Abstract
Objectives
The legume lectin family, one of the most extensively studied plant lectin families, has received increasing attention for the remarkable anti‐tumor activities of its members for binding specific cancer cell surface glycoconjugates. MicroRNAs, a class of small, non‐coding RNAs, control translation and stability of mRNAs at post‐transcriptional and translational levels. To date, accumulating evidence has revealed that microRNAs are involved in progression of a number of human diseases, especially cancers. However, the molecular manners of microRNA‐modulated apoptosis in legume lectin‐treated cancer cells are still under investigation.
Materials and methods
We performed in silico analyses to study the interactions between three typical legume lectins (ConA, SFL and SAL) and some specific sugar‐containing receptors (for example, EGFR, TNFR1, HSP70 and HSP90). Additionally, we predicted some relevant microRNAs which could significantly regulate these aforementioned targetreceptors and thus inhibiting down‐stream cancer‐related signaling pathways.
Results
The results showed that these three legume lectins could competitively bind sugar‐containing receptors such as EGFR, TNFR1, HSP70 and HSP90 in two ways, via anti‐apoptotic or survival pathways. On the one hand, the legume lectins could induce cancer cell death through triggering receptor‐mediated signaling pathways, which resulted from indirect binding between legume lectins and mannoses resided in receptors. On the other hand, direct binding between legume lectins and receptors could lead to steric hindrance, which would disturb efficient interactions between them, and thus, the legume lectins would induce cancer cell death by triggering receptor‐mediated signaling pathways. In addition, we identified several relevant microRNAs that regulated these targeted receptors, thereby ultimately causing cancer cell apoptosis.
Conclusions
These findings provide new perspectives for exploring microRNA‐modulated cell death in legume lectin‐treated cancer cells, which could be utilized in combination therapy for future cancer drug development.
Introduction
Plant lectins, a group of highly diverse non‐immune origin proteins ubiquitously distributed in various types of plant species, contain at least one non‐catalytic domain that enables them to selectively recognize and reversibly bind to specific free sugars or glycans presented on glycoproteins and glycolipids, without altering the structure of carbohydrate 1. According to their evolutionary status and carbohydrate‐binding specificities, plant lectins are divided into 12 different families 2. Among them, three major families, namely, proteins with legume lectin domains, type II ribosome‐inactivating proteins (RIPs II) and G. nivalis agglutinin (GNA)‐related lectins, have drawn increasing attention to themselves due to their significant functions in biological activities 3, 4.
As one of the most attention‐getting plant lectins, proteins with legume lectin domains are well studied for their multiple significant biological functions, such as being anti‐fungal, anti‐viral, and most notably by having anti‐tumour activities. Also, programmed cell death (PCD), referring to apoptosis and autophagy, plays important roles in animal maintenance of homeostasis, cell differentiation, cell population growth control, cell defence and more, jointly sealing the ultimate fate of cancer cells. Recent studies have revealed that proteins with legume lectin domains can induce apoptosis in PU5‐1.8 cells, human melanoma A375 cells, and human liver hepatocellular carcinoma HepG2 cells 5, 6, 7. They can also induce autophagic cell death in HeLa cells 8, and inhibit cell survival in U87 glioblastoma cells, by reducing angiogenesis 9, 10. In addition, the legume lectin family has been reported to stimulate cell immunity and generate an immune memory, resisting genotypically identical tumours. Both mechanisms of the two types of PCD induction by legume lectins shed light on potential applications of further lectins for new perspectives in cancer therapeutics.
MicroRNAs (miRNAs), highly conserved, non‐coding endogenous RNAs 18–25 nucleotides (nt) in length, are estimated to regulate ~30% of human gene expression at the post‐transcriptional level 11. Dysregulation of miRNAs is associated with a number of pathological processes, especially in cancer. Hitherto, it has been reported that miRNAs can act either as oncogenes or as tumour suppressors 12. Meanwhile, miRNAs have been well‐characterized to have influence on regulation of apoptosis in cancer; these studies might provide potential new therapeutic targets in malignancy 13, 14.
Of note, molecular docking is a widely used computational tool for predicting binding mode and affinity of complexes formed by molecules with known structures. Since computational docking is important for investigating ligand‐protein interactions and elucidating binding mechanisms 15, several pieces of research conducted through this method have been proposed. At least one previous study has reported modelling of three‐dimensional structures of GNA‐related lectins, and assessed affinities, stabilities and haemagglutinin of lectins in complex with influenza viruses via molecular docking and molecular dynamics simulations 16. A further study has suggested a hypothesis that carbohydrate‐binding motifs evolved from GNA‐related lectins may impact and bind their diverse sugar‐containing receptors, which would block several anti‐apoptotic or survival signalling pathways, ultimately leading to cancer cell death 17.
In the study described here, we used methods of bioinformatics to study typical legume lectins that could bind diverse sugar‐containing receptors, and thus block several anti‐apoptotic or survival signalling pathways. We have been able to identify a limited number of targeted miRNAs, which might negatively regulate these sugar‐containing receptors, thereby ultimately culminating in legume lectin‐induced apoptosis in cancer cells.
Materials and methods
Phylogenetic analysis and sequence alignment
All here‐reported legume lectin sequences were searched for in the NCBI database using ‘legume lectin’ as a keyword. In addition, the Sophora alopecuroides lectin (SAL) sequence was retrieved from the NCBI database to find its homology proteins by BLASTP (protein‐protein BLAST) in non‐redundant protein sequences (nr) in the database. Then, integrating all searched results, 99 sequences were achieved followed subsequently by, sequences which were not from the legume lectin family being deleted. Sequences from the same species, but bearing different amino acids composition, were further screened, and the sequence which possessed highest identity was chosen. Finally, 35 legume lectin sequences were achieved for further analyses.
These sequences were then aligned using ClustalW (version 2.1) program 18. The corresponding phylogenetic tree was constructed using a molecular evolutionary genetics analysis (MEGA5) package by using a neighbour‐joining method with default parameters 19. Reliability of each branch was evaluated using bootstrap methods (1000 times repeat), and the phylogenetic tree was visualized using the TreeView program 20.
Molecular docking
Molecular structures of concanavalin A (ConA), epidermal growth factor receptor (EGFR), heat shock protein 70 (HSP70) and heat shock protein 90 (HSP90) were downloaded from PDB (Protein Database Bank) (untethered EGFR: 1IV0, Tethered EGFR: 1YY9, ConA: IJN2, HSP70: 2E8A, and HSP90: 1UYM) respectively. Three‐dimensional structures of both Sophora flavescens lectin (SFL) and SAL were constructed using the SWISS‐MODEL server (http://swissmodel.expasy.org/) with the structure of Pterocarps angolensis lectin (PDB ID: 1UKG) as template; ions were then further added. Initial structures of the ligands were achieved in PubChem Compound database (http://www.ncbi.nlm.nih.gov/pccompound) from NCBI. Geometry of free ligands was further optimized before docking, including adding hydrogen ions and charges.
All lectins and ligands (sugars) were prepared using UCSF chimera 21, where proteins with negative charges were added, while corresponding ligands were added with negative or positive or even zero charge. Then, molecular docking calculations were performed using UCSF DOCK6.4 program with the flexible ligand docking method, in which the ligand would be allowed to be structurally rearranged in response to the receptor 22. Amber score was used to rescore topmost poses for each ligand. The PDB2PQR server was utilized to assign protonation state of lectin with AMBER forcefiled; PROPKA was applied to maintain protonation state at pH = 7.0 23, 24, 25. Protein–protein docking was carried out by ZDOCK and Hex Protein Docking Server respectively 26, 27.
Targeted miRNA prediction and classification
Since available prediction methods have strongly varying degrees of sensitivity and specificity, we thought that a combination of methods might provide better understanding of complex regulatory mechanisms that involve miRNAs. Thus, we took advantage of computational predictions by sources of three algorithmically different methods, TargetScan (stringent seed pairing, site type, site context, site number; option of ranking by likelihood of preferential conservation rather than site context) 28, MiRanda (moderately stringent seed pairing, site number, pairing to most of the miRNA) 29 and Diana‐MicroH (hybridization energy threshold rules) 30 respectively.
Results and discussion
Phylogenetic analysis of legume lectins
To trace evolutionary relationships between legume lectins, a phylogenetic evolutionary tree was constructed using the neighbour‐joining method. As shown in Fig. 1, the bootstrap of Canavalia gladiate lectin and Canavalia brasiliensis lectin was 57, while bootstrap of Canavalia ensiformis lectin and the group of Canavalia gladiate lectin and Canavalia brasiliensis lectin was 100. In addition, we can also illustrate from Fig. 1 that Medicago truncatula serves as the root of this tree.
Figure 1.
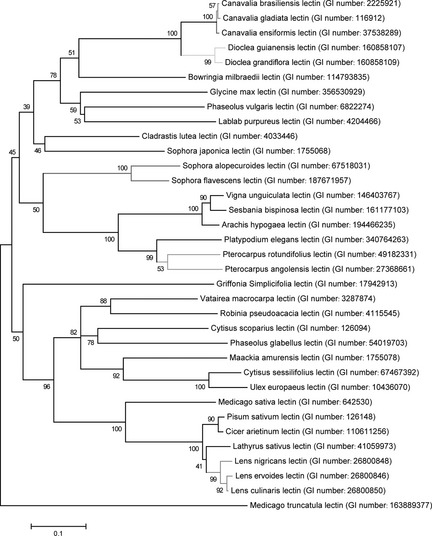
Phylogenetic analysis of present legume lectins from different species. Targeted legume lectins are coloured red and other lectins, which have higher sequence similarities are in chromatic colour.
Meanwhile, SFL also shows a close relationship with SAL. To further characterize sequence information among legume lectins in this group, sequence alignment was utilized as shown in (Fig. S1). Hitherto, it has been reported that legume lectins share similar amino acid sequences and tertiary structures, but have diverse quaternary structures, and that there are in the region of 50 legume lectin sequences that show pair‐wise sequence identities higher than 35% 31.
It has been reported that SFL, a mannose‐binding lectin, can induce apoptosis in HeLa cells, thus functioning as an anti‐tumour agent through a typical caspase‐dependent pathway 32. Since SAL showed the closest relationship with SFL, and to our knowledge, no studies have previously reported that SAL induced PCD in cancer, herein, we found SAL could potentially bind EGFR to block downstream anti‐apoptotic pathways eventually leading to cancer cell death, via bioinformatics analysis. In addition, ConA, a typical legume lectin, has been drawing much attention to itself for its potential therapeutic applications in cancer 7. Thus, based on the above‐mentioned reasons, three typical legume lectins including ConA, SFL and SAL were chosen for further study.
Affinity of legume lectin binding with different sugars
Carbohydrate‐binding activity of legume lectins depends on simultaneous presence of both a calcium ion and a transition metal ion 33, 34. Since the modelled structure of SFL and SAL bear almost the same structure as PAL which acts as a template, we speculated that both could possess active sites and ion‐binding sites similar to those of PAL. Thus, an ion was added in the modelled structure of SFL and SAL, to mimic manner of binding of PAL.
Molecular docking was used to assay carbohydrate‐binding capability of these three legume lectins. Accordingly, several typical sialic acids, glucoses and mannoses, including O‐sialic acid, α‐D‐Mannose, β‐D‐Mannose, methylmannoside, α‐1,2‐Mannobiose, β‐D‐Glucose, N‐acetyl‐D‐glucosamine, were chosen to bind with the three lectins respectively. Detailed information of these sugars is shown in (Table S1). Different amber scores are indicative of carbohydrate‐binding capabilities of these three lectins, in complex with diverse sugars (shown in Table 1). We inferred from Table 1 that ConA had best affinity with β‐D‐Mannose, amber score being −18.69. Although α‐D‐Mannose has almost the same structure as β‐D‐Mannose, exploration of binding sites of ConA in complex with α‐D mannose showed that key residues implicated in carbohydrate recognition were D208 and N14, while residues involved in carbohydrate binding of ConA in complex with β‐D mannose were D208 and L99. Thus, it is speculated that different residues implicated in mannose recognition might result in diverse binding capabilities. Although SAL and SFL have best affinities for N‐acetyl‐D‐glucosamine, with amber scores of −21.54 and −16.92, both SAL and SFL have relatively high binding abilities to α‐D‐Mannose and β‐D‐Mannose. Fig. 2 presents three legume lectin‐sugar complexes in the surface potential mode and stick‐ball model.
Table 1.
Molecular docking scores of ConA, SFL and SAL in complex with different types of sugars
| Lectin | Sugar type | Score |
|---|---|---|
| ConA | O‐sialic acid | −9.69 |
| α‐D‐Mannose | −9.21 | |
| β‐D‐Mannose | −18.69 | |
| methylmannoside | −17.81 | |
| α‐1,2‐Mannobiose | −1.40 | |
| β‐D‐Glucose | −12.16 | |
| N‐acetyl‐D‐glucosamine | −10.80 | |
| SFL | O‐sialic acid | −13.95 |
| α‐D‐Mannose | −16.50 | |
| β‐D‐Mannose | −15.25 | |
| methylmannoside | −13.70 | |
| α‐1,2‐Mannobiose | −3.87 | |
| β‐D‐Glucose | −8.14 | |
| N‐acetyl‐D‐glucosamine | −16.92 | |
| SAL | O‐sialic acid | −7.30 |
| α‐D‐Mannose | −17.69 | |
| β‐D‐Mannose | −16.49 | |
| methylmannoside | −19.61 | |
| α‐1,2‐Mannobiose | −3.46 | |
| β‐D‐Glucose | −18.66 | |
| N‐acetyl‐D‐glucosamine | −21.54 |
Figure 2.
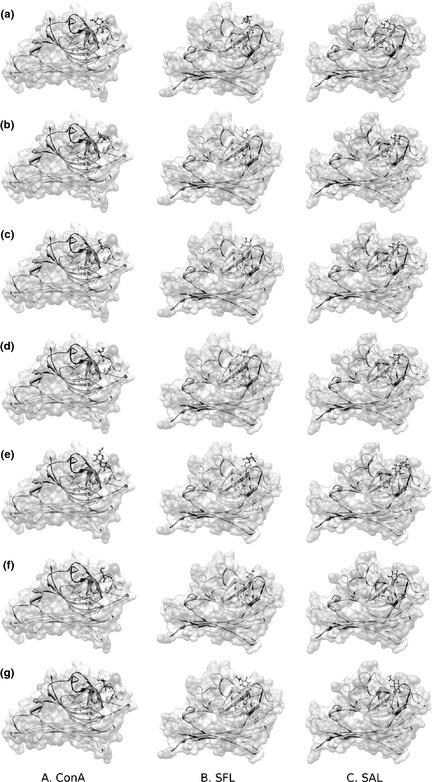
Overall modelling of the three typical legume lectins (ConA, SFL and SAL)‐sugar complexes: (a) O‐sialic acid, (b) α‐D‐Mannose, (c) β‐D‐Mannose, (d) Methylmannoside, (e) α‐1,2‐Mannobiose (f) β‐D‐Glucose (g) N‐acetyl‐D‐glucosamine. All complexes are presented in surface potential mode and in stick‐ball mode. In stick‐ball mode, the sugars are presented as purple sticks.
Of note, it is well known that ConA is a mannose/glucose‐binding specific lectin, and SFL is a mannose‐binding lectin. We have shown that these three legume lectins have stronger affinity to mannose. In addition, ConA and SAL have higher glucose affinity than does SFL (shown in Table 1). In this regard, we reasonably speculate that SAL might also be a mannose‐binding lectin. Since these three legume lectins have specific affinity to mannose, we speculate that they would bind mannose branches on cancer cell surfaces and block downstream anti‐apoptotic pathways, eventually leading to cancer cell death.
In this study, three typical legume lectins were demonstrated to have affinity to mannose, and a number of receptors, such as EGFR, TNFR1, HSP70 and HSP90 presenting on outer surfaces or within cancer cells were of the high mannose type. Glycosylation is a universal feature of activity of cancer cells, and aberrant glycosylation of N‐ or O‐linked glycoproteins is significantly related to tumour growth and metastasis 35, 36, 37. Legume lectins can bind specific glycoconjugates on surfaces of receptors resident within the cells, and subsequently result in conformation changes of receptors followed by initiating downstream signalling pathways. Thereby, legume lectins could induce cancer cell death by triggering receptor‐mediated signalling pathways, resultant from indirect binding between legume lectin and mannoses resident in the receptors. In combination with previous investigations 38, it is suggested that legume lectins can indeed bind mannose‐containing receptors, and this might play an essential role in inhibiting carcinogenesis, invasion and metastasis.
Anti‐apoptotic pathways inducable by sugar‐containing receptors
According to a number of recent studies, several sugar‐containing receptors (such as EGFR, TNFR1, HSP70 and HSP90) can mediate a number of anti‐apoptotic or survival pathways 39, 40. Here, we report that we found legume lectins that potentially could bind some receptors thus blocking downstream anti‐apoptotic pathways that would induce cancer cell death. We further elucidated these receptor‐mediated signalling pathways through data obtained from the literature and pathway databases 41, 42. In this regard, we selected the best legume lectins that have optimal anti‐tumour roles, through regulating several anti‐apoptotic or survival pathways.
We have shown that ConA, SAL and SFL may possess even closer affinities than some of the ligands of EGFR (there are several, for example EFG, TGF‐α, HB‐EGF) or have similar binding capabilities with them via ZDOCK (Table 2) and Hex Protein Docking Server (Table 3) respectively. In Table 2, the higher score demonstrated indicates stronger binding capacity, whereas in Table 3, the lower energy value indicates stronger binding capacity (Fig. S2a). Take EGFR (untethered monomer) for instance, the score (Table 2) and binding energy (Table 3) of ConA‐EGFR complex were 1590.03 and −922.29 kJ/mol respectively, indicating that EGFR possesses best affinity to ConA. Moreover, ConA has better affinity to EGFR than does that of ligands of EGFR itself, no matter whether EGFR was in untethered or tethered status. The other two lectins, SFL and SAL, also have stronger affinity to EGFR than do other ligands of EGFR itself.
Table 2.
Comparison between lectins and ligands by binding EGFR, TNFR1, HSP70 and HSP 90 using ZDOCK
| Protein | Ligand | Score |
|---|---|---|
|
EGFR (tethered monomer) |
ConA | 1582.57 |
| SFL | 1426.58 | |
| SAL | 1446.04 | |
| EGF | 1400.56 | |
| TGF‐α | 1249.26 | |
| HB‐EGF | 1180.40 | |
|
EGFR (untethered monomer) |
ConA | 1590.03 |
| SFL | 1436.52 | |
| SAL | 1561.46 | |
| EGF | 1470.22 | |
| TGF‐α | 1339.99 | |
| HB‐EGF | 1197.63 | |
| TNFR1 | ConA | 1190.15 |
| SFL | 1162.19 | |
| SAL | 1240.79 | |
| TNF | 1376.22 | |
| HSP70 | ConA | 1390.56 |
| SFL | 1164.94 | |
| SAL | 1271.39 | |
| HSP90 | ConA | 1306.28 |
| SFL | 1267.24 | |
| SAL | 1159.85 |
Table 3.
Comparison between plant lectins and ligands via binding EGFR, TNFR1, HSP70 and HSP 90 using Hex Protein Docking Server
| Protein | Ligand | Energy (kJ/mol) |
|---|---|---|
|
EGFR (tethered monomer) |
ConA | −890.45 |
| SFL | −989.69 | |
| SAL | −882.89 | |
| EGF | −710.36 | |
| TGF‐α | −724.15 | |
| HB‐EGF | −733.63 | |
|
EGFR (untethered monomer) |
ConA | −922.29 |
| SFL | −883.50 | |
| SAL | −782.28 | |
| EGF | −830.28 | |
| TGF‐α | −887.85 | |
| HB‐EGF | −712.77 | |
| TNFR1 | ConA | −772.75 |
| SFL | −846.80 | |
| SAL | −696.51 | |
| TNF | −936.61 | |
| HSP70 | ConA | −600.77 |
| SFL | −787.077 | |
| SAL | −823.19 | |
| HSP90 | ConA | −663.21 |
| SFL | −605.29 | |
| SAL | −596.54 |
Types of epidermal growth factor receptor, with the potential of binding ligands, are common cell surface receptors (bearing high mannose‐type glycan) which function in mediating its downstream signalling pathways 43. A close correlation has been demonstrated in previous pieces of research, between EGFR overexpression and a variety of epithelial cancers – such as those of breast, head and neck, colon, kidney, lung, pancreas and prostate 44, 45.
Herein, legume lectins, ConA, SFL and SAL were demonstrated to compete directly with ligands in binding with growth factor binding sites on the specific receptor, thereby suppressing EGFR‐related anti‐apoptotic pathways, including EGFR‐Ras‐Raf‐PI3K‐Akt and EGFR‐JAK‐STAT pathways 46. PI3K is activated by EGFR and then activates Akt, which has inhibitive impact on a number of pro‐apoptotic factors such as BAD, caspase 9 and FOXO1 47. Moreover, EGFR phosphorylation can activate a number of STATs such as STAT1, STAT3 and STAT5, which subsequently translocate into the cell nucleus to mediate gene expression relating to cell proliferation, transformation and oncogenesis 48. In addition, domain II dimerization interface can be blocked by occupation of the inhibitor, leading to incomplete receptor dimerization and activation. On the basis of the above‐mentioned mechanisms and a certain number of pathways included, we may speculate that these types of binding of legume lectins and EGFR, potentially switch off anti‐apoptotic pathways, such as Ras‐Raf and PI3K‐Akt pathways, thus playing pro‐apoptotic roles in cancer cells.
Furthermore, tumour necrosis factor (TNF) is a type II transmembrane protein with an intracellular amino terminus. Tumour necrosis factor receptors (TNFRs) including TNFR1 and TNFR2 may recruit corresponding proteins and activate multiple signalling pathways downstream 49. Binding between ligand and TNFR1 activates AP1 transcription factors or IκB kinases, which in turn, induce production of a range of inflammatory mediators and growth factors. Subsequently, nuclear factor‐κB (NF‐κB) is activated, inducing inhibitors of apoptosis. Thus, we indicate that lectins binding to TNFR1 possibly block anti‐apoptotic pathways which would induce cancer cell apoptosis. From Table 2 and Table 3, we observe that TNF has stronger binding capability to TNFR1 than lectins such as ConA, SAL and SFL; however, these lectins still have affinity for TNFR1 (Fig. 2b).
Heat shock proteins (HSPs) compose a family of proteins induced in cells under stress. They function as protein chaperones in helping refolding of misfolded proteins or in elimination of irreversibly damaged proteins, thus consequently promoting cell survival in stress conditions 50. Recent studies have revealed that quantities of HSPs are usually abnormally high in various types of cancer cells and have relevance to tumour cell proliferation, differentiation, invasion and metastasis 51. Based on the aforementioned studies, some typical legume lectins can bind with HSP70 and HSP90 thus impeding their anti‐apoptotic pathways in cancer cells. It is speculated that the three legume lectins in this study may potentially bind with HSP70 and HSP90, and switch off anti‐apoptotic pathways 38. Docking results in Table 2 and Table 3 revealed that ConA, SAL and SFL could possibly bind HSP70 and HSP90 (Fig. S2b). Inferring from Table 2, both HSP70 and HSP90 have highest affinity to ConA with scores of 1390.56 and 1306.28, respectively, and in Table 3, the most important ligand of HSP70 was SAL while the best ligand of HSP90 was ConA.
Summarizing from the above‐mentioned results, direct binding between legume lectins and receptors could possibly lead to steric hindrance, which might disturb efficient interaction between them. Thus, legume lectins might induce cancer cell death through triggering receptor‐mediated signalling pathways.
Over the last few years, plant lectins have been adopted for differentiating malignant tumours from benign ones and as alternative cancer therapy with reduction of side effects compared to adjuvant agents in chemotherapy and radiotherapy 52, 53, 54. Legume lectins, a large family of homologous carbohydrate‐binding proteins, show widely different carbohydrate specificities and quaternary structures, but share strong similarity of their amino acid sequences and tertiary structures 34. According to previous research, ConA can induce mitochondrial apoptosis, p73‐Foxola‐Bim apoptosis and BNIP3‐mediated mitochondrial autophagy, eventually causing tumour cells death. In addition, ConA can inhibit cancer cell survival via IKK‐NF‐κB‐COX‐2, SHP‐2‐MEK‐1‐ERK, and SHP‐2‐Ras‐ERK caused anti‐angiogenesis 55, 56.
Sophora flavescens lectin, SFL can lead to HeLa cell apoptotic death through the caspase‐dependent pathway, and its molecular mechanisms might involve the death‐receptor pathway 32. A further typical legume lectin, Phaseolus coccineus lectin can induce caspase‐dependent apoptosis in murine fibrosarcoma L929 cells 57. According to our studies, SAL may share common anti‐cancer activity with SFL, suggesting that legume lectins might possess some identical or similar biological activities and molecular mechanisms.
Thus, ConA, SFL and SAL might impede receptor‐mediated anti‐apoptotic or survival signalling pathways, such as those mediated by EGFR, TNFR1‐mediated signalling pathways, HSP70 and HSP90 family‐involved signalling pathways (Fig. 3). These studies provide novel insights for application of legume lectins as potential anti‐neoplastic drugs.
Figure 3.
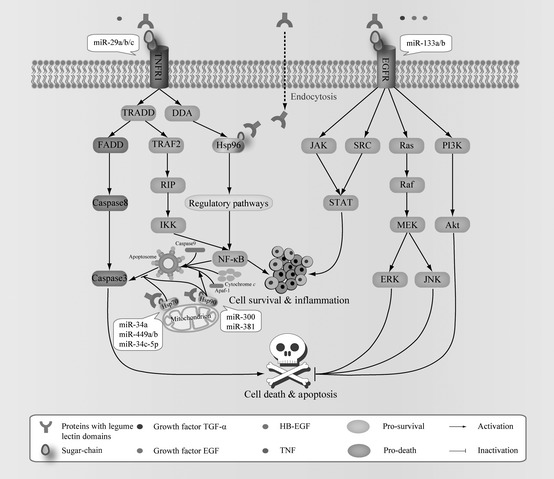
The three typical legume lectins, ConA, SFL and SAL which may impede receptor‐mediated anti‐apoptotic or survival signalling pathways, such as EGFR‐mediated signalling pathways, TNFR1‐mediated signalling pathways, HSP70 and HSP90 family‐involved signalling pathways.
Prediction of microRNAs targeting receptors
On the basis of the above‐mentioned receptors, we firstly utilized Diana‐MicroH, MiRanda and TargetScan to predict miRNAs targeting the EGFR, TNFR1 HSP70 and HS90 respectively. Due to different algorithms, diverse results might be achieved. Due to huge differences between these three predicted methods, we combined results into consensus results. We assumed that a combination of methods would profoundly mitigate the problem of discovering false positives and negatives and only accounted for potential interactions 58.
For example EGFR, we predicted 7 miRNAs through Diana‐MicroH, 34 miRNAs through MiRanda and 145 miRNAs through TargetScan. Subsequently, we integrated these miRNAs into a consensus result. Results of the prediction are two miRNAs, including hsa‐miR‐133a and hsa‐miR‐133b; these two miRNAs may negatively regulate EGFR‐mediated signalling pathways. All prediction results are shown in Table 4 and Fig. 4. Information of all predicted miRNA sequences is shown in Table S2. Together, these results indicate that these targeted miRNAs may play fundamental roles for regulation of some sugar‐containing receptors in cancer.
Table 4.
Consensus results of predicted miRNA targeting receptors
| Gene name | Protein name | Consensus result |
|---|---|---|
| EGFR | EGFR | has‐miR‐133a/b, has‐miR‐7, has‐miR‐27a/b, has‐miR‐302a, has‐miR‐107, has‐miR‐539, has‐miR‐141, has‐miR‐200a |
| TNFRSF1A | TNFR1 | has‐miR‐29a/b/c |
| HSPA1A | HSP70 | has‐miR‐590‐3p, has‐miR‐146a, has‐miR‐34a, has‐miR‐449a/b, has‐miR‐34c‐5p |
| HSPA1B | has‐miR‐34a, has‐miR‐34c‐5p, has‐miR‐449a/b, has‐miR‐424, has‐miR‐495 | |
| HSP90AB1 | HSP90 | has‐miR‐300, has‐miR‐381, has‐miR‐448, has‐miR‐153, has‐miR‐485‐5p, has‐miR‐214 |
Figure 4.
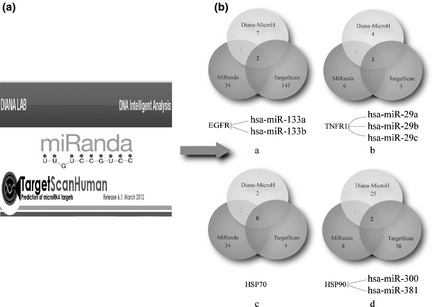
Prediction of miRNAs targeting several receptors via combinational methods including Diana‐MicroH, MiRanda and Targetscan. (a) Three prediction software of miRNA. (b) MiRNA prediction results of four receptors: (i) EGFR, (ii) TNFR1, (iii) HSP70, (iv) HSP90.
MicroRNAs, an abundant class of negative gene regulators, have been shown to be involved in cell proliferation, differentiation and apoptosis. Notably, miRNAs can act in roles of either tumour suppressors or control oncogenes; thus, miRNAs can limit expression of important cancer‐related genes and might be utilized for diagnosis and treatment of cancer. In this study, we predicted several relevant miRNAs, which might be involved in regulation of some sugar‐containing receptors. More importantly, identification of miRNAs that are critically involved in legume lectin‐treated cancer cells may open new avenues for combination therapeutic strategies to improve treatment outcomes of cancer. Overall, these miRNAs and legume lectins could be used conjunctively in future cancer therapies.
Conclusion
Hitherto, accumulating evidence has demonstrated that legume lectins, such as ConA and SFL can lead to cancer cell death through regulation of PCD pathways. Based upon docking results as described above, we clarified that legume lectins might potentially bind corresponding sugar‐containing receptors, such as EGFR, TNFR1, HSP70 and HSP90. Subsequently, we found that these three legume lectins could bind these receptors which may regulate their downstream pathways implicated in miRNA regulation. To our knowledge, this is the first time it is reported that SAL could induce cancer cell death, in which several miRNAs were up‐regulated, inhibiting expression of several receptors. As compensation, legume lectins could also bind to these receptors, as well as induce cancer cell death via blocking several anti‐apoptotic or survival signalling pathways.
In this study, we built a phylogenetic analysis to show the evolutionary relationship of legume lectins. Then, we carried out a series of in silico analyses of three legume lectins including ConA, SFL and SAL for exploring carbohydrate‐binding capability, via docking experiments. Subsequently, we simulated protein‐protein interaction between the lectins and some receptors commonly overexpressed in cancer cells, to assay the possibility of binding between them. In addition, we predicted some miRNAs, which could significantly associate with appropriate receptors and thus inhibit downstream cancer‐related signalling pathways, thereby, eventually causing cancer cell death. These inspiring findings may provide a firm perspective for development of combined therapeutic approaches, based on use of miRNA‐modulated cell death in legume lectin‐treated cancers. As discussed above, we propose that the legume lectins and miRNAs could be considered in combination therapy for future cancer therapeutics.
Supporting information
Fig. S1 Sequence alignments of 35 legume lectins.
Fig. S2 The three typical legume lectins may bind some sugar‐containing receptors on or inside cancer cells, thus blocking downstream anti‐apoptotic or survival pathways.
Table S1. Detailed information concerning the sugars.
Table S2. Information of predicted miRNA sequences.
Acknowledgements
We are grateful to Miss W. Li (University College London) for providing her constructive suggestions on this work. We also thank Dr. J. Li (Ohio University), C. Li, and H. Xu (Sichuan University) for their critical reviews on the manuscript. This work was supported in part by National Natural Science Foundation of China (No. 30970643, No. 81173093 and No. J1103518).
Z. Shi, N. An and S. Zhao contributed equally to this work.
References
- 1. Peumans WJ, Van Damme EJ, Barre A, Rouge P (2001) Classification of plant lectins in families of structurally and evolutionary related proteins. Adv. Exp. Med. Biol. 491, 27–54. [DOI] [PubMed] [Google Scholar]
- 2. Van Damme EJ, Nakamura‐Tsuruta S, Smith DF, Ongenaert M, Winter HC, Rouge P et al (2007) Phylogenetic and specificity studies of two‐domain GNA‐related lectins: generation of multispecificity through domain duplication and divergent evolution. Biochem. J. 404, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Mejia EG, Bradford T, Hasler C (2003) The anticarcinogenic potential of soybean lectin and lunasin. Nutr. Rev. 61, 239–246. [DOI] [PubMed] [Google Scholar]
- 4. Meyer A, Rypniewski W, Szymanski M, Voelter W, Barciszewski J, Betzel C (2008) Structure of mistletoe lectin I from viscum album in complex with the phytohormone zeatin. Biochim. Biophys. Acta 1784, 1590–1595. [DOI] [PubMed] [Google Scholar]
- 5. Liu B, Li CY, Bian HJ, Min MW, Chen LF, Bao JK (2009) Antiproliferative activity and apoptosis‐inducing mechanism of concanavalin A on human melanoma A375 cells. Arch. Biochem. Biophys. 482, 1–6. [DOI] [PubMed] [Google Scholar]
- 6. Liu B, Bian HJ, Bao JK (2010) Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett. 287, 1–12. [DOI] [PubMed] [Google Scholar]
- 7. Li CY, Xu HL, Liu B, Bao JK (2010) Concanavalin A, from an old protein to novel candidate anti‐neoplastic drug. Curr. Mol. Pharmacol. 3, 123–128. [DOI] [PubMed] [Google Scholar]
- 8. Chang CP, Yang MC, Liu HS, Lin YS, Lei HY (2007) Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma model. Hepatology 45, 286–296. [DOI] [PubMed] [Google Scholar]
- 9. Lei HY, Chang CP (2009) Lectin of concanavalin A as an anti‐hepatoma therapeutic agent. J. Biomed. Sci. 16, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Battelli MG (2004) Cytotoxicity and toxicity to animals and humans of ribosome‐inactivating proteins. Mini Rev. Med. Chem. 4, 513–521. [DOI] [PubMed] [Google Scholar]
- 11. Fu LL, Wen X, Bao JK, Liu B (2012) MicroRNA‐modulated autophagic signaling networks in cancer. Int. J. Biochem. Cell Biol. 44, 733–6. [DOI] [PubMed] [Google Scholar]
- 12. Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH (2011) MicroRNA regulation of core apoptosis pathways in cancer. Eur. J. Cancer 47, 163–174. [DOI] [PubMed] [Google Scholar]
- 13. Gandellini P, Profumo V, Folini M, Zaffaroni N (2011) MicroRNAs as new therapeutic targets and tools in cancer. Expert. Opin. Ther. Targets 15, 265–279. [DOI] [PubMed] [Google Scholar]
- 14. Cho WC (2010) MicroRNAs in cancer – from research to therapy. Biochim. Biophys. Acta 1805, 209–217. [DOI] [PubMed] [Google Scholar]
- 15. Chang SS, Huang HJ, Chen CY (2011) Two birds with one stone? Possible dual‐targeting H1N1 inhibitors from traditional Chinese medicine. PLoS Comput. Biol. 7, e1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu HL, Li CY, He XM, Niu KQ, Peng H, Li WW et al (2012) Molecular modeling, docking and dynamic simulation of GNA‐related lectins for potential prevention in influenza virus (H1N1). J. Mol. Model. 18, 27–37. [DOI] [PubMed] [Google Scholar]
- 17. Yu QJ, Li ZY, Yao S, Ming M, Wang SY, Liu B et al (2011) In silico analysis of molecular mechanisms of Galanthus nivalis agglutinin‐related lectin‐induced cancer cell death from carbohydrate‐binding motif evolution hypothesis. Appl. Biochem. Biotechnol. 165, 1037–1046. [DOI] [PubMed] [Google Scholar]
- 18. Higgins DG, Sharp PM (1988) CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73, 237–244. [DOI] [PubMed] [Google Scholar]
- 19. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12, 357–358. [DOI] [PubMed] [Google Scholar]
- 21. Lang PT, Brozell SR, Mukherjee S, Pettersen EF, Meng EC, Thomas V et al (2009) DOCK 6: combining techniques to model RNA‐small molecule complexes. RNA 15, 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G et al (2007) PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 35, W522–W525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H, Robertson AD, Jensen JH (2005) Very fast empirical prediction and rationalization of protein pKa values. Proteins 61, 704–721. [DOI] [PubMed] [Google Scholar]
- 24. Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA (2004) PDB2PQR: an automated pipeline for the setup of Poisson‐Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718. [DOI] [PubMed] [Google Scholar]
- 26. Chen R, Li L, Weng Z (2003) ZDOCK: an initial‐stage protein‐docking algorithm. Proteins 52, 80–87. [DOI] [PubMed] [Google Scholar]
- 27. Macindoe G, Mavridis L, Venkatraman V, Devignes MD, Ritchie DW (2010) HexServer: an FFT‐based protein docking server powered by graphics processors. Nucleic Acids Res. 38, W445–W449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Betel D, Wilson M, Gabow A, Marks DS, Sander C (2008) The microRNA.org resource: targets and expression. Nucleic Acids Res. 36, D149–D153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maragkakis M, Alexiou P, Papadopoulos GL, Reczko M, Dalamagas T, Giannopoulos G et al (2009) Accurate microRNA target prediction correlates with protein repression levels. BMC Bioinformatics 10, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manoj N, Suguna K (2001) Signature of quaternary structure in the sequences of legume lectins. Protein Eng. 14, 735–745. [DOI] [PubMed] [Google Scholar]
- 32. Liu Z, Liu B, Zhang ZT, Zhou TT, Bian HJ, Min MW et al (2008) A mannose‐binding lectin from Sophora flavescens induces apoptosis in HeLa cells. Phytomedicine 15, 867–875. [DOI] [PubMed] [Google Scholar]
- 33. Brewer CF, Brown RD 3rd, Koenig SH (1983) Metal ion binding and conformational transitions in concanavalin A: a structure‐function study. J. Biomol. Struct. Dyn. 1, 961–997. [DOI] [PubMed] [Google Scholar]
- 34. Loris R, Hamelryck T, Bouckaert J, Wyns L (1998) Legume lectin structure. Biochim. Biophys. Acta 1383, 9–36. [DOI] [PubMed] [Google Scholar]
- 35. Li M, Song L, Qin X (2010) Glycan changes: cancer metastasis and anti‐cancer vaccines. J. Biosci. 35, 665–673. [DOI] [PubMed] [Google Scholar]
- 36. Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS (1987) Beta 1–6 branching of Asn‐linked oligosaccharides is directly associated with metastasis. Science 236, 582–585. [DOI] [PubMed] [Google Scholar]
- 37. Ueno T, Ohtawa K, Kimoto Y, Sakurai K, Kodera Y, Hiroto M et al (2000) Polyethylene glycol‐modified concanavalin A as an effective agent to stimulate anti‐tumor cytotoxicity. Cancer Detect. Prev. 24, 100–106. [PubMed] [Google Scholar]
- 38. Xu Z, Zhou X, Lu H, Wu N, Zhao H, Zhang L et al (2007) Comparative glycoproteomics based on lectins affinity capture of N‐linked glycoproteins from human chang liver cells and MHCC97‐H cells. Proteomics 7, 2358–2370. [DOI] [PubMed] [Google Scholar]
- 39. Saint‐Guirons J, Zeqiraj E, Schumacher U, Greenwell P, Dwek M (2007) Proteome analysis of metastatic colorectal cancer cells recognized by the lectin helix pomatia agglutinin (HPA). Proteomics 7, 4082–4089. [DOI] [PubMed] [Google Scholar]
- 40. Ciocca DR, Calderwood SK (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10, 86–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Agarwal P, Searls DB (2009) Can literature analysis identify innovation drivers in drug discovery? Nat. Rev. Drug Discov. 8, 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bauer‐Mehren A, Furlong LI, Sanz F (2009) Pathway databases and tools for their exploitation: benefits, current limitations and challenges. Mol. Syst. Biol. 5, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu SL, Kim J, Hancock WS, Karger B (2005) Extended Range Proteomic Analysis (ERPA): a new and sensitive LC‐MS platform for high sequence coverage of complex proteins with extensive post‐translational modifications‐comprehensive analysis of beta‐casein and epidermal growth factor receptor (EGFR). J. Proteome Res. 4, 1155–1170. [DOI] [PubMed] [Google Scholar]
- 44. Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7, 301–311. [DOI] [PubMed] [Google Scholar]
- 45. Salomon DS, Brandt R, Ciardiello F, Normanno N (1995) Epidermal growth factor‐related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 19, 183–232. [DOI] [PubMed] [Google Scholar]
- 46. Wang SY, Yu QJ, Zhang RD, Liu B (2011) Core signaling pathways of survival/death in autophagy‐related cancer networks. Int. J. Biochem. Cell Biol. 43, 1263–1266. [DOI] [PubMed] [Google Scholar]
- 47. Nyati MK, Morgan MA, Feng FY, Lawrence TS (2006) Integration of EGFR inhibitors with radiochemotherapy. Nat. Rev. Cancer 6, 876–885. [DOI] [PubMed] [Google Scholar]
- 48. Sharma SV, Bell DW, Settleman J, Haber DA (2007) Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 7, 169–181. [DOI] [PubMed] [Google Scholar]
- 49. Balkwill F (2009) Tumour necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371. [DOI] [PubMed] [Google Scholar]
- 50. Khalil AA, Kabapy NF, Deraz SF, Smith C (2011) Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim. Biophys. Acta 1816, 89–104. [DOI] [PubMed] [Google Scholar]
- 51. Jego G, Hazoume A, Seigneuric R, Garrido C (2010) Targeting heat shock proteins in cancer. Cancer Lett. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 52. Gorelik E, Galili U, Raz A (2001) On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 20, 245–277. [DOI] [PubMed] [Google Scholar]
- 53. Schumacher K, Schneider B, Reich G, Stiefel T, Stoll G, Bock PR et al (2003) Influence of postoperative complementary treatment with lectin‐standardized mistletoe extract on breast cancer patients. A controlled epidemiological multicentric retrolective cohort study. Anticancer Res. 23, 5081–5087. [PubMed] [Google Scholar]
- 54. Valentiner U, Fabian S, Schumacher U, Leathem AJ (2003) The influence of dietary lectins on the cell proliferation of human breast cancer cell lines in vitro . Anticancer Res. 23, 1197–1206. [PubMed] [Google Scholar]
- 55. Ruhul Amin AR, Oo ML, Senga T, Suzuki N, Feng GS, Hamaguchi M (2003) SH2 domain containing protein tyrosine phosphatase 2 regulates concanavalin A‐dependent secretion and activation of matrix metalloproteinase 2 via the extracellular signal‐regulated kinase and p38 pathways. Cancer Res. 63, 6334–6339. [PubMed] [Google Scholar]
- 56. Li WW, Yu JY, Xu HL, Bao JK (2011) Concanavalin A: a potential anti‐neoplastic agent targeting apoptosis, autophagy and anti‐angiogenesis for cancer therapeutics. Biochem. Biophys. Res. Commun. 414, 282–286. [DOI] [PubMed] [Google Scholar]
- 57. Chen J, Liu B, Ji N, Zhou J, Bian HJ, Li CY et al (2009) A novel sialic acid‐specific lectin from phaseolus coccineus seeds with potent antineoplastic and antifungal activities. Phytomedicine 16, 352–360. [DOI] [PubMed] [Google Scholar]
- 58. Wuchty S, Arjona D, Li A, Kotliarov Y, Walling J, Ahn S et al (2011) Prediction of associations between microRNAs and gene expression in glioma biology. PLoS ONE 6, e14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Sequence alignments of 35 legume lectins.
Fig. S2 The three typical legume lectins may bind some sugar‐containing receptors on or inside cancer cells, thus blocking downstream anti‐apoptotic or survival pathways.
Table S1. Detailed information concerning the sugars.
Table S2. Information of predicted miRNA sequences.


