, 34. Previous studies have demonstrated that miR‐375 inhibits several types of digestive cancers 28, 30. We also found that miR‐375 is specifically expressed in islet cells of the pancreas and associated with pancreatic development. However, there was still lack of information about the specific function and mechanism of miR‐375 in porcine PSCs.
To dissect the function of miR‐375 in porcine PSCs, we transfected porcine PSCs with the miR‐375 mimic or inhibitor and analysed the changes in phenotype and molecular expression associated with cell proliferation and apoptosis. The results from these analyses indicate that miR‐375 inhibits proliferation and promotes apoptosis of porcine PSCs. Specifically, flow cytometry analysis showed that the population of cells at S phase after miR‐375 mimic treatment is reduced, while the apoptosis rate is elevated. Furthermore, the BrdU assay and analysis of the cell proliferation marker expression confirmed that miR‐375 inhibits proliferation and promotes apoptosis of porcine PSCs at the molecular level.
In addition, to analyse the role of miR‐375 in the regulation of PSC differentiation, we induced differentiation into insulin‐producing cells using our previously developed protocol and examined the effect of miR‐375 overexpression 6. We found that overexpression of miR‐375 in PSCs restrains cell differentiation into insulin‐secreting cells, as judged by morphology, DTZ staining, expression of cell‐specific markers and the level of insulin secretion. Our results demonstrated that miR‐375 overexpression not only decreases the expression of C‐peptide and insulin, but also reduces insulin secretion of islet‐like cell mass after porcine PSC differentiation. Interestingly, it was previously reported that in human mesenchymal stem cells (MSCs) and embryonic stem cells (ESCs), miR‐375 is beneficial for the production of insulin‐producing cells 27, 35, 36, 37, 38. The differential effects of miR‐375 on human MSCs, ESCs and PSCs might be caused by differences in cell types and/or other undefined factors.
Increasing evidences has shown that several classes of miRNAs are expressed and play essential roles in pancreatic cell development. Generally, miRNAs function using their seeding sequences to bind to the 3′UTR sequences of target genes 39, 40. In our study, we demonstrated that the 3′UTR of porcine PDK1 contains a putative target sequence of miR‐375. We showed that miR‐375 overexpression specifically decreases PDK1 expression in PSCs at the protein level. Thus, miR‐375 might target PDK1 in porcine PSCs to inhibit cell proliferation and promote apoptosis. Indeed, we found that miR‐375 modulates phosphorylation of Akt, a protein kinase downstream of PDK1, in PSCs (Fig. 5d,e). Together, we identified a critical role of miR‐375 in regulating PSC fate (Fig. 6), a novel mechanism for enhancing islet integrity and a potential innovative strategy for diabetes therapy involving the use of PSCs.
Figure 6.
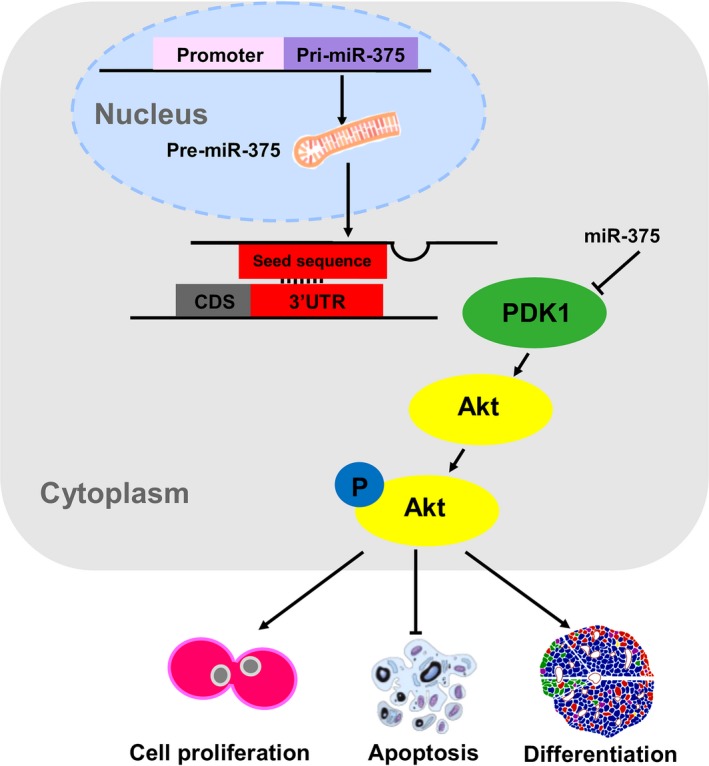
Diagram illustrating the mechanisms of miR‐375 regulating PSC proliferation, apoptosis and differentiation. miR‐375 achieved its effect through complementary base‐pairing with the 3′UTR of Pdk1 mRNA and directly repressed its translation in porcine PSC. Furthermore, miR‐375 could induce cell apoptosis and decrease the proliferation, even inhibit cell differentiation into β cell by depressing the Akt signaling pathway.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC, 31101775), the Basal Research Fund of Northwest A&F University (2452015034), the Science and Technology Foundation for Selected Overseas Chinese Scholar, the Ministry of Human Resources and Social Security (No. A289021502), the Scientific Research Foundation for Returned Scholars, Chinese Ministry of Education (K308021501).
References
- 1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 103, 137–149. [DOI] [PubMed] [Google Scholar]
- 2. Nasr MB, D'Addio F, Usuelli V, Tezza S, Abdi R, Fiorina P (2015) The rise, fall, and resurgence of immunotherapy in type 1 diabetes. Pharmacol. Res. 98, 31–38. [DOI] [PubMed] [Google Scholar]
- 3. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J et al (2010) Prevalence of diabetes among men and women in China. N. Engl. J. Med. 362, 1090–1101. [DOI] [PubMed] [Google Scholar]
- 4. Stanley EG, Elefanty AG (2008) Building better beta cells. Cell Stem Cell 2, 300–301. [DOI] [PubMed] [Google Scholar]
- 5. Cao H, Chu Y, Lv X, Qiu P, Liu C, Zhang H et al (2012) GSK3 inhibitor‐BIO regulates proliferation of immortalized pancreatic mesenchymal stem cells (iPMSCs). PLoS ONE 7, e31502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao H, Chu Y, Zhu H, Sun J, Pu Y, Gao Z et al (2011) Characterization of immortalized mesenchymal stem cells derived from foetal porcine pancreas. Cell Prolif. 44, 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogers SA, Chen F, Talcott M, Faulkner C, Thomas JM, Thevis M (2007) Long‐term engraftment following transplantation of pig pancreatic primordia into non‐immunosuppressed diabetic rhesus macaques. Xenotransplantation 14, 591–602. [DOI] [PubMed] [Google Scholar]
- 8. Alvarez‐Garcia I, Miska EA (2005) MicroRNA functions in animal development and human disease. Development 132, 4653–4662. [DOI] [PubMed] [Google Scholar]
- 9. Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E et al (2002) Frequent deletions and down‐regulation of micro‐ RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 99, 15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couzin J (2008) MicroRNAs make big impression in disease after disease. Science 319, 1782–1784. [DOI] [PubMed] [Google Scholar]
- 11. Duroux‐Richard I, Presumey J, Courties G, Gayc S, Gordeladze J, Jorgensen C et al (2011) MicroRNAs as new player in rheumatoid arthritis. Joint Bone Spine 78, 17–22. [DOI] [PubMed] [Google Scholar]
- 12. Mohan R, Mao Y, Zhang S, Zhang Y‐W, Xu C‐R, Gradwohl G et al (2015) Differentially expressed microRNA‐483 confers distinct functions in pancreatic beta‐ and alpha‐cells. J. Biol. Chem. 7, 19955–19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sebastiani G, Po A, Miele E, Ventriglia G, Ceccarelli E, Bugliani M et al (2015) MicroRNA‐124a is hyperexpressed in type 2 diabetic human pancreatic islets and negatively regulates insulin secretion. Acta Diabetol. 52, 523–530. [DOI] [PubMed] [Google Scholar]
- 14. Stefani G, Slack FJ (2008) Small non‐coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 9, 219–230. [DOI] [PubMed] [Google Scholar]
- 15. Bunt Mvd, Gaulton KJ, Parts L, Moran I, Johnson PR, Lindgren CM et al (2013) The miRNA profile of human pancreatic islets and beta‐cells and relationship to type 2 diabetes pathogenesis. PLoS ONE 8, e55272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, MacDonald PE et al (2004). A pancreatic islet‐specific microRNA regulates insulin secretion. Nature 432, 226–230. [DOI] [PubMed] [Google Scholar]
- 17. Ballian N, Brunicardi FC (2007) Islet vasculature as a regulator of endocrine pancreas function. World J. Surg. 31, 705–714. [DOI] [PubMed] [Google Scholar]
- 18. Joglekar MV, Joglekar VM, Hardikar AA (2009) Expression of islet‐specific microRNAs during human pancreatic development. Gene Expr. Patterns 9, 109–113. [DOI] [PubMed] [Google Scholar]
- 19. Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P et al (2009) miR‐375 maintains normal pancreatic alpha‐ and beta‐cell mass. Proc. Natl. Acad. Sci. USA 106, 5813–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng J, Wang LY, Xu L, Wang H, Liu P, Bu S et al (2013) Gender‐dependent miR‐375 promoter methylation and the risk of type 2 diabetes. Exp. Ther. Med. 5, 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higuchi C, Nakatsuka A, Eguchi J, Teshigawara S, Kanzaki M, Katayama A et al (2015) Identification of circulating miR‐101, miR‐375 and miR‐802 as biomarkers for type 2 diabetes. Metabolism 64, 489–497. [DOI] [PubMed] [Google Scholar]
- 22. Li X (2014) MiR‐375, a microRNA related to diabetes. Gene 533, 1–4. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Q, Xiao X, Li M, Li W, Yu M, Zhang H et al (2014) miR‐375 and miR‐30d in the effect of chromium‐containing Chinese medicine moderating glucose metabolism. J. Diabetes Res. 2014, 862473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ouaamari AE, Baroukh N, Martens GA, Lebrun P, Pipeleers D, Obberghen EV (2008) miR‐375 targets 3‐phosphoinositide‐dependent proteinkinase‐1 and regulates glucose‐induced biological responses in pancreatic beta‐cells. Diabetes 57, 2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang ZW, Men T, Feng RC, Li YC, Zhou D, Teng CB (2013). miR‐375 inhibits proliferation of mouse pancreatic progenitor cells by targeting YAP1. Cell. Physiol. Biochem. 32, 1808–1817. [DOI] [PubMed] [Google Scholar]
- 26. Zhou J, Song S, He S, Zhu X, Zhang Y, Yi B et al (2014). MicroRNA‐375 targets PDK1 in pancreatic carcinoma and suppresses cell growth through the Akt signaling pathway. Int. J. Mol. Med. 33, 950–956. [DOI] [PubMed] [Google Scholar]
- 27. Latreille M, Herrmanns K, Renwick N, Tuschl T, Malecki MT, McCarthy MI et al (2015) miR‐375 gene dosage in pancreatic ß‐cells: implications for regulation of ß‐cell mass and biomarker development. J. Mol. Med. (Berl) 93, 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen PS, Su JL, Hung MC (2012) Dysregulation of MicroRNAs in cancer. J. Biomed. Sci. 19, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruan K, Fang X, Ouyang G (2009) microRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 285, 116–126. [DOI] [PubMed] [Google Scholar]
- 30. Song S, Zhou J, He S, Zhu D, Zhang Z, Zhao H et al (2013) Expression levels of microRNA‐375 in pancreatic cancer. Biomed. Rep. 1, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He X, Han W, Hu SX, Zhang MZ, Hua JL, Peng S (2015) Canonical Wnt signaling pathway contributes to the proliferation and survival in porcine pancreatic stem cells (PSCs). Cell Tissue Res. 362, 18. [DOI] [PubMed] [Google Scholar]
- 32. Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS (2003) MicroRNA targets in Drosophila. Genome Biol. 5, R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kapsimali M, Kloosterman WP, Ewart DB, Rosa F, Plasterk RH, Wilson SW (2007) MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 8, R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewis BP, Shih LH, Jones‐Rhoades MW, Bartel DP, Burge CB (2003) Prediction of mammalian MicroRNA targets. Cell 115, 787–798. [DOI] [PubMed] [Google Scholar]
- 35. Jafarian A, Taghikani M, Abroun S, Allahverdi A, Lamei M, Lakpour N et al (2015) The generation of insulin producing cells from human mesenchymal stem cells by MiR‐375 and Anti‐MiR‐9. PLoS ONE 10, e0128650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lahmy R, Soleimani M, Sanati MH, Behmanesh M, Kouhkan F, Mobarra N (2014) MiRNA‐375 promotes beta pancreatic differentiation in human induced pluripotent stem (hiPS) cells. Mol. Biol. Rep. 41, 2055–2066. [DOI] [PubMed] [Google Scholar]
- 37. Nathan G, Kredo‐Russo S, Geiger T, Lenz A, Kaspi H, Hornstein E et al (2015) MiR‐375 promotes redifferentiation of adult human β cells expanded in vitro. PLoS ONE 10, e0122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaer A, Azarpira N, Vahdati A, Karimi M, Shariati M (2014) miR‐375 induces human decidua basalis‐derived stromal cells to become insulin‐producing cells. Cell. Mol. Biol. Lett. 19, 483–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pasquinelli AE, Hunter S, Bracht J (2005) MicroRNAs: a developing story. Curr. Opin. Genet. 15, 200–205. [DOI] [PubMed] [Google Scholar]
- 40. Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]


