Abstract
Abstract. Objectives: Adipose tissue in vocal fold lipoinjection is currently used to treat patients affected by laryngeal hemiplegia or anatomical defects. The aim of this study has been to evaluate the efficacy of this clinical strategy, by long‐term follow‐up of the patients and to investigate whether the fat samples used to treat them contain a stem cell population with a wide differentiation potential. Materials and methods: Fat samples harvested from 12 patients affected by severe breathy dysphonia who had undergone vocal fold lipoinjection were analysed by immunocytochemistry, by flow cytometry and reverse transcription‐polymerase chain reaction, and the isolated adipose derived mesenchymal stem cells (ADMSCs) were evaluated in order to define their ability to produce soluble factors possibly involved in tissue regeneration, and to differentiate towards different lineages. Results: ADMSCs were efficiently and successfully isolated from all of the samples. They were positive for SSEA‐4, an embryonic marker recently identified on bone marrow MSCs and which could explain their high differentiation plasticity. Molecular analysis showed that these cells also expressed Oct‐4, Runx‐1 and ABCG‐2, which characterize the stem cell state, and a number of other specific lineage markers. Flow cytometry revealed mesenchymal markers expressed on ADMSCs and identified a subpopulation characterized by CD146+/34−/45− cells consistent with perivascular/pericyte‐like cells. Osteogenic, adipogenic and endothelial tissue differentiation were obtained. Conclusions: Our results confirmed the therapeutic efficacy of this clinical approach and showed that adipose tissue, administered to patients in order to restore glottic competence, contains mesenchymal stem cells.
INTRODUCTION
Mesenchymal stem cells (MSC) are an adherent, fibroblast‐like cell population found in bone marrow (BM) as well as blood, muscle, dermis and adipose tissue. In this regard, fat is a source of uncommitted MSCs that can be easily expanded and, over the last few years, autologous fat has been used in some clinical applications for novel cell‐based therapies (Zuk et al. 2002; Barry & Murphy 2004; Schäffler & Buchler 2007).
The main characteristic of adult stem cells is to give rise to specialized cell types of the tissue in which they reside, but they can also form specialized cells of other tissues. A number of studies have recently compared the properties of MSCs collected from BM, adipose tissue and cord blood (De Ugarte et al. 2003; Wagner et al. 2005; Kern et al. 2006). Adipose tissue is an ideal source of autologous stem cells, particularly in comparison with the traditional BM procurement procedure, as it is easily obtainable by lipoaspiration under local anesthesia with minimal discomfort for the patient, and its MSC content is adequate for clinical‐grade cell manipulation in regenerative medicine.
One interesting clinical application is treatment of vocal fold scarring, which disrupts the viscoelastic layered structure of the lamina propria, increases stiffness of the vibratory structure and glottic incompetence, and is the most frequent cause of a poor voice after vocal fold injury. A number of new therapeutic strategies has been developed in animal models, including the use of synthetic extracellular matrix components, hydrogel constructs and hyaluronic acid, although the results obtained in vocal fold medialization are quite controversial and not completely satisfactory, mainly because changes in these molecules affect the viscoelasticity of scarred vocal folds (Hirano 2005). However, other strategies (including cell therapy) seem to have considerable therapeutic potential and, over the last 10 years, adipose tissue has been used to treat vocal fold medialization in cases of glottic incompetence mainly due to laryngeal hemiplegia (LH) (Shindo et al. 1996; McCulloch et al. 2002; Laccourreye et al. 2003; Cantarella et al. 2005).
The aim of this study was to explore whether fat tissue used to treat patients affected by LH or anatomical defects of the vocal folds contains a stem cell population that could be thought to be participating in long‐term fat graft survival and in improvement in glottic competence, obtained in these patients after lipoinjection.
MATERIALS AND METHODS
Patients
The study involved 12 subjects aged 16–66 years, who were affected by severe breathy dysphonia due to LH (n = 9) or bilateral vocal fold defects (one case of scarring and two of sulcus glottidis). It was approved by our hospital's ethics committee, and all of the subjects gave their informed consent.
Surgical technique
Under strictly sterile conditions after the donor site had been infiltrated with lidocaine and epinephrine 1 : 100 000, fat tissue was harvested from the lower abdomen by means of curetting during suction under moderate negative pressure, using a 50‐mL disposable syringe connected to a 2‐holed 4.0 mm blunt cannula. Contents of the syringe were poured into one or two 10‐mL test tubes (depending on the amount of fat), which were then centrifuged at 956 g for 3 min in order to separate blood and liquid fat from the fat cells. The material forms three layers: an oily upper layer of ruptured adipocytes, a middle layer of fat cells and a lower layer of blood and lidocaine. The oily layer was gently aspirated with a needle, whereas the middle layer was collected and placed in the barrel of a pistol with a bayonet needle of 1 mm in diameter. Some of the fat was used for autologous injection into one to four sites of the vocalis muscle, under direct microlaryngoscopy, and some of the processed tissue was used for isolation and characterization of adipose derived mesenchymal stem cells (ADMSCs).
Evaluation of voice outcome
All patients underwent preoperative videolaryngoscopy, and a perceptual voice evaluation was made using yarameters of G (grade of dysphonia), R (roughness), B (breathiness), A (astenicity) and S (strain).
Patients also underwent measurement of maximal phonation time and multidimensional voice program acoustic voice analysis (MDVP), and were administered the voice handicap index questionnaire. The considered acoustic parameters were jitter (percentage), shimmer (percentage) and noise‐to‐harmonic ratio. All of the measurements were repeated 1 month after surgery and then at 3‐month intervals; the mean duration of follow‐up was 21 months (range 13–30).
ADMSC culture and expansion
Samples of lipoaspirate obtained from patients who received autologous transplantation were used for laboratory studies. Raw lipoaspirates were extensively washed with sterile phosphate‐buffered saline (PBS; Invitrogen, Carlsdad, CA, USA) in order to remove contaminating debris and red blood cells, and then treated with 0.075% collagenase (type A; Roche, Mannheim, Germany) in PBS for 30 min at 37 °C with gentle agitation. Collagenase was inactivated by an equal volume of Dulbecco's modified Eagle's medium‐low glucose (DMEM‐LG) (Invitrogen) supplemented with 20% foetal bovine serum (FBS) (Biochrom AG, Berlin, Germany), and the suspension was centrifuged at low speed for 10 min. The stromal vascular fraction was re‐suspended overnight in DMEM‐LG/10% FBS/1% penicillin‐streptomycin (Sigma‐Aldrich, St. Louis, MO, USA), after which the non‐adherent fraction was removed, and adherent cells were cultured for 2 weeks (Zuk et al. 2002). Cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2, and were passaged at 60–70% confluence. At passage 3, the ADMSCs were extensively characterized by flow cytometry, immunofluorescence and reverse transcription‐polymerase chain reaction (RT‐PCR), and their potentiality was evaluated by their ability to differentiate towards different lineages.
We also tested how different media influenced the fate of ADMSCs. After initial centrifugation, the lower‐density solid phase was collected and digested at 37 °C for 45 min with 0.075% w/v collagenase. After neutralization, the stromal‐vascular fraction was cultured in the presence of high‐DMEM + 20% FBS (A) or EGM‐2 (Cambrex, Walkersville, MD, USA) (B) for 1 week. The non‐adherent fraction was removed after 24 h. After initial expansion, (A) cells were kept under the same culture conditions, whereas (B) cells were split and kept in EGM‐2 (B) or cultured in the presence of high‐DMEM + 20% FBS (C). In order to evaluate different commitment obtained using mesenchymal versus endothelial differentiation media, co‐expression of Ulex europaeus agglutinin I (UEA) and CD146 was detected by immunocytochemistry.
RNA isolation and RT‐PCR
Total RNA from 1 × 106 ADMSCs before and after differentiation was extracted using the RNeasy Mini Kit (Qiagen AG, Hilden, Germany), and contaminating genomic DNA was further eliminated by DNase (Qiagen) digestion according to the manufacturer's instructions. Total RNA was eluted in a final volume of 40 µL and its quality, integrity and size distribution was assessed by optical density (absorbance at 260/280 nm and ratio of >1.8). RT‐PCR was performed using 800 ng of total RNA and GoTaq DNA polymerase (Promega, Madison, WI, USA), and 4 ng of cDNA were used for each PCR assay of different adipogenic (PPARgamma2), osteogenic (osteocalcin), myogenic (MyoD, myogenin, MRF4, Myf‐5, desmin), hepatic (alfa Feto protein, albumin, cytokeratin (CK) 18, CK19, CYP1B1, CYP2B6), endothelial (VE‐cadherin, von Willebrand factor) and stem cell markers (Oct‐4, Runx‐1, Rex‐1, Sox‐2, hTert, FGF‐4, Pax‐7, PDX‐1, ABCG2, CXCR4); positive controls were obtained from the corresponding foetal tissues. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as a normalizing housekeeping gene. Primers were constructed on the basis of published human sequences, and were selected using version 1.5 of Primer Express software available from Applied Biosystems (Foster City, CA, USA; Table 1). Each set of oligonucleotides was designed to span two different exons. The samples were loaded on 1.5% agarose gel.
Table 1.
Primer sequences used for RT‐PCR. The primers were constructed on the basis of published human sequences, and selected using version 1.5 of Primer Express software available from Applied Biosystems
| Gene | Primer sequence | Gene | Primer sequence |
|---|---|---|---|
| Osteocalcin | F – gcagagtccagcaaaggtg | PDX1 | F – ctgcctttcccatggatgaa |
| R – atgtggtcagccaactcgtc | R – aagttcaacatgacagccagc | ||
| PPARgamma2 | F – gctgaatccagagtccgctg | ABCG2 | F – gcttgcaacaaccatgacgaa |
| R – caaactcaaacttgggctcc | R – gccagttgtaggctcatccaa | ||
| MyoD | F – agcactacagcggcgact | PAX7 | F – ctttgccgctaccaggagac |
| R – gcgactcagaaggcacgtc | R – tcgatgctgtgtttggcct | ||
| Myf–5 | F – cagtcctgtctggtccagaa | von Willebrand | F – tgtcaagigacgtgicagca |
| R – gaactagaagcccctggag | R – gcagtagaaatcgtgcaacg | ||
| Myogenin | F – cagcgaaigcagctctcaca | Ve Cadherin | F – tgtctgtttgttgaggaccc |
| R – agttgggcatggtttcatctg | R – aagtggtagaaaggctgctg | ||
| MRF4 | F – ggctctcctttgtatccagg | CXCR4 | F – aggtagcaaagtgacgccga |
| R – cctlagccgttatcacgagc | R – aagtaccagtttgccacggc | ||
| Desmin | F – gatggaataccgacaccaga | Rex‐1 | F – aacatgagccagcaactgaag |
| R – ggtaggtggcaatctccaca | R – agaaatcatcccctccgagag | ||
| CK18 | F – gctggagagcaaaatccgg | FGF‐4 | F – ctactgcaacgtgggcatc |
| R – cctccttgagagcctcgaict | R – acatgccggggtacttgtag | ||
| CK19 | F – gaactccaggattgtcctgca | hTert | F – gagaacaagctgtttgcggg |
| R – aaccaggcttcagcatccttc | R – ggcatctgaacaaaagccgt | ||
| alfa Feto Protein | F – ttccagaacctgtcacaagctg | Sox‐2 | F – accagaaaaacagcccgga |
| R – tctcctctgcaacagtgctcat | R – tcatgagcgtcttggttttcc | ||
| Albumin | F – ggttgatgtgatgtgcactgc | Runx‐1 | F – tcactgtgatggctggcaat |
| R – tcccttcatcccgaagttcat | R – ctgcatctgactctgaggctga | ||
| CYP1B1 | F – gcctttatcctctctgcggaa | Oct‐4 | F – acatgtgtaagctgcggcc |
| R – acaaagctggagaagcgcat | R – gttgtgcatagtcgctgcttg | ||
| CYP2B6 | F – atggaaaccgctggaaggi | GAPDH | F – gcttgtcatcaatggaaatccc |
| R – gccccaggaaagiatttcaaga | R – tccacacccatgacgaacatg |
Flow cytometric analysis
ADMSCs were extensively characterized by flow cytometry at passage 3, before starting any other characterisation or differentiation steps. They were washed in PBS solution and stained for 20 min at room temperature in the dark with the following directly coupled mouse–antihuman antibodies: CD45 PC7 (Beckman Coulter, Fullerton, CA, USA), CD34 PE (Becton Dickinson, San Jose, CA, USA), CD146 FITC (Biocytex, Marseille, France), CD90 FITC (Becton Dickinson), α‐SMA FITC (Sigma‐Aldrich), NG2 PE (Immunotech, Marseille, France), CD133 PE (Miltenyi, Bergisch Gladbach, Germany), CD56 PE (Chemicon, Temecula, CA, USA), CD44 FITC (Sigma), CD105 PE (Biocytex), CD73 PE (Becton Dickinson), CXCR4 APC (Becton Dickinson), LNGFR PE (Becton Dickinson), HLA‐ABC FITC (Becton Dickinson) and HLA‐DR (Becton Dickinson). Cells were also incubated with the following primary mouse antihuman antibodies: BB9 (Becton Dickinson), Desmin (Dako Cytomation, Carpinteria, CA, USA) and SSEA‐4 (Chemicon; 1 : 25) and, after washing with a secondary rat–antimouse antibody, IgG1 PE and FITC (Exalpha, Carlsbad, CA, USA). Isotype immunoglobulins IgG1 PE‐FITC (Chemicon), IgG1 PC7 (Beckman Coulter), IgG1 APC (Becton Dickinson) were used as negative controls under the same conditions. When required, cells were permeabilized with 0.1% Triton X‐100 (Sigma). After staining, they were washed once with PBS containing 0.1% of bovine serum albumin (Sigma). For each sample, at least 50 000 list mode events were acquired using a Cytomics FC500 (Beckman Coulter); all plots were generated using CXP analysis software.
Immunofluorescence assay
Passage 3 ADMSCs were plated on a Laboratory‐Tek chamber slide (Nalge Nunc Int., Rochester, NY, USA), and were fixed with absolute methanol (–20 °C) for 5 min. After washing with PBS (Gibco, Grand Island, NY, USA), they were permeabilized with 0.1% Triton X‐100 when required, blocked for 30 min at room temperature with PBS containing 2% of bovine serum albumin, and then incubated for 1 h in the dark with unconjugated primary mouse–antihuman monoclonal antibodies SSEA‐4 (Chemicon; 1 : 25), von Willebrand factor (vWF, Clone F8/86, Dako Cytomation; 1 : 25) and with Ulex europaeus agglutinin I (Vector Laboratories, Burlingame, CA, USA; 1 : 50), and then conjugated with a secondary rat–antimouse antibody IgG1 FITC or PE (Exalpha). The same population was also stained with conjugated mouse–antihuman antibodies VE‐cadherin FITC (Chemicon; 1 : 25) and CD146 FITC (Chemicon; 1 : 100). Cells were counterstained with 4′,6‐diamidino‐2‐phenylindole dihydrochloride (Roche Diagnostic Corporation, Indianapolis, IN, USA) for 15 min in the dark at room temperature. Moreover, after endothelial differentiation and after culture with different media, they were characterized for von Willebrand factor and VE‐cadherin, or for CD146 and UEA. FITC‐ and PE‐conjugated goat antimouse IgG secondary antibodies (Chemicon, 1 : 1000) were used for the negative controls.
After incubation, coverslipped slides were examined using a fluorescence microscope (Eclipse 80i, Nikon, Tokyo, Japan), and micrographs were taken using DS camera control (DS‐5 m, Nikon). Images were acquired using a Nikon Digital Slide DS‐L1, and were merged and analysed using Adobe Photoshop 5.5 software (Microsoft Corporation, Washington DC, USA).
Multiplexed sandwich ELISA
A multiplexed sandwich ELISA that allows quantitative chemiluminescent measurement of seven proteins per well (HB‐EGF, TPO, PDGF, KGF, HGF, FGF, ANG2; their lower detection limit was 1.8; 5.9; 1.0; 1.0; 3.1; 2.0; 4.9 pg/mL, respectively) (SearchLight proteome array, Pierce‐Endogen Boston Technologies Center, Woburn, MA, USA) was used in order to evaluate ADMSCs’ production of soluble factors. Briefly, supernatants were collected from confluent ADMSC cultures at passage 3 immediately stored at –20 °C until evaluation. Each well of the microplate was pre‐spotted with target protein‐specific antibodies and 50 µL of standards, and samples were added to the plate for one hour at RT with shaking at 200 r.p.m. After washing away unbound proteins, 50 µL of biotinylated antibodies were added to the plate for 30 min at RT with shaking at 200 r.p.m. Excess biotinylated antibodies was washed away, and 50 µL of streptavidin‐horseradish peroxidase (SA‐HRP) was added to the plate for 30 min at room temperature with shaking at 200 r.p.m.
In order to identify the signal, we used SuperSignal ELISA femto chemiluminescent substrate (Pierce, Rockford, IL, USA); the luminescent signal was recorded within 10 min using a cooled CCD camera and amount of each target protein was analysed using Microsoft Excel 2000 (Microsoft Corporation) as recommended by the manufacturers. The culture medium, DMEM + 10% FBS, was also tested as a blank and the value obtained was subtracted from the amount of factor present in cell‐conditioned medium.
Cell lineage differentiation
ADMSCs were differentiated into adipogenic, osteogenic and endothelial cell lineages. For differentiation experiments, passage 3 ADMSCs were cultured in different media, which were replaced twice a week.
In order to promote adipogenic differentiation, ADMSCs were plated at 20 × 103 cells/cm2, and were cultured for 2 weeks in the presence of human MSC adipogenic induction medium (Cambrex). Medium was replaced every 3–4 days for 15 days. Cells were stained with Oil red O solution (Sigma) (three parts of stock solution 0.5% in isopropanol, and two parts distilled water), in order to detect the presence of lipid vacuoles.
For osteogenic differentiation, 20 × 103 cells/cm2 were grown for 2 weeks in the presence of human MSC osteogenic medium (Cambrex). Osteocytes were stained with alizarin red in order to detect presence of calcium deposits.
For endothelial differentiation, 5 × 103 cells/well were cultured for 10 days on fibronectin chamber slides in the presence of DMEM (Invitrogen) and human vascular endothelial growth factor (PeproTech EC; 10 ng/mL), after which medium was replaced by EGM‐2 medium (Cambrex) for 3 weeks. Immunofluorescence and RT‐PCR procedures were performed after differentiation.
RESULTS
Clinical outcome
Videolaryngoscopy demonstrated improved glottic closure in all 12 patients and voice perceptual evaluation showed significant improvement in all three parameters of the GRBAS score: G (P = 0.0197), R (P = 0.0115) and B (P = 0.0437).
MDVP acoustic analysis revealed reduction in the mean values of jitter (from 4.48 ± 3.73% preoperatively to 2.16 ± 1.32%) and shimmer (from 11.01 ± 8.42% to 5.99 ± 3.07%), and mean noise‐to‐harmonic ratio changed from 0.18 ± 0.06 to 0.15 ± 0.05, thus indicating greater voice signal stability and reduced noise. Maximal phonation time improved in all cases, with mean values increasing from 7.08 ± 3.60 to 13.00 ± 5.62 s (P = 0.0023), and mean voice handicap index score significantly decreased from 67.08 ± 29.47 to 35.08 ± 24.41 (P = 0.0004).
All patients showed significant voice improvement. Results remained stable during the long‐term follow‐up (mean 21 months; range 13–30).
ADMSC characterization before differentiating treatments
ADMSC morphology
After expansion, all culture samples gave rise to MSCs with spindle‐shaped morphology in confluent wave‐like layers, which could be replated for at least 12 passages (Fig. 1a).
Figure 1.
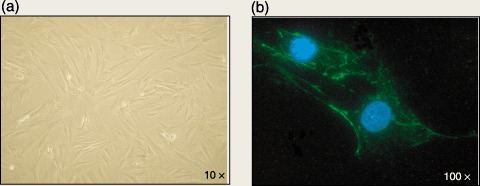
(a) Morphological appearance of human ADMSCs at passage 3: a heterogeneous population of cells with spindle‐shaped morphology (×10 magnification). (b) Fluorescence image of ADMSCs after immunostaining with mouse–antihuman monoclonal antibody SSEA‐4 (green). Nuclei counterstained with DAPI (blue) (×100 magnification).
Molecular profile
Reverse transcription‐polymerase chain reaction analysis showed that ADMSCs were positive for desmin, osteocalcin, PPARgamma2, CK18 and CK19, CYP1B1, CYP2B6, von Willebrand factor and VE‐cadherin, and negative for MyoD, myogenin, MRF‐4, Myf‐5, alphafoeto protein, albumin, PDX1, PAX7, CXCR4, FGF4, hTERT and SOX2. They also expressed Oct‐4, Runx‐1, Rex‐1 and ABCG‐2, which characterize the undifferentiated stem cell state (Fig. 2).
Figure 2.
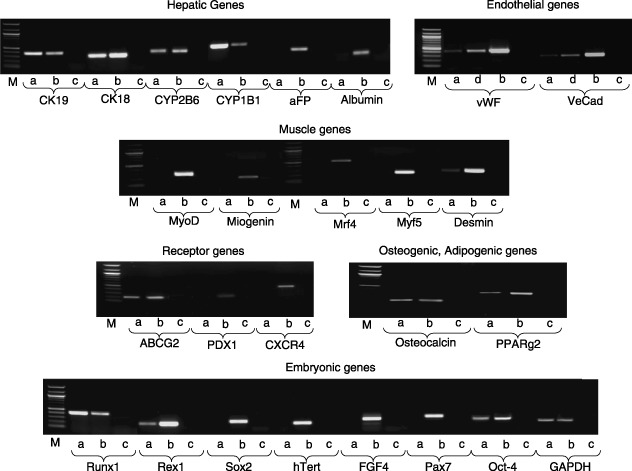
RT‐PCR on total RNA. ADMSCs were characterized for expression of several hepatic, endothelial, muscle, receptor, osteogenic, adipogenic and embryonic genes. (a) Passage 3 ADMSCs. (b) Positive control obtained from corresponding foetal tissues. (c) Negative control. (d) ADMSCs after endothelial differentiation. M, Marker.
ADMSC phenotype
Interestingly, ADMSCs were positive for the embryonic marker SSEA‐4, confirmed by flow cytometry, (Fig. 1b), and negative for endothelial markers such as von Willebrand factor and VE‐cadherin.
Moreover, flow cytometry showed that they were positive for CD44, CD90, CD105, CD73, SSEA4 and HLA‐ABC; weakly positive for CD34; and negative for CD56, CD133, HLA‐DR, CXCR4, LNGFR, BB9 and desmin (Fig. 3). They also expressed CD146 (around 4%), α‐SMA but not CD45 nor NG2, which characterize a multipotent adult stem cell population consistent with a perivascular/pericyte‐like phenotype. The high level of α‐SMA (Fig. 3) confirmed the ability of these cells to differentiate towards smooth muscle cell lineage, as recently reported by Lee et al. (2006b).
Figure 3.
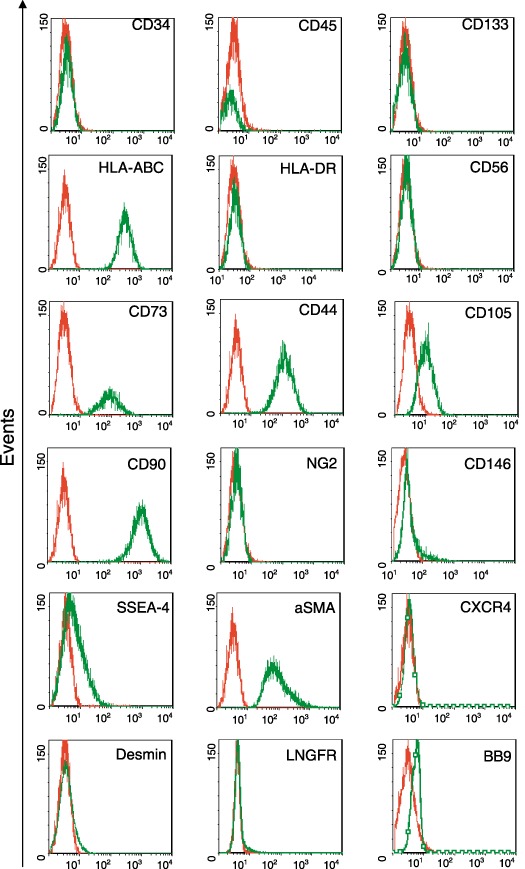
Flow cytometicy analysis. ADMSCs were extensively characterized by flow cytometry at passage 3, before starting any other characterization or differentiation.
Multiplexed sandwich ELISA
ADMSCs secreted high levels of TPO, substantial amounts of KGF and HGF and only minimal amounts of HB‐EGF, FGF and PDGF. No ANG2 was secreted (Fig. 4).
Figure 4.
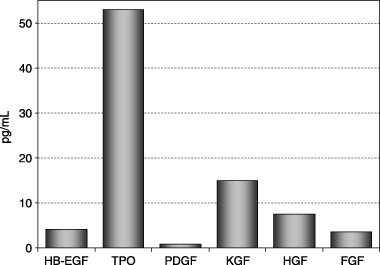
Secretion of HB‐EGF, TPO, PDGF, KGF, HGF and FGF by ADMSCs at passage 3 was measured using a multiplexed sandwich ELISA.
ADMSC expansion in the presence of different media
The aim of this part of the study was to determine how different media influence the fate and expansion of stem cells present in adipose tissue. To this end, the stromal‐vascular fraction was cultured in the presence of: (A) DMEM; (B) EGM2 for 1 week, and then DMEM; or (C) EGM2. After 3 weeks, all cells were stained for CD146 and UEA in order to investigate possible differences of commitment. A few (A) cells were weakly positive for CD146 (Fig. 5aII) but negative for Ulex (Fig. 5aIII), and showed typical mesenchymal morphology (Fig. 5aI). A large majority of (B) cells were strongly positive for CD146 (Fig. 5bII), negative for UEA (Fig. 5bIII) and their morphology was consistent with mesenchymal features (Fig. 5bI). Most of the (C) cells co‐expressed CD146 and UEA (Fig. 5cII and 5cIII), and had typical endothelial morphology (Fig. 5cI).
Figure 5.
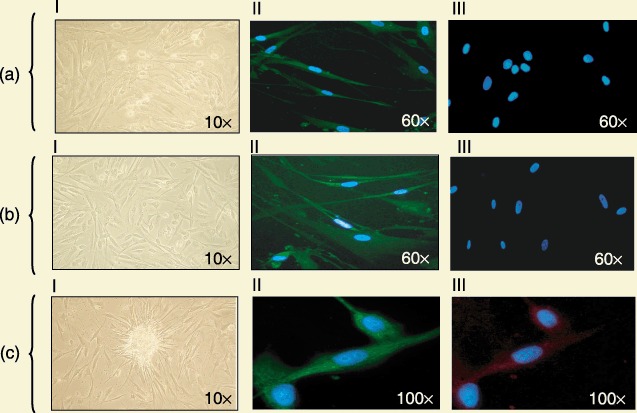
After culturing the stromal‐vascular fraction for three weeks in the presence of different media, cells showed differing commitments. (a) A few cells were weakly positive for CD146 (Fig. 5aII, green) but negative for UEA (Fig. 5aIII, red) upon immunofluorescence (×60 magnification), and showed typical mesenchymal morphology (Fig. 5aI) (×10 magnification). (b) A large number of cells were strongly positive for CD146 (Fig. 5bII, green) and negative for UEA (Fig. 5bIII, red) on immunofluorescence (×60 magnification), and their morphology showed mesenchymal features (Fig. 5bI) (×10 magnification). (c) Most cells co‐expressed CD146 and UEA (Fig. 5cII, green, and Fig. 5cIII, red) on immunofluorescence (×60 magnification), and had typical endothelial morphology (Fig. 5cI) (×10 magnification). Nuclei counterstained with DAPI (blue).
ADMSC characterization after differentiating treatments
The potential of ADMSCs isolated from adult subcutaneous adipose tissue was demonstrated by culturing the cells in media promoting their differentiation into osteogenic, adipogenic and endothelial lineages.
Osteogenic differentiation
During osteogenic differentiation, ADMSCs showed extensive areas of mineralization indicated by staining with the calcium‐specific marker alizarin red (Fig. 6a). RT‐PCR showed that the cells were osteocalcin‐positive.
Figure 6.
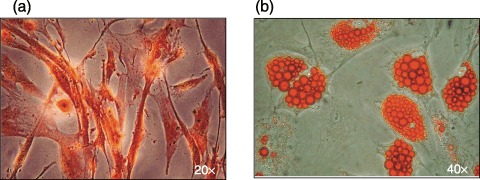
(a) Alizarin red staining showing an area of mineralization on ADMSCs cultured in osteogenic differentiating medium (×20 magnification). (b) Oil red O staining showing accumulation of lipid vacuoles in ADMSCs after adipogenic treatment (×40 magnification).
Adipogenic differentiation
ADMSCs were induced to accumulate lipid vacuoles that stained positive with the triglyceride‐specific dye oil red O and could be detected by light microscopy (Fig. 6b).
Endothelial differentiation
After being cultured with specific endothelial differentiation media, ADMSCs showed typical endothelial morphology (Fig. 7a). Immunofluorescence indicated that they were positive for von Willebrand factor and VE‐cadherin, which are endothelial markers (Fig. 7b,c), thus confirming committed differentiation.
Figure 7.
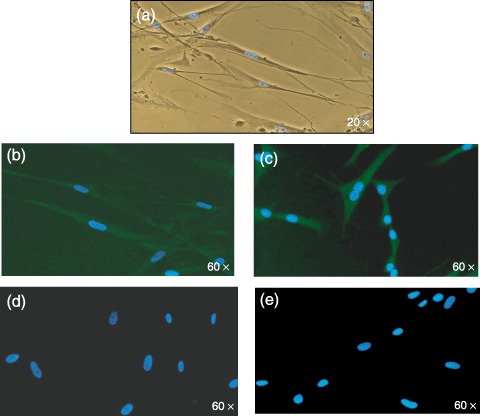
(a) Morphology of ADMSCs after exposure to endothelial conditioning medium (×20 magnification). Fluorescence image of ADMSCs after immunostaining with mouse–antihuman monoclonal antibodies for von Willebrand factor (b) and VE‐cadherin (c). (d) and (e) are the negative controls. Nuclei counterstained with DAPI (blue) (×60 magnification).
DISCUSSION
The aim of this study was to investigate whether adipose tissue in vocal fold lipoinjections used to treat patients affected by LH or anatomical defects, contains a stem cell population that may play a role in long‐term fat graft survival. To this end, we isolated a stem cell population with mesenchymal features from adipose tissue samples of patients undergoing vocal fold repair, and explored its characteristics and multilineage potential.
First, we confirmed the presence of MSCs in the adipose tissue in accordance with recent findings (Zuk et al. 2002; Strem et al. 2005), and showed that the isolated cells expressed many genes typical of mesodermal and non‐mesodermal lineages (Fig. 2) and a number of mesenchymal markers (Fig. 3), before the differentiation treatments. In particular, we identified a CD146+/34−/45−subpopulation consistent with perivascular/pericyte‐like cells, thus showing that adult human ADMSCs include a multipotential stem cell population that can be intimately associated with the cells surrounding blood vessels (Zannettino et al. 2008). It is worth noting that ADMSCs found in fat tissue were positive for some genes characteristic of embryonic and adult stem cells (Oct‐4, Runx‐1 and ABCG‐2), which underline their possible multipotency (Fig. 2).
We also found that ADMSCs were positive for SSEA‐4, an early embryonic glycolipid antigen that is commonly used as a marker of undifferentiated pluripotent human embryonic stem cells and embryos at the cleavage to blastocyst stage, and which have recently been identified in an adult BM mesenchymal stem cell population (Gang et al. 2007). This could explain their high differentiation plasticity (1, 3).
Capacity for multilineage differentiation of the expanded human ADMSCs was also tested. After being cultured under specific osteogenic, adipogenic and endothelial conditions, they showed morphological and phenotypical characteristics of different mesodermal and non‐mesodermal cell lineages. We also found that they secrete multiple soluble angiogenic factors involved in haematopoiesis, vasculogenesis and epithelial formation. This secretory profile shows that human ADMSCs offer an appealing cell‐based approach that may have therapeutic effects in various clinical settings, as recently proposed and demonstrated in animal models (Nakagami et al. 2005; Rehman et al. 2007; Wu et al. 2007).
These results demonstrated that patients affected by LH or laryngeal defects and treated with this clinical approach based on fat tissue administration, showed restoration of normal voice levels confirmed by long‐term follow‐up.
Second, our findings demonstrated that all samples administered to these patients, contained MSCs exhibiting a high proliferative potential, very primitive stemness characteristics and an interesting secretion profile. Some recent in vivo animal model studies confirm that damaged tissues such as injured vocal folds can be regenerated by MSC tissue engineering (Kanemaru et al. 2003, 2005; Hertegard et al. 2006; Lee et al. 2006a). These recent data and our findings, taken together, could let us hypothesize that these ADMSCs found in fat tissue could play a role even in human tissue regeneration.
However, it is crucial to investigate appropriate MSC culture conditions (i.e. media, sera and further parameters) for clinical application very carefully (Mannello & Tonti 2007), as we have demonstrated that different culture reagents can give rise to different cell phenotypes. Given all of the above, human ADMSCs are a highly flexible and very promising potential source of multipotent cells that can be used for regenerative somatic cell therapy in a variety of vocal fold disorders, as well as for tissue engineering.
ACKNOWLEDGEMENTS
This study was supported by grants from the Italian Ministry of Health (Progetto Ricerca Finalizzata 2005; Progetto a concorso 2006 and 2007, Ex Art. 56), the Istituto Superiore di Sanità (Malattie Neurodegenerative), and the 6FP EU Project – THERCORD.
(Viviana Lo Cicero, Elisa Montelatici) These authors contributed equally to the study.
REFERENCES
- Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 36, 568–584. [DOI] [PubMed] [Google Scholar]
- Cantarella G, Mazzola RF, Domenichini E, Arnone F, Maraschi B (2005) Vocal fold augmentation by autologous fat injection with lipostructure procedure. Otolaryngol. Head Neck Surg. 132, 239–243. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH (2003) Comparison of multi‐lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 17, 101–109. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Bosnakovski D, Figueiredo CS, Visser JW, Perlingeiro RC (2007) SSEA‐4 identifies mesenchymal stem cells from bone marrow. Blood 109, 1743–1751. [DOI] [PubMed] [Google Scholar]
- Hertegard S, Cedervall J, Svensson B, Forsberg K, Maurer FH, Vidoska D, Olivius P, Ahrlund‐Richter L, Le Blanc K (2006) Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope 116, 1248–1254. [DOI] [PubMed] [Google Scholar]
- Hirano S (2005) Current treatment of vocal fold scarring. Curr. Opin. Otolaryngol. Head Neck Surg. 13, 915–920. [DOI] [PubMed] [Google Scholar]
- Kanemaru S, Nakamura T, Omori K, Kojima H, Magrufov A, Hiratsuka Y, Hirano S, Ito J, Shimizu Y (2003) Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann. Otol. Rhinol. Laryngol. 112, 915–920. [DOI] [PubMed] [Google Scholar]
- Kanemaru S, Nakamura T, Yamashita M, Magrufov A, Kita T, Tamaki H, Tamura Y, Iguchi F, Kim TS, Kishimoto M, Omori K, Ito J (2005) Destiny of autologous bone marrow‐derived stromal cells implanted in the vocal fold. Ann. Otol. Rhinol. Laryngol. 114, 907–912. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood or adipose tissue. Stem Cells 24, 1294–1301. [DOI] [PubMed] [Google Scholar]
- Laccourreye O, Papon JF, Kania R, Crevier‐Buchman L, Brasnu D, Hans S (2003) Intracordal injection of autologous fat in patients with unilateral laryngeal nerve paralysis: long‐term results from the patient's perspective. Laryngoscope 113, 541–545. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Wang SG, Lee JC, Jung JS, Bae YC, Jeaon HJ, Kim HW, Lorenz RR (2006a) The prevention of vocal fold scarring using autologous adipose tissue‐derived stromal cells. Cells Tissues Organs 184, 198–204. [DOI] [PubMed] [Google Scholar]
- Lee WC, Rubin JP, Marra KG (2006b) Regulation of alpha‐smooth muscle actin protein expression in adipose‐derived stem cells. Cells Tissue Organs 183, 80–86. [DOI] [PubMed] [Google Scholar]
- Mannello F, Tonti GA (2007) Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder‐free; medium with fetal calf serum, human serum, or enriched plasma; serum‐free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells 25, 1603–1609. [DOI] [PubMed] [Google Scholar]
- McCulloch TM, Andrews BT, Hoffman HT, Graham SM, Karnell MP, Minnick C (2002) Long‐term follow‐up of fat injection laryngoplasty for unilateral vocal cord paralysis. Laryngoscope 112, 1235–1238. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y (2005) Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue‐derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 25, 2542–2547. [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, Merfeld‐Clauss S, Temm‐Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL (2007) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298. [DOI] [PubMed] [Google Scholar]
- Schäffler A, Buchler C (2007) Concise review: adipose tissue‐derived stromal cells – basic and clinical implications for novel cell‐based therapies. Stem Cells 25, 818–827. [DOI] [PubMed] [Google Scholar]
- Shindo ML, Zaretsky LS, Rice DH (1996) Autologous fat injection for unilateral vocal fold paralysis. Ann. Otol. Rhinol. Laryngol. 105, 602–606. [DOI] [PubMed] [Google Scholar]
- Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH (2005) Multipotential differentiation of adipose tissue‐derived stem cells. Keio J. Med. 54, 132–141. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W (2005) Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 33, 1402–1416. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25, 2648–2659. [DOI] [PubMed] [Google Scholar]
- Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S (2008) Multipotential human adipose‐derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo . J. Cell Physiol 214, 413–421. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]


