Abstract
Abstract. Objectives: Islet‐like clusters (ILCs), differentiated from human embryonic stem cells (hESCs), were characterized both before and after transplantation under the kidney capsule of streptozotocin‐induced diabetic immuno‐incompetent mice. Materials and methods: Multiple independent ILC preparations (n = 8) were characterized by immunohistochemistry, flow cytometry and cell insulin content, with six preparations transplanted into diabetic mice (n = 42), compared to controls, which were transplanted with either a human fibroblast cell line or undifferentiated hESCs (n = 28). Results: Prior to transplantation, ILCs were immunoreactive for the islet hormones insulin, C‐peptide and glucagon, and for the ductal epithelial marker cytokeratin‐19. ILCs also had cellular insulin contents similar to or higher than human foetal islets. Expression of islet and pancreas‐specific cell markers was maintained for 70 days post‐transplantation. The mean survival of recipients was increased by transplanted ILCs as compared to transplanted human fibroblast cells (P < 0.0001), or undifferentiated hESCs (P < 0.042). Graft function was confirmed by secretion of human C‐peptide in response to an oral bolus of glucose. Conclusions: hESC‐derived ILC grafts continued to contain cells that were positive for islet endocrine hormones and were shown to be functional by their ability to secrete human C‐peptide. Further enrichment and maturation of ILCs could lead to generation of a sufficient source of insulin‐producing cells for transplantation into patients with type 1 diabetes.
INTRODUCTION
Type 1 diabetes is a disease in which β‐cells of the pancreas are destroyed by an autoimmune mechanism. Use of exogenous insulin to treat diabetes is life‐saving; however, it does not truly mimic the body's natural response to blood glucose. As a result, serious complications, such as diabetic retinopathy, nephropathy and neuropathy, can occur (Shamoon et al. 1993). Pioneered by the Edmonton protocol, islet cell transplantation is currently considered to be an important method of therapy (Shapiro et al. 2000). However, scarcity of cadaveric islets prevents this form of treatment from benefiting most patients with type 1 diabetes.
Human embryonic stem cells (hESCs) are self‐renewing pluripotent cells obtained from the inner cell mass of pre‐implantation embryos (Thomson et al. 1998; Reubinoff et al. 2000) that can be maintained in vitro indefinitely while remaining karyotypically and phenotypically normal and stable (Amit et al. 2000). These cells have the ability to develop into cells of all three embryonic germ layers (Thomson et al. 1998; Amit et al. 2000; Reubinoff et al. 2000) and can be grown in an undifferentiated state without the need of mouse embryonic feeder (MEF) cells (Xu et al. 2001). Although many groups have been able to develop protocols for differentiation of mouse embryonic stem cells (mESCs) into insulin‐positive cells (Houard et al. 2003; Moritoh et al. 2003; Blyszczuk et al. 2004; Miyazaki et al. 2004; Fujikawa et al. 2005), there has been only limited success in differentiating hESCs into endocrine precursors. Protocols have been described for differentiation of hESCs into definitive endoderm (D’Amour et al. 2005) and, more recently, into insulin expressing β‐like cells (D’Amour et al. 2006). Although the insulin‐positive cells generated in this latter report released C‐peptide in response to various secretory stimuli, their minimal response to glucose suggests that they were not fully functional β‐cells. In addition, no in vivo data regarding survival and function of these hESC‐derived β‐like cells were presented in their study.
We have previously developed a multistep protocol to differentiate hESCs into insulin‐producing islet‐like clusters (ILCs) in serum‐free conditions without the use of feeder cell layers (Jiang et al. 2007a). We have shown that this differentiation process appeared to recapitulate some aspects of pancreatic differentiation during development, including the temporal pattern of pancreatic gene expression. Within these ILCs, we have found cells positive for markers of the endocrine pancreas plus ductal epithelium. In addition to insulin, ILCs also contain human C‐peptide and glucagon‐positive cells, and release C‐peptide in response to elevated concentrations of glucose.
In the present study, we have characterized the hormone content and functionality of these ILCs, both before and after transplant under the kidney capsule, of streptozotocin‐treated diabetic mice. These studies show that the hESC‐derived ILCs successfully engrafted and continued to express C‐peptide, insulin, glucagon and somatostatin‐positive cells. The cells that expressed pancreatic endocrine markers within ILCs are likely to represent an immature phenotype as they contained significantly less insulin than adult β‐cells; however, they responded to high glucose challenge in vivo and extended the survival of graft recipients.
MATERIALS AND METHODS
Cell culture and in vitro differentiation of hESCs
H1 hESCs were plated on Matrigel (Invitrogen, Burlington, Canada) coated plates and were cultured in mouse embryonic fibroblast‐derived conditioned medium (MEF‐CM), supplemented with 8 ng/mL of basic fibroblast growth factor (bFGF; R&D Systems, Minneapolis, MN, USA) as described previously (Xu et al. 2001). Differentiation was carried out in four stages using RPMI 1640 as the base medium as described previously (Jiang et al. 2007a). Briefly, confluent hESCs were cultured in RPMI medium supplemented with 2% B27 (Invitrogen) and 4 nm activin A (R&D Systems) and 1 mm sodium butyrate (Sigma‐Aldrich Canada, Oakville, ON, Canada) for 1 day, followed by 0.5 mm sodium butyrate and activin A for 6 days. At the end of this first stage, the cells were dissociated with 200 unit/mL collagenase IV (Invitrogen) and were transferred into ultra‐low attachment 6‐well plates (Corning, St. Louis, MO, USA). Cells were fed with fresh medium containing 2 ng/mL bFGF, 20 ng/mL epidermal growth factor and 100 ng/mL Noggin every 2–3 days for 2 weeks, followed by an additional week with epidermal growth factor and Noggin only. For the final week of differentiation, cell clusters were cultured with fresh medium containing 0.5% bovine serum albumin, 10 mm nicotinamide (Sigma‐Aldrich), and 50 ng/mL insulin‐like growth factor II (R&D Systems) for 5 days, followed by 2 days without insulin‐like growth factor II.
The data contained in this report have been obtained from eight independent preparations of this differentiation procedure. Six preparations were used for both in vitro characterization experiments and transplantation studies and two preparations were used exclusively for in vitro analysis.
Human fibroblasts (BJ cells, Dr. Woody Wright, University of Texas Southwestern Medical School, Dallas, TX, USA) were grown in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum (Invitrogen).
Characterization of hESC‐derived ILC grafts
For immunohistochemistry (IHC) and immunofluorescence (IF), ILCs were fixed overnight in Z‐fix (Anatech Ltd., Battle Creek, MI, USA) and then were embedded in 2% low‐melting‐point agarose (Sigma). The agar plugs or recovered graft‐bearing kidneys were then processed and embedded in paraffin wax, after which 3 µm sections were cut and collected on HistoBond slides (Marienfeld, Lauda‐Königshofen, Germany). IHC was performed on the samples as previously described previously (Korbutt et al. 1996). Briefly, sections were rehydrated in water, followed by the quenching of endogenous peroxidases using a solution of 20% hydrogen peroxide in methanol, for 6 min. For cytokeratin 19 (CK19) and PDX‐1 staining, antigen retrieval was performed by microwaving the slides for 15 min at 80% power using either 10 mm sodium citrate solution (pH 6) or 10 mm Tris 1 mm ethylenediaminetetraacetic acid (pH 9). Blocking was performed with 20% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 15 min at room temperature. Primary antibody concentrations were as follows: 1 : 1000 guinea pig anti‐insulin (Dako Cytomation, Missisauga, Canada), 1 : 50 mouse anti‐cytokeratin 19 (Dako), 1 : 50 rabbit anti‐synaptophysin (Dako), 1 : 1000 rabbit anti‐human somatostatin (Dako), 1 : 5000 mouse anti‐glucagon (Sigma), 1 : 500 mouse anti‐human C‐peptide, specific for human C‐peptide of human pro‐insulin (Cedarlane Laboratory Ltd., Hornby, Canada), 1 : 100 rabbit anti‐human PC1/3 and rabbit anti‐human PC2 (Chemicon International Inc., Temecula, CA, USA), 1 : 5000 rabbit anti‐PDX‐1 (generously donated by Dr. Chris Wright) and 1 : 200 PDX‐1 (Geron Corp., Menlo Park, CA, USA). Whole guinea pig or rabbit IgG (1 : 100, Jackson ImmunoResearch Laboratories) and mIgG1 (1 : 200, Cedarlane) were used as controls. Primary antibody incubation was either 30 min at room temperature or overnight at 4 °C (PDX‐1), followed by three washes in phosphate‐buffered saline (PBS). Secondary antibody was applied for 20 min at room temperature. After washes in PBS, sections were incubated with avidin‐biotin enzyme complex for 40 min at room temperature (Vector Laboratories, Burlingame, CA, USA), then washed three times in PBS, and developed using diaminobenzidine as the chromagen (Signet Laboratories Inc., Dedham, MA, USA). Sections were counterstained with Harris’ haematoxylin, and coverslipped using Entellan mounting media (Electron Microscopy Sciences, Hattlefield, PA, USA). For IF staining, the same protocol was conducted; however, the quench step was eliminated, and secondary antibodies were applied for 1 h at room temperature followed by three washes in PBS and coverslipping using Prolong Antifade mounting media (Invitrogen). IHC and IF slides were visualized using an Axioscope II microscope equipped with an AxioCam MRc and HBS 100 W AttoArc 2 light source. Images were analysed using AxioVision 3.1 software (Carl Zeiss, Gottingen, Germany). ILCs were also assessed by electron microscopy and by fluorescence‐activated cell sorting (FACS) analysis using methods described previously (Korbutt et al. 1996; Jiang et al. 2007a).
Enumeration of ILC equivalents and insulin content
To account for variability in cluster size between different ILC preparations, cell clusters were counted using a 100‐micron grid and were normalized for size, with the mean size and cluster volume referred to as one islet‐like cluster equivalent (IE). This count was performed so that regardless of average size of ILCs from multiple preparations, transplanted tissue volume would always be 2500 IE, which equates to roughly 5 million cells.
Cell insulin content of the ILCs was assessed by RIA (Diagnostic Products Corp., Los Angeles, CA, USA) on a minimum of five replicate aliquots (100 IE each) after extraction in 2 mm acaetic acid containing 0.25% bovine serum albumin (Korbutt et al. 1996). In order to determine the number of total cells and β‐like cells per ILC, we measured total DNA content per ILC (Korbutt et al. 1996), which was then divided by 6.1 pg DNA per human cell to calculate total cell number, and multiplied the mean percent C‐peptide positive cells by this number to establish the absolute number of β‐like cells per transplant.
Transplantation of ILCs into diabetic mice and metabolic follow‐up
Aliquots of either 2500 IE of differentiated ILCs or 5 × 106 human fibroblasts (hFCs) or undifferentiated hESCs (hESCs) were aspirated into polyethylene tubing (P‐90), pelleted by centrifugation, and gently placed under the left kidney capsule of streptozotocin‐induced (Sigma, 175 mg/kg) diabetic C57BL6 Rag‐1/‐1 mice (Jackson Laboratories, Bar Harbour, ME, USA) with the aid of a micromanipulator syringe (Korbutt et al. 1996). Once the tubing was removed, the capsulotomy was cauterized with a disposable high‐temperature cautery pen. Mice were monitored for blood glucose levels (MediSense glucose meter) using blood from the tail vein once a week. A total of 42 animals were transplanted from six separate ILC preparations, and 28 additional animals were transplanted with either human fibroblasts or undifferentiated hESCs. Animals were euthanized when deemed unhealthy by exhibiting specific symptoms, including lethargy, lack of righting reflex, development of necrotic tail, excessive lack of grooming, or abdominal fluid retention, or at previously determined time points (2, 4, 6, 8 or 9 weeks) according to the guidelines of the Canadian Council on Animal Care. On the day of euthanization, healthy animals were fasted for a period of 3 h, followed by an administered oral bolus (3 mg/g body weight) of a 50% solution of dextrose (Abbott Laboratories Ltd., Montreal, Canada). When 15 min had elapsed, the animals were subjected to halothane treatment and their blood was harvested via cardiac puncture. The blood was allowed to clot, then was centrifuged to collect serum, which was analysed using a human‐specific ultra‐sensitive C‐peptide ELISA kit (Mercodia Inc., Salem, NC, USA) as per manufacturers instructions.
Statistical analysis
Continuous data were expressed as mean ± standard error of the mean (SEM). Means of independent groups were compared by an independent samples t‐test. Means of multiple groups were compared by one‐way analysis of variance (anova) with Tukey honestly significant difference post hoc analysis to compare individual groups using SPSS 14.0 (SPSS Inc., Chicago, IL, USA). Kaplan Meier curves were generated and compared using the log‐rank (Mantel‐Cox) test.
RESULTS
Composition of differentiated hESC‐derived ILC grafts
Human embryonic stem cells were differentiated into ILCs using a multistep protocol described previously (Jiang et al. 2007a). Briefly, the differentiation protocol had been divided into four stages, in which the cells developed from definitive endoderm (day 8), to pancreatic endoderm (day 15), and finally to pancreatic exocrine/endocrine (day 29) and ILCs (days 35–36), at which point samples were taken for in vitro analysis. As previously reported in Jiang et al. (2007a), these hESC‐derived ILCs were shown to express pancreatic hormones, by IHC, including insulin (Fig. 1a), glucagon (Fig. 1b) and human C‐peptide (Fig. 1c). However, pancreatic polypeptide and islet amyloid polypeptide (amylin) were not detected (data not shown). These clusters also expressed other pancreatic markers, including ductal epithelial ductal cell marker CK19 (Fig. 1f), neuroendocrine marker synaptophysin and insulin transcription factor PDX‐1 (data not shown). ILCs were also positive for prohormone convertase (PC) 1/3 and 2 (Fig. 1d,e), both of which are expressed normally in mature islets as they are responsible for endocrine hormone processing. Analysis of serial sections of these ILCs indicated that in most ILCs expression of pancreatic hormones occurred either within the same regions of a single cluster or possibly co‐localized in the same cells (Fig. 1a–c). When ILC grafts were examined after 1‐day post‐transplant, the great majority of insulin‐positive cells also expressed human C‐peptide, while a smaller proportion co‐expressed insulin/glucagon (Fig. 2). Furthermore, electron micrographs of ILCs demonstrated β‐like cells exhibiting both immature (granule surrounded by a halo) and mature (dense granule) secretory vesicles typical for those seen in adult pancreatic β‐cells (Tooze et al. 1991) (Fig. 3, indicated by solid arrowhead or hollow arrow).
Figure 1.
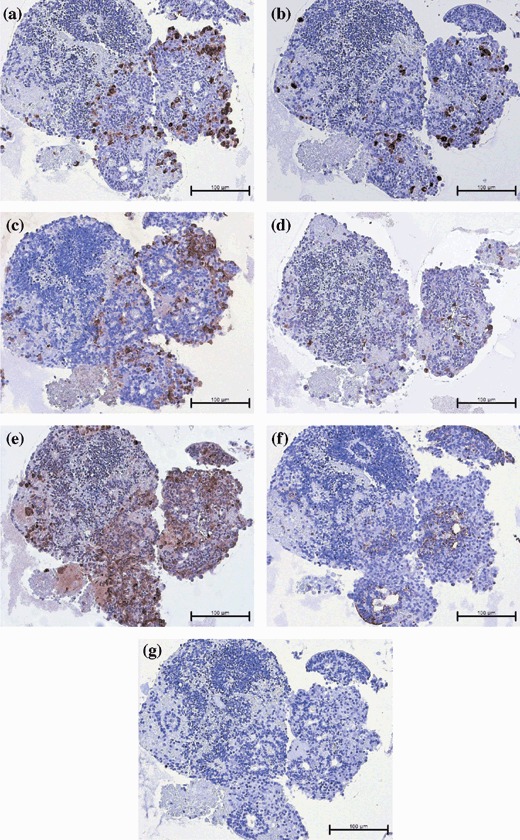
Photomicrographs of hESC‐derived ILCs after 36 days of in vitro differentiation and immediately prior to transplantation. Serial sections stained by immunohistochemistry for: (a) insulin, (b) glucagon, (c) human C‐peptide, (d) pro‐hormone convertase 1/3, (e) pro‐hormone convertase 2, (f) cytokeratin‐19 and (g) representative isotype control. Magnification ×200; scale bar indicates 100 microns.
Figure 2.
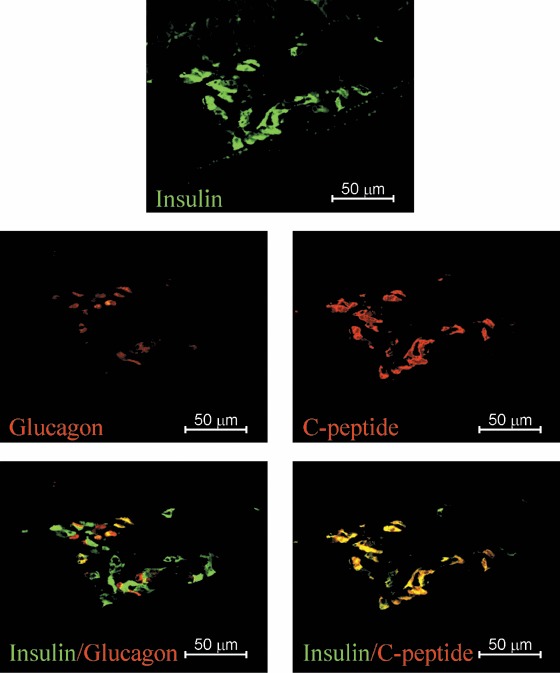
Photomicrographs of a hESC‐derived ILC graft‐bearing kidney removed 1‐day post‐transplantation. Serial sections double stained with anti‐insulin (green) and antiglucagon (red) or double stained with antihuman‐C‐peptide (red) and anti‐insulin (green), specific antibodies as indicated. Magnification ×400; scale bar indicates 50 microns.
Figure 3.
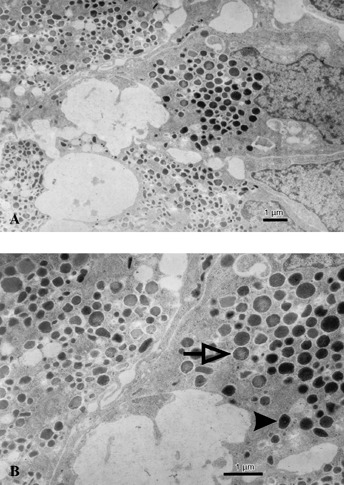
Electron photomicrographs of hESC‐derived ILCs after 36 days of in vitro differentiation and prior to transplantation. β‐like cells within the ILC appear well granulated, structurally intact containing both mature (solid arrow) and immature (open arrow) secretory granules, typical for adult islet β‐cells. Scale bar indicates 1 micron.
Based on DNA content of ILCs (6.1 pg DNA/cell; Table 1), we calculated that a graft of 2500 IE contained 4.8 × 106 cells. We have previously reported that using our procedure, 2–8% of hESCs differentiated into human C‐peptide‐positive cells (referred to as β‐like cells in Table 1) as determined by FACS analysis (Jiang et al. 2007a). Based on these analyses, we estimated that each graft contained roughly 0.25 × 106β‐like cells. Similarly, because the differentiated ILCs contained on average 0.36 ng insulin per IE, we estimated that each β‐like cell contained 3.6 pg of insulin, and that a graft of 2500 IE would contain approximately 900 ng insulin.
Table 1.
Characterization of human embryonic stem cell‐derived insulin‐like clusters (ILC) grafts by insulin content, DNA content and FACS prior to transplantation. Values are means ± SE from n = 3 independent ILC preparations
| DNA (ng) | Content per ILC IE | C‐peptide positive (%) | β‐Like cells | |
|---|---|---|---|---|
| Insulin (ng) | Total cells | |||
| 12 ± 0.62 | 0.36 ± 0.23 | 1900 ± 100 | 5.3 ± 0.01 | 101 ± 5.4 |
Transplantation of ILCs into diabetic mice
The number of animals transplanted with ILCs, hFCs or undifferentiated hESCs as well as their post‐surgical outcome are described in detail in Table 2. Briefly, of the 42 animals transplanted with differentiated ILCs, seven (17%) were found dead during the 11 week follow‐up period, and five (12%) were euthanized due to illness before or after 50 days post‐transplant, respectively. Of the 30 remaining mice, 11 and 19 healthy mice were euthanized for assessment before or after 50 days post‐transplant, respectively. Of 21 animals transplanted with hFCs, nine animals (43%) were found dead over the course of the follow‐up and 12 (57%) were euthanized due to poor health. Similarly, of six mice implanted with undifferentiated hESCs, four (67%) were found dead and two (33%) euthanized due to sickness. When excluding those animals euthanized for assessment at pre‐determined time points less than 50 days post‐transplant, differentiated ILC transplanted mice had a mean survival of 55 ± 2.6 days. This number may be artificially low, as all of the animals were euthanized at the end of the experiment irrespective of their healthy status. According to the guidelines of the Canadian Council for Animal Care, endpoints must be determined in advance, as death cannot be considered as an endpoint in an experiment of this kind. Those mice receiving hFCs or undifferentiated hESCs had a mean survival of 23.4 ± 4.9 and 35.2 ± 3.1 days, respectively (Fig. 4a). None of these animals survived beyond 56 days, compared to the ILC group, in which 77 days was the final euthanization point. This increase in survival of the ILC‐transplanted group compared to the hFC group was statistically significant (P < 0.042) whereas the difference in survival between the hFC and undifferentiated hESC group was not (P < 0.351) (Fig. 4a). When assessing the proportion of recipients surviving beyond 50 days post‐transplant, 78.6% of the ILC‐implanted animals survived beyond 50 days, compared to only 23.8% and 0% in the hFC and undifferentiated hESC groups. In addition to this, a Kaplan‐Meier survival curve (Fig. 4b) using the log‐rank (Mantel‐Cox) test of the equality of survival distributions for the different levels of group denotes a significant difference of P < 0.0001 between the three groups of animals transplanted.
Table 2.
Description of the number of animals transplanted. Three groups transplanted were insulin‐like clusters (ILCs), human fibroblast cell line (hFCs) or undifferentiated human embryonic stem cells (uhESCs). Animals were either found dead, euthanized due to illness, or, if healthy, euthanized at defined time points either before or after 50 days post‐transplant
| Group | Total transplants | Found dead | Euthanized due to illness | Euthanized <50 days | Euthanized >50 days |
|---|---|---|---|---|---|
| ILC | 40 | 7 | 5 | 11 | 19 |
| hFCs | 21 | 9 | 12 | N/A | N/A |
| uhESC | 6 | 4 | 2 | N/A | N/A |
Figure 4.
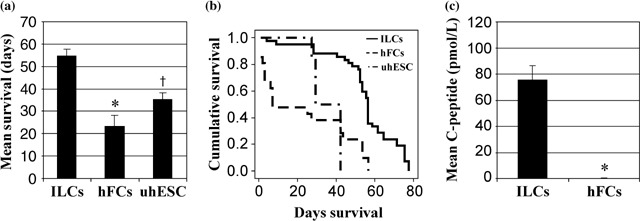
Mean survival (a) and the Kaplan Meier curve of survival (b) of mice transplanted with either hESC derived ILCs (n = 42), a human fibroblast cell line (hFCs, n = 21) or undifferentiated hESC (uhESC, n = 6). For the Kaplan Meier curve (4B), the log‐rank (Mantel‐Cox) test of the equality of survival distributions for the different levels of group denotes P < 0.0001. Of the 42 animals transplanted with ILCs, 7 were found dead at various time points, 19 healthy mice were euthanized at defined time points after 50 days post‐transplant then 3 and 2 mice were euthanized due to illness before or after 50 days post‐transplant, respectively. Lastly, 11 healthy mice in this group were euthanized at predetermined time points before 50 days post transplant and were not included in this graph. Human serum C‐peptide was also determined in mice transplanted with ILCs (n = 12) or control mice implanted with hFCs (n = 9, c). *P < 0.0001, †P < 0.042.
After streptozotocin administration, all recipients exhibited blood glucose levels above 20.0 mmol/L at the time of transplant. Transplantation of 2500 IE of differentiated hESC‐derived ILCs did not result in a significant reduction in blood glucose, as all recipients had values ranging from 18.0 to 35 mmol/L. Meanwhile, mice that had been implanted with either human fibroblasts or undifferentiated hESCs as a control had blood glucose values above 35 mmol/L (data not shown). In addition, when recipients of differentiated ILCs were given an oral bolus of glucose (3 mg/g body weight), serum collected from these mice (n = 12) at 15 min post‐administration contained human serum C‐peptide with values ranging from 23.86 to 124.81 pmol/L. In contrast, no measurable human C‐peptide was detected in the serum of mice transplanted with either human fibroblasts (n = 9; Fig. 4c) or undifferentiated hESCs (n = 1; data not shown). In the absence of an oral bolus of glucose, ILC‐transplanted recipients did not secrete perceptible C‐peptide, indicating that ILCs secreted C‐peptide in response to elevated levels of glucose (data not shown).
Immunohistochemical examination of ILC grafts revealed that CK19 was expressed in 83% of the recovered ones (Fig. 5), with the majority of staining occurring in regions of the graft that were structurally similar to ducts of adult human pancreas (Fig. 6h). Insulin‐positive cells were found in 16 of the 23 grafts (ranging between 40 and 64 days post‐transplant) (Fig. 6a), of which 18 were shown to be positive for human C‐peptide (5, 6). Similarly, PDX‐1 expression was detected in 16 of the ILC grafts (Fig. 5) and staining was located in or near cells expressing CK19 and synaptophysin (Fig. 6g). We found that 13 of the 23 grafts were positive for both insulin and PDX‐1, while three were positive for insulin and PDX‐1 independently (Fig. 5). It was also shown that glucagon‐positive cells were observed in 12 of 23 grafts (5, 6), while somatostatin staining was seen in 8 of 23 grafts (5, 6). We also observed that within or adjacent to many CK19‐positive ductal regions (indicated by arrows), small foci of insulin, glucagon and C‐peptide‐positive cells were often seen (Fig. 6a,c,h). In those grafts with large numbers of cells positive for pancreatic hormones, there was also prevalent expression of PC 1/3 and PC2 (Fig. 6d,e).
Figure 5.
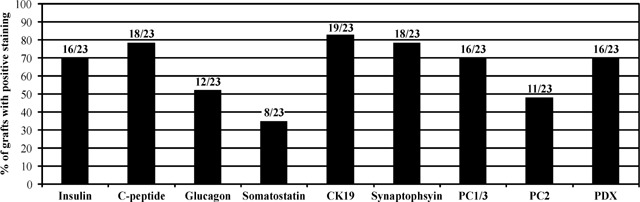
Morphological assessment of differentiated ILC‐derived grafts in mice following at least 40 days post‐transplantation. Data are expressed as percentage of grafts with positive staining for specific pancreatic markers from a total of 23 mice.
Figure 6.
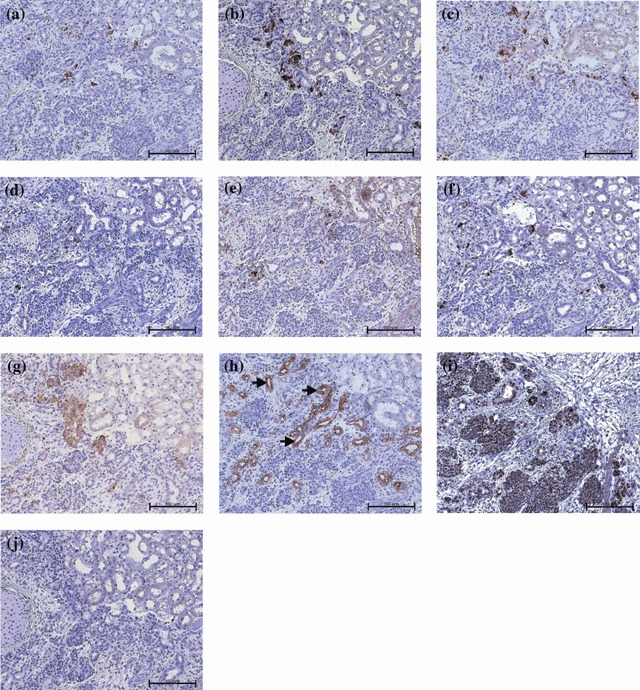
Photomicrographs of differentiated ILC‐derived grafts at 56 days post‐transplantation. (a) Insulin, (b) glucagon, (c) human C‐peptide, (d) pro‐hormone convertase 1/3, (e) pro‐hormone convertase 2, (f) somatostatin, (g) synaptophysin, (h) cytokeratin‐19, (I) PDX‐1 and (j) representative isotype control. Arrows indicate areas of cytokeratin‐19 staining which are comparable to the ductal human pancreas. Magnification ×200; scale bar indicates 100 microns.
DISCUSSION
Differentiation of embryonic stem cells into insulin‐producing and glucose‐responsive β‐cells has been an active and controversial field of study. In experiments involving mESCs, it was found that protocols modified from those producing neurones and neuronal precursors could also produce cells which were positive for insulin (Lumelsky et al. 2001). However, there is now published evidence that the insulin‐positive cells produced by these protocols are, in fact, neuronal precursors and not of a pancreatic endocrine lineage (Sipione et al. 2004), with insulin immunoreactivity due to uptake of insulin present in the culture medium (Rajagopal et al. 2003; Hansson et al. 2004).
Subsequently, new protocols using activin A (Shi et al. 2005) have been developed to differentiate mESCs into cells that can function in a manner similar to pancreatic β‐cells in vitro and in vivo. There have been fewer protocols to differentiate hESCs, three of which are based on spontaneous differentiation (Assady et al. 2001; Brolen et al. 2005; Xu et al. 2006) and two on a modified version of the Lumelsky protocol (Segev et al. 2004; Baharvand et al. 2006). Only recently has a protocol been developed to differentiate hESCs into definitive endoderm through the use of activin A and bFGF (D’Amour et al. 2005) and, even more recently, into insulin‐expressing cells through stepwise growth factor application (D’Amour et al. 2006).
An additional drawback of previous methods to differentiate hESCs into large numbers of functional insulin‐secreting cells is that most methods rely on the use of MEF as feeders in order to maintain the undifferentiated state of the hESC lines. We have developed a protocol to grow hESCs without the use of feeders for multiple passages, while retaining the fundamental characteristics of undifferentiated cells (Xu et al. 2001). In addition, our differentiation protocol is conducted without the use of serum components (Jiang et al. 2007a).
Each stage of differentiation used to produce the ILCs in the present study has been extensively characterized in vitro (Jiang et al. 2007a). In this previous study (Jiang et al. 2007a), we measured the ability of these ILCs to respond to glucose in vitro by a C‐peptide‐static incubation assay, and observed a stimulation index after 3.5 h of 3.3, compared to undifferentiated hESCs, which are not responsive to high glucose. In multiple independent differentiations, we have further observed that ILCs contain cells that express islet hormones and other pancreatic cell markers, including PDX‐1, suggesting that these cells are of a pancreatic endocrine or ductal progenitor lineage. The presence of three pancreatic endocrine‐derived hormones within these ILCs implies that their composition is similar to their islet counterparts, which contain β, α and δ cells, producing insulin, glucagon and somatostatin, respectively. Mature islets function as a whole organ to maintain normoglycaemia in the human body and it is not yet known if there are greater benefits associated with transplantation of a pure β‐cell population as opposed to whole islets.
It should be noted that there are still a number of cells within the ILCs that are as yet uncharacterized; it is possible that the removal of these cells could improve the function of our ILCs in vivo. In some animals, we observed large outgrowths of tissues containing cells that expressed the undifferentiated cell marker Oct3/4, indicating that ILCs contained an undifferentiated and proliferating stem cell population (data not shown), a phenomenon that has been described in many experiments involving embryonic stem cells transplants (Niwa et al. 2000; Bieberich et al. 2004; Fujikawa et al. 2005). Due to this, we were unable to take our experiments much further than 11–12 weeks, as the animals at this point exhibited swollen abdomens. It is interesting to note that in experiments involving transplantation of human embryonic pancreas, a similar phenomenon is seen, as the graft grows 10‐fold in size within 8–12 weeks and continues to grow another 10‐fold by 33–38 weeks post‐transplant (Castaing et al. 2001). Future transplantation experiments will have to be performed to determine whether removal of such Oct3/4+ cells from ILCs decreases the outgrowth and, thus, provides a better environment for further differentiation and survival of β‐like cells to induce euglycaemia.
In the present study, transplantation of hESC‐derived ILCs into diabetic mice did not significantly reduce blood glucose levels. Mice were implanted with 2500 IE of differentiated ILCs that contained a total of 4.8 × 106 cells with approximately 0.25 × 106 of these being β‐like cells (i.e. only 5.3% total β‐like cells). One possible explanation concerning why 2500 IE were unable to achieve normoglycaemia in these mice is that an insufficient β‐cell mass was transplanted. In our experience, a transplant of 2000 human islet equivalents is necessary to reverse hyperglycaemia in diabetic mice. Assuming that 2000 human islets are composed of approximately 2.0 × 106 cells (1000 cells per islet) with a proportion of ~60%β‐cells, a mass of 1.2 × 106β‐cells is needed to reverse diabetes in mice. Thus, our ILC grafts consisting of only 0.25 × 106β‐like cells were comprised of a β‐cell mass that was approximately 5‐fold too low to achieve euglycaemia. Another possibility is that many of the β‐like cells within transplanted ILCs were not functionally mature. For example, mature human islets may contain approximately 20 ng (3.44 pmol) of insulin per islet, whereas a human foetal islet contains approximately 0.26 ng (0.05 pmol) per islet cell cluster (Hayek & Beattie 1997). We have observed as high as 0.82 ng (0.16 pmol) of insulin per ILC, which compares more than favourably with that of a human foetal islet. Low cellular insulin content of insulin‐producing cells within hESC‐derived ILCs, in comparison to β‐cells of adult islets, is likely to be a reflection of their immature status. This immaturity is further supported by low expression of PC 1/3 and lack of expression of its subsequent product amylin, which are both seen in mature β‐cells (Piper et al. 2004), and by presence both before and after transplant, of cells which were positive for C‐peptide (pro‐insulin) but not for insulin. It is important to note, that in the case of human foetal pancreas, a 500‐IEC transplant can normalize a diabetic animal in 2–3 months (Tuch 1991), with C‐peptide serum levels after glucose challenge at 12 weeks post‐transplant in the region of 1200 pmol/L (Hayek & Beattie 1997). There have also been experiments involving transplantation of human embryonic pancreas beneath the kidney capsule of diabetic mice in which the grafts did not reverse hypoglycaemia until 3 months post‐transplant (Castaing et al. 2001). Unfortunately, we did not see the same effect with our ILC transplants, even though our pre‐transplant insulin content compared favourably with human foetal islets. A potential reason for this is the comparatively heterogeneous makeup of our ILCs in comparison with human foetal islets. Due to this, it is hoped that improving the proportion of pancreatic precursor cells within the ILCs, and removing undifferentiated or non‐pancreatic cells would be highly beneficial to both the ability of ILCs to fully differentiate in vivo and to their potential as a long‐term solution to hyperglycaemia.
We therefore hypothesize that in our study, combination of low levels of insulin and potentially a lack of fully processed insulin being produced and secreted by the ILC grafts may not be adequate to normalize blood glucose values in the mice, but are sufficient to improve their overall health and survival. Functionality of ILCs in vivo is corroborated by the fact that the majority of animals transplanted with differentiated ILCs that survived longer than 42 days were comparatively healthy, and contained grafts that were positive for human C‐peptide and insulin. Further evidence that ILC grafts were functional and secreting insulin post‐transplantation is detection of human C‐peptide in the serum of mice following a glucose challenge. A recent report (Emamaullee et al. 2005) demonstrated that a suboptimal mass of 1000 human islets transplanted into diabetic mice, results in human serum C‐peptide levels of 50–100 pmol/L of human C‐peptide in response to glucose. In our study, the human serum C‐peptide levels of mice transplanted with 2500 ILCs ranged from 24 to 125 pmol/L, indicating that the surviving β‐like cells in these ILC grafts were functional. Optimized differentiation protocols that further increase the proportion of β‐like cells within ILCs as well as cell insulin content and functional maturation would most likely increase our ability to normalize blood glucose values following transplantation into diabetic mice.
In a recent study (Jiang et al. 2007b), differentiated hESCs were transplanted into diabetic animals and were shown to lower blood glucose levels in 9 of 19 mice. In that report (Jiang et al. 2007b), the criteria for induced diabetes was a blood glucose level of only > 13.9 mm. In six mice that had successful transplants, mean blood glucose levels were 18 mm prior to transplant and decreased to 11 mm over 6 weeks. Moreover, this study (Jiang et al. 2007b) used a multiple low‐dose streptozotocin‐induced diabetic model which is known to result in pancreatic regeneration (Hartmann et al. 1989).
In a further study, blood glucose levels were successfully reduced in six mice as early as 4 days following transplantation of differentiated hESCs that did not express insulin at the time of transplantation (Shim et al. 2007). In addition, this report employs human embryonic stem cell lines (Miz‐hES4 and Miz‐hES6) which are not cleared for research in North America by either the National Institute of Health or the Canadian Institute for Health Research, making any comparison of the differentiation procedures challenging at best. Furthermore, the differentiation procedure used in this study includes the use of foetal bovine serum in the early stages, which we have eliminated completely from our procedure. This protocol also included a medium containing insulin (ITS) during the end stages of their differentiation procedure, which may have affected survival and the blood glucose levels of the animals shortly after transplantation. Furthermore, this study did not provide thorough data to indicate the proportion of differentiated cells which expressed human C‐peptide prior to transplant, or any indication of their prospective insulin content.
In summary, our key findings include extended survival of ILC‐transplanted animals, whose grafts express insulin, C‐peptide, PDX‐1 and other markers of pancreatic islets, and clear evidence of glucose‐stimulated human C‐peptide secretion in vivo by the grafts. With further improvements to this protocol and development of a method to remove residual undifferentiated tissue, a large‐scale means of providing suitable tissue for transplant to patients with type I diabetes should be feasible in the near future.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Lynette Elder and James Lyon for technical support, and Dr. Eric Lehr for his help with the statistical analysis. We would also like to acknowledge Dr. Joseph R. Gold and Dr. Jackie Lee for their help with editing of the manuscript. We also thank Dr. Woody Wright for providing the human fibroblast cell line and Dr. Chris Wright for providing the PDX‐1 antibody. G. S. Korbutt is an Alberta Heritage Foundation for Medical Research Senior Scholar.
REFERENCES
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz‐Eldor J, Thomson JA (2000) Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271–278. [DOI] [PubMed] [Google Scholar]
- Assady S, Maor G, Amit M, Itskovitz‐Eldor J, Skorecki KL, Tzukerman M (2001) Insulin production by human embryonic stem cells. Diabetes 50, 1691–1697. [DOI] [PubMed] [Google Scholar]
- Baharvand H, Jafary H, Massumi M, Ashtiani SK (2006) Generation of insulin‐secreting cells from human embryonic stem cells. Dev. Growth Differ. 48, 323–332. [DOI] [PubMed] [Google Scholar]
- Bieberich E, Silva J, Wang GH, Krishnamurthy K, Condie BG (2004) Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell‐derived neural transplants. J. Cell Biol. 167, 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyszczuk P, Asbrand C, Rozzo A, Kania G, St Onge L, Rupnik M, Wobus AM (2004) Embryonic stem cells differentiate into insulin‐producing cells without selection of nestin‐expressing cells. Int. J. Dev. Biol. 48, 1095–1104. [DOI] [PubMed] [Google Scholar]
- Brolen GKC, Heins N, Edsbagge J, Semb H (2005) Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin‐producing beta‐cell‐like cells. Diabetes 54, 2867–2874. [DOI] [PubMed] [Google Scholar]
- Castaing M, Peault B, Basmaciogullari A, Casal I, Czernichow P, Scharfmann R (2001) Blood glucose normalization upon transplantation of human embryonic pancreas into beta‐cell‐deficient SCID mice. Diabetologia 44, 2066–2076. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE (2006) Production of pancreatic hormone‐expressing endocrine cells from human embryonic stem cells. Nat. Biotech. 24, 1392–1401. [DOI] [PubMed] [Google Scholar]
- Emamaullee JA, Rajotte RV, Liston P, Korneluk RG, Lakey JRT, Shapiro AMJ, Elliott JF (2005) XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes 54, 2541–2548. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE (2005) Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell‐derived insulin‐producing cells. Am. J. Pathol. 166, 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Tonning A, Frandsen U, Petri A, Rajagopal J, England MCO, Heller RS, Hakansson J, Fleckner J, Skold HN, Melton D, Semb H, Serup P (2004) Artifactual insulin release from differentiated embryonic stem cells. Diabetes 53, 2603–2609. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Besch W, Zuhlke H (1989) Spontaneous recovery of streptozotocin diabetes in mice. Exp. Clin. Endocrinol. 93, 225–230. [DOI] [PubMed] [Google Scholar]
- Hayek A, Beattie GM (1997) Experimental transplantation of human fetal and adult pancreatic islets. J. Clin. Endocrinol. Metab. 82, 2471–2475. [DOI] [PubMed] [Google Scholar]
- Houard N, Rousseau GG, Lemaigre FP (2003) HNF‐6‐independent differentiation of mouse embryonic stem cells into insulin‐producing cells. Diabetologia 46, 378–385. [DOI] [PubMed] [Google Scholar]
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS (2007a) Generation of insulin‐producing islet‐like clusters from human embryonic stem cells. Stem Cells 25, 1940–1953. [DOI] [PubMed] [Google Scholar]
- Jiang W, Shi Y, Zhao DX, Chen S, Yong J, Zhang J, Qing TT, Sun XN, Zhang P, Ding MX, Li DS, Deng HK (2007b) In vitro derivation of functional insulin‐producing cells from human embryonic stem cells. Cell Res. 17, 333–344. [DOI] [PubMed] [Google Scholar]
- Korbutt GS, Elliott JF, Ao ZL, Smith DK, Warnock GL, Rajotte RV (1996) Large scale isolation, growth, and function of porcine neonatal islet cells. J. Clin. Invest. 97, 2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, Mckay R (2001) Differentiation of embryonic stem cells to insulin‐secreting structures similar to pancreatic islets. Science 292, 1389–1394. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yamato E, Miyazaki J (2004) Regulated expression of pdx‐1 promotes in vitro differentiation of insulin‐producing cells from embryonic stem cells. Diabetes 53, 1030–1037. [DOI] [PubMed] [Google Scholar]
- Moritoh Y, Yamato E, Yasui Y, Miyazaki S, Miyazaki J (2003) Analysis of insulin‐producing cells during in vitro differentiation from feeder‐free embryonic stem cells. Diabetes 52, 1163–1168. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct3/4 defines differentiation, dedifferentiation or self‐renewal of ES cells. Nat. Genet. 24, 372–376. [DOI] [PubMed] [Google Scholar]
- Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA (2004) Beta cell differentiation during early human pancreas development. J. Endocrinol. 181, 11–23. [DOI] [PubMed] [Google Scholar]
- Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA (2003) Insulin staining of ES cell progeny from insulin uptake. Science 299, 363. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A (2000) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro . Nat. Biotechnol. 18, 399–404. [DOI] [PubMed] [Google Scholar]
- Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz‐Eldor J (2004) Differentiation of human embryonic stem cells into insulin‐producing clusters. Stem Cells 22, 265–274. [DOI] [PubMed] [Google Scholar]
- Shamoon H, Duffy H, Fleischer N, Engel S, Saenger P, Strelzyn M, Litwak M, Wylierosett J, Farkash A, Geiger D, Engel H, Fleischman J, Pompi D, Ginsberg N, Glover M, Brisman M, Walker E, Thomashunis A, Gonzalez J, Genuth S, Brown E, Dahms W, Pugsley P, Mayer L, Kerr D, Landau B, Singerman L, Rice T, Novak M, Smithbrewer S, Mcconnell J, Drotar D, Woods D, Katirgi B, Litvene M, Brown C, Lusk M, Campbell R, Lackaye M, Richardson M, Levy B, Chang S, Heinheinemann M, Barron S, Astor L, Lebeck D, Brillon D, Diamond B, Vasilasdwoskin A, Laurenzi B, Foldi N, Rubin M, Flynn T, Reppucci V, Heise C, Sanchez A, Whitehouse F, Kruger D, Kahkonen D, Fachnie J, Fisk J, Carey J, Cox M, Ahmad B, Angus E, Campbell H, Fields D, Croswell M, Basha K, Chung P, Schoenherr A, Mobley M, Marchiori K, Francis J, Kelly J, Etzwiler D, Callahan P, Hollander P, Castle G, Bergenstal R, Spencer M, Nelson J, Bezecny L, Roethke C, Orban M, Ulrich C, Gill L, Morgan K, Laechelt J, Taylor F, Freking D, Towey A, Lieppman M, Rakes S, Mangum J, Cooper N, Upham P, Jacobson A, Crowell S, Wolfsdorf J, Beaser R, Ganda O, Rosenzweig J, Stewart C, Halford B, Friedlander E, Tarsy D, Arrigg P, Sharuk G, Shah S, Wu G, Cavallerano J, Poole R, Silver P, Cavicchi R, Fleming D, Marcus J, Griffiths C, Cappella N, Nathan D, Larkin M, Godine J, Lynch J, Norman D, Mckitrick C, Haggen C, Delahanty L, Anderson E, Lou P, Taylor C, Cros D, Folino K, Brink S, Abbott K, Sicotte K, Service FJ, Schmidt A, Rizza R, Zimmerman B, Schwenk W, Mortenson J, Ziegler G, Lucas A, Hanson N, Sellnow S, Pach J, Stein D, Ickhoff E B, Woodwick R, Tackmann R, Trautmann J, Rostvold J, Link T, Dyck P, Daube J, Colligan R, Windebank A, King J, Colwell J, Wood D, Mayfield R, Picket J, Chitwood M, Billings D, Dabney Y, Buse J, King L, Vale S, Thompson T, Bohm B, Lyons T, Hermayer K, Rice A, Molitch M, Schaefer B, Johnson C, Lyons J, Metzger B, Cohen B, Nishida T, Parque K, Yusim V, Moore M, Jampol L, Dineen K, Stahl J, Richine L, Weinberg D, Loose I, Kushner M, Morrison A, Jalbert A, Tildesley H, Leung S, Begg I, Johnson D, Lalani S, Kennedy T, Meadows G, Kolterman O, Lorenzi G, Jones K, Goldbaum M, Swenson M, Lyon R, Giotta M, Kadlec K, Reed R, Kirsch L, Goodman J, Cahill S, Clark T, Abram R, Sayner L, Ochabski R, Gloria R, Birchler G, Grant J, Grasse B, Christle L, Abreu B, Grant I, Heaton R, Zeitler R, Sivitz W, Bayless M, Schrott H, Olson N, Tindal B, Snetselaar L, Mueller D, Dudler A, Swartzendruber J, Hoffman R, Macindoe J, Kramer J, Weingeist T, Kimura A, Stone E, Grout T, Fountain C, Karakas S, Vogel C, Montague P, Keyser D, Mennen S, Doggett C, Rose G, Devet K, Muhle P, Kowarski A, Ostrowski D, Levin P, Chalew S, Hylton J (1993) The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986. [DOI] [PubMed] [Google Scholar]
- Shapiro JAM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238. [DOI] [PubMed] [Google Scholar]
- Shi Y, Hou LL, Tang FC, Jiang W, Wang PG, Ding MX, Deng HK (2005) Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three‐step approach with activin A and all‐trans retinoic acid. Stem Cells 23, 656–662. [DOI] [PubMed] [Google Scholar]
- Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, Mckay R., Kim JH (2007) Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia 50, 1228–1238. [DOI] [PubMed] [Google Scholar]
- Sipione S, Eshpeter A, Lyon JG, Korbutt GS, Bleackley RC (2004) Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia 47, 499–508. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Tooze SA, Flatmark T, Tooze J, Huttner WB (1991) Characterization of the immature secretory granule, an intermediate in granule biogenesis. J. Cell Biol. 115, 1491–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch BE (1991) Reversal of diabetes by human fetal pancreas – optimization of requirements in the hyperglycemic nude mouse. Transplantation 51, 557–562. [DOI] [PubMed] [Google Scholar]
- Xu CH, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK (2001) Feeder‐free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971–974. [DOI] [PubMed] [Google Scholar]
- Xu X, Kahan B, Forgianni A, Jing P, Jacobson L, Browning V, Treff N, Odorico J (2006) Endoderm and pancreatic islet lineage differentiation from human embryonic stem cells. Cloning Stem Cells 8, 96–107. [DOI] [PubMed] [Google Scholar]


