Summary
Aim
This study investigated whether histamine could play a protective role in pathophysiological response of spinal cord injury (SCI) and regulate the glial scar formation.
Methods
Functional assessment and histological analyses were performed to investigate the effect of histamine after SCI. Histidine decarboxylase knockout (HDC −/−) mice were used to confirm the action of histamine. Selective antagonists for H1 and H2 receptors were utilized in vivo and in vitro to verify the functional properties of histamine on astrogliosis.
Results
The local administration of histamine significantly attenuated the tissue damage and glial scar formation after SCI. In particular, the astrogliosis and neurocan expression found around the lesion were significantly suppressed by histamine. Immunofluorescent staining for neurofilament showed that histamine promoted axonal growth across the glial scar. The HDC −/− mice, lacking in endogenous histamine, showed lower behavior score, increased lesion size and astrogliosis, as compared with the wild types. The effect of histamine on locomotor recovery and reactive astrogliosis is reversed by H1 receptor antagonist but not H2 receptor antagonist.
Conclusions
Our results indicate that histamine significantly improved the chronic locomotor recovery via attenuating astrogliosis after SCI by stimulating histamine H1 receptor. This study highlights a therapeutic potential of histamine and its related drugs for SCI.
Keywords: Astrocyte, Histamine, Scar, Spinal cord injury
Introduction
Spinal cord injury (SCI) is a serious high disabling disease which results in motor and sensory dysfunction, and even ends people's lives, yet no effective therapeutic approaches have been developed so far. The complicated pathophysiological process in and around the lesion site limits the development of agents. The mechanisms of injury to the spinal cord, including the “primary injury” and “secondary injury”, determined the extent of tissue injury and dysfunction of body. The primary injury results in structural disturbance for physical and mechanical trauma 1, followed by secondary injury comprising an inflammatory process and formation of glial scar 2.
Glial cells especially astrocytes are contributed to the glial scar formation around the lesion cavity 3. Upon the stimulation of inflammatory factors after SCI, astrocytes are activated and proliferated along with the overexpression of glial fibrillary acidic protein (GFAP) and hypertrophied morphology change 4. Although reactive astrocytes may play a number of beneficial effects by restricting inflammation and promoting wound healing 5, 6, glial scar is regarded as one of the major factors blocking spontaneous axon regeneration 7. The suppression of glial scar may reduce tissue damage and improve functional recovery 8. The mice deficient for both GFAP and vimentin recovered substantial locomotor function after a severe SCI 9. For these reasons, targeting astrogliosis becomes a therapeutic strategy for SCI.
Some studies have shown that astrocytes separated from mammalian brain and spinal cord express histamine receptors 10, 11. Our previous studies revealed that histamine can upregulate expressions of astrocytic glutamine synthetase (GS) and glutamate transporter GLT‐1, resulting in neuroprotection against excitotoxicity 12, 13. These data indicate that histamine may be a regulator of astrocyte function during CNS injury. Numerous studies have proved that histamine participated in ischemic brain pathology. The results both in vivo and in vitro showed that histamine exerts protective effect in brain ischemic pathology 14, probably involving targeting to astrocytes 12. In SCI, it has been reported that histamine level increases in the traumatized segment at 2 h after injury 15, which may contribute to the increase of the sensitivity of vessels to other vasoactive substances 16. However, the role of histamine in the functional recovery after SCI, especially its action on glial scar formation remains unclear.
This study is aimed to investigate whether histamine could exert a neuroprotective activity in pathophysiological response of SCI and regulate glial scar formation using two independent approaches including exogenous application of histamine and knockout of endogenous histamine.
Materials and Methods
Animals
All experiments were performed according to the National Institute of Health Guide for the Care and Use of Laboratory Animals and were in accordance with the Animal Care and Use Committee of School of Medicine, Zhejiang University. Adult female Sprague–Dawley rats weighing 220–250 g were used. Both wild‐type (HDC+/+) and HDC−/− female C57BL/6 mice weighing 22–27 g were used. From 15 day before experiments until sacrifice, HDC−/− mice were fed with special chow low in histamine (0.5 μg/g).
Surgical Procedures
Chloral hydrate (400 mg/kg, intraperitoneally, i.p.) was used to anesthetize animals. The T10 laminectomy was exerted to expose spinal cord. The right hemisection was performed with the tip of an iridectomy scissors (cut twice to make sure complete disconnection), and the injury of dorsal blood vessel should be avoided. This hemisection model was chosen because it created a controlled lesion location. Furthermore, this model facilitates the formation of glial scars in a consistent manner. Immediately after the injury, histamine was applied directly (0.1 nmol or 1 nmol in 10 μL) at the injured site using gelatin sponge (gelfoam, Jinling, Nanjing, China) as a carrier. The animals in vehicle‐SCI group were topically treated with saline‐soaked gelfoam after SCI. In the antihistamine receptor treatment groups, diphenhydramine (2, 10 mg/kg) or cimetidine (2, 10 mg/kg) was injected 30 min i.p. before operation and once daily for the next 1 weeks. After operation, animals were return to individual cages at 22–25°C and injected with penicillin sodium (80,000 U/day) for up to next 7 days. Manually pressing on bladders was performed twice daily until return of reflexive bladder control.
Locomotion Recovery Assessment
Open‐Field Scoring
Hindlimb motor behavior was estimated with the Basso–Beattie–Bresnahan (BBB) 17 scale before the operation and at 1, 7, 10, 14, 21, and 28 days postoperation (dpo) in rats. The Basso mouse scale (BMS) 18 was used to estimate the behavior recovery for all mice at 7, 14 dpo. Briefly, animals were placed individually in an open field so that they can move freely at least for 4 min. Two independent observers scored the performance of ipsilateral hindlimb.
Footprint Analysis
Footprint analysis was performed following the procedure of Karimi‐Abdolrezaee et al. 19. Briefly, the forepaws of the animals were inked with red color, and their hindpaws were inked with dark‐blue color. The animals were allowed to walk along a runway (7 cm width, 100 cm length) which was previously covered with blank article. Hindlimb footprints were used to determine the aberrant gait by calculating the angle rotation (AR) and interlimb coordination (ILC). The AR was assessed by testing the angle formed by two lines, one connecting the third toe and center of the paw pad; the other one connecting the stride line and center of the paw pad. The ILC was assessed by measuring the distance between the central pads of the forelimb and hindlimb on ipsilateral side of the body.
Histology and Immunohistochemistry
At different time point, animals were killed and perfused with 4% paraformaldehyde (PFA). A 1.5‐cm‐long segment containing lesion epicenter was separated, postfixed overnight and then cryoprotected in 30% sucrose for processing into 16 μm longitudinally frozen sections by acryostat (SM2000R, LEICA, Wetzlar, Germany). For HE stain, the sections were stained with hematoxylin solution (2 min) and followed by eosin (10–20 second). For Luxol fast blue (LFB) stain, the sections were stained with LFB solution (Solvent blue 38, Sigma‐Aldrich, St. Louis, MO, USA) at 56°C overnight. The stained images were used to assess the preserved myelinated area using the Image J software. For immunofluorescence, primary antibodies were added onto sections at 4°C overnight as follows: mouse anti‐GFAP (1:300, EMD Millipore, Billerica, MA, USA), rabbit anti‐GFAP (1:300, Boster, Wuhan, China), rabbit antineurocan (1:200, Boster), and mouse anti‐NF200 (1:100, Abcam, Cambridge, UK). After washing with phosphate‐buffered saline repeatedly, sections were incubated sequentially in Alexa 488 anti‐rabbit IgG (1:400, Invitrogen, Eugene, OR, USA) or Alexa 594 anti‐mouse IgG (1:400, Invitrogen) for 2 h. Images were acquired under fluorescent microscopy (Olympus BX61, Tokyo, Japan). Using Image‐Pro Plus program, the mean fluorescence intensity of images (four areas per section) was assessed (corrected by cell number) and normalized to obtain relative ratios that were compared between experimental groups. To estimate the thickness and volume of glial scar, 3, 3′‐diaminobenzidine (DAB) histochemistry staining of GFAP was performed in serial sections spaced 200 μm apart using a SABC kit (Boster), and DAB was used as a chromogenic agent. Mean thickness of glial scar was calculated from fixed four areas per section. The scar volume was estimated according to the Cavalieri principle 20.
Western Blot Analysis
To quantitate the GFAP protein levels, animals were killed and perfused with ice‐cold saline at 2 week after surgery. Five millimeter length of spinal cord segments containing lesion epicenter was quickly taken out and then lysed with homogenization buffer. Equal amount (40 μg) of tissue lysates was separated by 10% gels and transferred onto a nitrocellulose membrane. The membranes were incubated with primary antibodies (mouse anti‐GAPDH or rabbit anti‐GFAP) at 4°C overnight followed by IRDye 800 anti‐rabbit or IRDye 700 anti‐mouse antibodies for 2 h. Images were captured by the Odyssey infrared imaging system (LI‐COR Biosciences, Lincoln, NE, USA).
Cell Scratch Injury
Primary astrocyte cultures were separated from cerebral cortex of 1 day postnatal Sprague–Dawley rats as described 21. The GFAP‐positive cells in these cultures were more than 95% (data not shown). Astrocytes were plated on PLL‐coated slides in 24‐well plates. After the cells were grown to confluence, mechanical injury was performed using a sterile pipette tip (200 μL).
Proliferation Assay
For labeling the proliferation cells, 5‐bromo‐2‐deoxyuridine (BrdU) incorporation assay was performed as described 22. After scratching injury, different concentrations of histamine (10−7–10−5 M) were added into astrocyte cultures for 24 h. And 10 μM BrdU (Sigma‐Aldrich) was added for the last 18 h before the experiment was finished. After fixed with 4% PFA, astrocytes were incubated in 1 M HCl and then neutralized using 0.1 M sodium borate (pH 8.5). Then, the slices were incubated with anti‐BrdU antibody (1:600, Sigma‐Aldrich) and sequentially secondary antibody (Alexa 594 anti‐mouse IgG, 1:400, Invitrogen). Images were captured under fluorescent microscopy. Cell proliferation was estimated as ratio of BrdU+ cells to DAPI+ cells. To investigate the antiproliferation effect of histamine, histamine H1‐specific antagonist diphenhydramine (10−6‐10−4 M), and H2 specific antagonist cimetidine (10−6–10−4M) were added 30 min before histamine. After treatment with or without histamine for 24 h, cell proliferation index was determined as above.
Statistical Analysis
The Student t‐test was used for single comparison, one‐way ANOVA followed by Tukey's post hoc tests was used for multiple comparisons, and repeated measures two‐way ANOVA followed by least‐significant difference's post hoc tests was used to analyze the locomotor behavioral data. All values are expressed as means ± SEM.
Results
Exogenous Delivery of Histamine Reduced Lesion Size and Improved Hindlimb Functions after SCI
To investigate whether histamine plays a relevant role in the pathophysiology following SCI, we first evaluated the morphological changes and recovery of motor function after topical delivery of histamine following SCI (Figure 1). It has been reported that following incomplete SCI a large cyst with amorphous material, trabeculae and liquid surrounded by a glial scar has formed at 4 weeks, and remained stable even at 8 and 16 weeks after SCI 23. Moreover, many studies have shown a strong relationship between the severity of SCI and the recovery of motor functions based on behavioral assessments 24. So, the histological outcomes and functional recovery were assessed at 4 weeks after SCI. The lesion cavitation measured from HE staining and GFAP immunostaining was significantly smaller in histamine treatment (1 nmol) group compared with SCI group at 4 weeks after SCI (P < 0.01). Moreover, histamine delivery markedly increased the LFB‐stained myelinated area which was probably related to increased preservation of myelin and/or increased remyelination (P < 0.05, Figure 1A,B).
Figure 1.
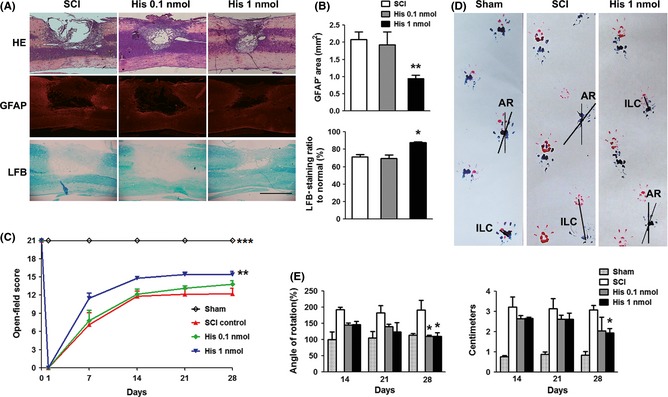
Protective effect of histamine on spinal cord injury. (A) Representative photographs from HE, GFAP, and LFB staining at 28 days postoperation (dpo). Scale bars: 1 mm. (B) The GFAP − area and LFB‐positive ratio in both groups. n = 5. *P < 0.05; **P < 0.01 versus the SCI group. (C) The Basso–Beattie–Bresnahan (BBB) scores at 1, 7, 14, 21, and 28 dpo. n = 4 for sham group, n = 6 for other groups. **P < 0.01; ***P < 0.001 versus the SCI group. (D) Representative photographs of footprints from sham, SCI, and histamine rats on 28 dpo. (E) Quantitative analyses of the hindlimb angle rotation (AR) and interlimb coordination (ILC) at 28 dpo in both groups. n = 4 for sham group, n = 6 for other groups. *P < 0.05 versus the SCI group.
To determine whether histamine improved functional recovery after SCI, two independent behavioral tasks, including 21‐point BBB locomotor rating scale and footprint analysis, were used. Functional recovery was evaluated before operation and 1, 7, 14, 21, 28 dpo. Before operation, all the animals showed normal locomotion, corresponding to a BBB scale of 21. On the 1 dpo, the animals dragged the injured hindlimb, and their behavior score was 0. During the first 14 dpo, recovery of locomotion proceeded rapidly and then maintained at a relatively slower pace thereafter. Compared with SCI group, animals from two histamine groups recovered faster during the entire experimental period. One nmol dose of histamine was more effective than 0.1 nmol dose. It significantly improved the functional recovery compared with SCI group (P < 0.01, two‐way ANOVA, Figure 1C).
To analyze the animals' gait patterns after injury, AR and ILC were estimated in the footprint test. During the first week, animals in all the groups dragged their right hindpaw, so we examined the footprint test from 14 dpo. Relative to the SCI animals, rats which have received histamine (1 nmol) revealed a markedly behavior improvement in AR and ILC at 28 dpo after injury (P < 0.05, Figure 1D,E), suggesting that histamine can greatly improve the gait and coordination of forelimb and hindlimb foot placements.
Exogenous Histamine Delivery Attenuated the Reactive Astrogliosis after SCI
GFAP is regarded as a sensitive and reliable marker of astrocyte activation after CNS injury 8, so immunohistochemistry analysis of GFAP expression was performed. It has been reported that astrocytic glial scar is fully established by 14 dpo 25, and our results indicated that locomotion recovery reached a plateau around 2 weeks by BBB score after operation (Figure 1C), so we took 14 dpo as a time point for investigating the extent of astrogliosis. Severe spinal cord trauma resulted in excessively reactive astrogliosis around the lesion site as shown in Figure 2A. Compared with that in 1 mm proximal area, the GFAP signal intensity was much stronger and the number of GFAP‐labeled astrocytes in perilesion dramatically increased (Figure 2A). Histamine markedly reduced the number of reactive astrocytes, downregulated GFAP expression in immunofluorescent staining, and reversed their morphological alteration compared with the SCI group (Figure 2A,B). Furthermore, the expression of GFAP was confirmed by Western blot analysis. We found that histamine markedly attenuated the GFAP expression induced by SCI (P < 0.05, Figure 2C). To test whether histamine impacts the glial scar formation, the thickness of scar was measured by DAB staining of GFAP. The results showed that the histamine treatment could significantly reduce the mean thickness of scar (P < 0.01) compared with SCI group. As it has been reported that the volume of the glial scar negatively correlates with functional recovery and neurogenesis in the SCI 26, the volume of glial scar was also estimated. The data showed that histamine treatment reduced the scar volume (P < 0.05, Figure 2D,E) compared with SCI group.
Figure 2.
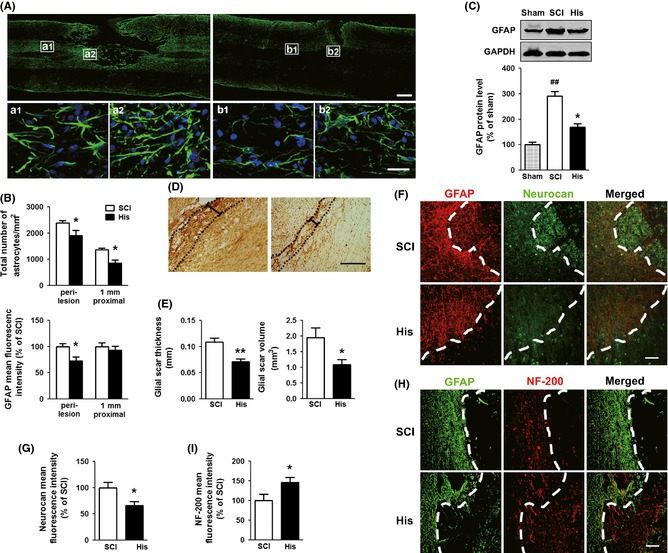
Effect of histamine on reactive astrogliosis in the damaged spinal cord. The astrocytic gliosis of the rats under the treatment of normal saline or histamine (1 nmol) using gelfoam was analyzed by immunohistochemistry and Western blot examination of GFAP at 14 dpo. (A) Representative photographs from GFAP immunostaining. The areas at 1 mm proximal (a1, b1) and close to lesion site (a2, b2) in upper panel are enlarged in the lower panel. Scale bars: 500 μm in upper panel; 25 μm in lower panel. (B) Quantitative analyses of reactive astrogliosis around the lesion site and 1 mm proximal to the epicenter, including the number of astrocytes, and the GFAP mean fluorescence intensity which has been corrected by cell number. n = 5. *P < 0.05 versus SCI group. (C) Western blot analysis of GFAP expression. n = 3. ## P < 0.01 versus sham group, *P < 0.05 versus SCI group. (D) Representative photographs from DAB straining of GFAP which contain glial scar (dotted lines). The black calibration bar indicates glial scar thickness. Scale bars: 200 μm. (E) Quantitative analyses of thickness and volume glial scar. n = 5. **P < 0.01 versus SCI group, *P < 0.05 versus SCI group. (F) Representative photographs containing astrocytic fronts (dashed lines) from neurocan immunostaining. Scale bars: 100 μm. (G) Quantitative analysis of neurocan mean fluorescence intensity. n = 5. *P < 0.05 versus SCI group. (H) Representative photographs containing astrocytic fronts (dashed lines) from neurofilament (NF‐200) immunofluorescence on spinal cord sections. Scale bars: 100 μm. (I) Quantitative analysis of NF‐200 staining intensity. n = 5. *P < 0.05 versus SCI group.
The CSPGs secreted from reactive astrocytes are the main inhibitory constituents for axon regeneration. Neurocan, one of the main scar‐associated CSPGs, was highly expressed within the lesion core after injury (Figure 2F,G). Compared with SCI group, the mean fluorescence intensity of neurocan was significantly downregulated by 33.9% after histamine treatment (P < 0.05), indicating that histamine can inhibit the expression of neurocan.
We then asked whether histamine can promote axonal growth through reducing glial scar. In the SCI group, neurofilament (NF‐200)‐labeled axons were lost in the epicenter of injury. By contrast, in histamine‐treated group a number of NF‐200‐labeled fibers were present around the lesion site and grown beyond the glial front (dashed lines in Figure 2H). The density of axons was highly increased adjacent to the lesion site compared with SCI group. These data indicated that histamine can inhibit the formation of glial scar, which probably facilitates axonal regeneration.
Functional Recovery and Reactive Astrogliosis were Aggravated in HDC–/– Mice after SCI
To verify the role of histamine in the pathophysiological response after SCI, we investigated the effect of the absence of histamine on SCI using HDC knockout mice. Wild‐type and HDC−/− mice underwent a right hemisection after T10 laminectomy, and the lesion area and functional recovery were evaluated for 2 weeks. After hemisection, the injured spinal cord showed structural disturbance from HE staining in both groups. We found that GFAP− area and the loss of white matter were significantly less in wild‐type mice than that in HDC−/− mice (Figure 3A–C). We next determined the reactive astrogliosis in HDC knockout mice by GFAP immunostaining. It showed that endogenous absence of histamine increased the number of astrocytes (P < 0.05) and GFAP expression (P < 0.05) around the lesion compared with wild types (Figure 3D,E). The thickness and the volume of glial scar were increased in HDC−/− group (Figure 3F,G). Although no discernible differences in BMS behavior performances were found between wild‐type and HDC−/− mice before operation, HDC−/− mice had significantly lower scores in BMS compared with that of wild‐type mice after SCI (Figure 3H).
Figure 3.
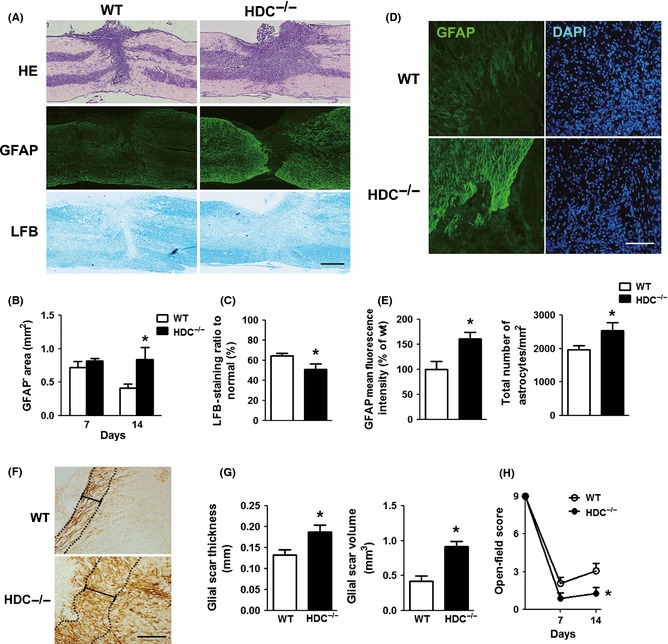
Aggravation of spinal cord damage in HDC −/− mice after SCI. (A) Representative photographs from HE, GFAP, and LFB staining at 14 days. Scale bars: 1 mm. (B, C) Analyses of GFAP – area and LFB‐positive ratio in both groups. n = 4. *P < 0.05 versus the wild type. (D) Representative images from GFAP immunostaining at the lesion site on 14 dpo. Scale bars: 100 μm. (E) Quantitative analyses of the number of astrocytes and GFAP fluorescence mean intensity (corrected by cell number). n = 4. *P < 0.05 versus the wild type. (F) Representative images containing glial scar from DAB staining of GFAP at 14 dpo. Scale bars: 200 μm. (G) Quantitative analyses of thickness and volume of glial scar. n = 4. *P < 0.05 versus the wild type. (H) The Basso mouse scale (BMS) of SCI model mice at 7, 14 dpo. n = 8 for wild type, n = 7 for HDC −/−. *P < 0.05 versus the wild type.
Histamine Inhibits Reactive Astrogliosis via Stimulating Histamine H1 Receptor in vivo and in vitro
Histamine action is believed to be mediated by binding to its specific cell‐surface receptors. H1 receptor antagonist diphenhydramine (10 mg/kg, i.p.) reversed the inhibitory properties of histamine on astrogliosis assessed by the number of astrocytes and GFAP expression, and partly reversed the neuroprotective effect of histamine on locomotor recovery measured by open‐field score (P < 0.01, two‐way ANOVA). The thickness and volume of scar were significantly increased in rats treated with diphenhydramine, compared with histamine treatment (Figure 4E,F). However, cimetidine, a selective H2 antagonist, had no effect on the inhibition of glial scar formation induced by histamine even at a high dose of 10 mg/kg (Figure 4), indicating that histamine impedes astrogliosis through activating histamine H1 receptor but not H2 receptor.
Figure 4.
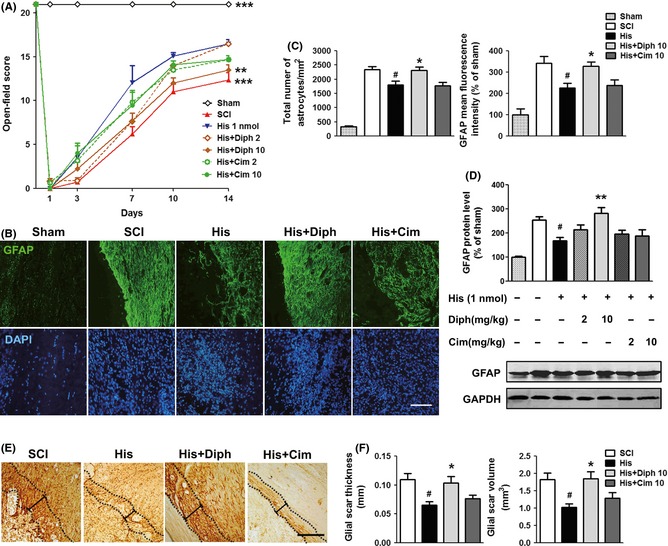
Inhibitory effect of histamine on reactive astrogliosis via stimulating histamine H1 receptor in SCI. (A) The BBB scores at 1, 3, 7, 10, and 14 dpo. n = 6. **P < 0.01; ***P < 0.001 versus the histamine group. (B) Representative photographs from GFAP immunostaining of the lesion site at 14 dpo. (C) Quantitative assessments of the total number of astrocytes and GFAP fluorescence mean intensity. n = 4. # P < 0.05 versus SCI group, *P < 0.05 versus histamine group. (D) Western blot analysis of GFAP level in spinal cord. n = 3. # P < 0.05 versus SCI group, **P < 0.01 versus histamine group. (E) Representative photographs containing glial scar from DAB straining of GFAP. Scale bars: 200 μm. (F) Quantitative assessments of thickness and volume of glial scar. n = 4. # P < 0.05 versus SCI group, *P < 0.05 versus histamine group.
To further confirm the effect of histamine on astrogliosis via H1 receptor, we used in vitro “scratch‐wound” model. Treatment of 10‐5 M histamine resulted in a suppression of proliferation after scratched by a plastic pipette tip, showing 23% less BrdU+/DAPI+ cells than controls (P < 0.05; Figure 5A,B). The antiproliferation effect of histamine was reversed by anti‐H1 antagonist, but not anti‐H2 antagonist, further suggesting that histamine possessed the ability to inhibit astrocyte proliferation via stimulation of H1 receptor (Figure 5C). Moreover, both the anti‐H1 antagonist and anti‐H2 antagonist had no effect on the astrocyte proliferation in controls.
Figure 5.
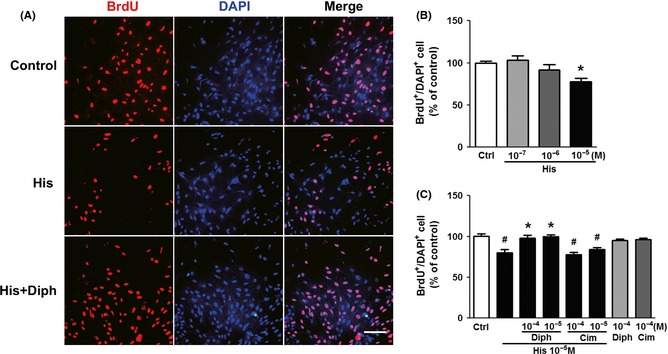
Inhibitory effect of histamine on the proliferation of reactive astrocytes in scratch injury model in vitro. (A) Representative photographs from BrdU immunostaining around the lesion site. Scale bar: 100 μm. (B) Quantitative assessments of BrdU‐positive astrocytes administrated with different concentrations of histamine at 24 h after scratch. *P < 0.05 versus control. (C) Quantitative assessments of BrdU‐positive astrocytes treated with histamine (10−5 M) and its receptor antagonists (Diph 10−4, 10−5 M, Cim10−4, 10−5 M) for 24 h. # P < 0.05 versus control, *P < 0.05 versus histamine (10−5M) group. Experiments were repeated three times independently.
Discussion
So far, the role of histamine in pathophysiological response of SCI is still unclear. In this study, we investigated the effect of histamine on long‐term recovery after SCI using several methods, including histological examination and behavioral evaluation. Our results showed that histamine markedly decreased lesion area, inhibited glial scar formation, and improved functional recovery even 28 day after SCI. It is likely that exogenous delivery of histamine may be a therapeutic strategy for functional recovery following SCI.
There are some reports regarding histamine's action at early stage after SCI, which suggested that histamine can mediate the edema formation within 5 h 27, 28. In contrast to that, our data indicated that exogenous histamine provided prominent neuroprotection in the long‐term injury of SCI. To confirm the role of histamine, we used HDC knockout mice to reveal the role of endogenous histamine in SCI. We found that HDC−/− mice, which are absent of histamine, displayed increased astrocyte activation, larger lesion size, impaired myelination, and behavior recovery compared with wild‐type mice. Therefore, these results at least indicated that the endogenous histamine also plays a protective role in the pathophysiological response of SCI.
It is well accepted that excessive scar tissue formation is a major impediment for axonal regeneration both physically and chemically 3, while ablation of the proliferation of scar‐forming astrocytes or removing inhibitory CSPGs secreted from reactive astrocytes have been proved to ameliorate functional deficit after injury 29, 30. So, manipulation of astrocytic glial scar may facilitate the axon regeneration and locomotor recovery after SCI. In this study, we demonstrated that physical barriers, including astroglial proliferation, GFAP upregulation, and glial scar formation, were markedly reduced after topical administration with histamine in the hemisection model. In the “scratch injury model”, we also found that histamine inhibited the proliferation of astrocytes. Furthermore, we evaluated the expressions of neurocan and phosphacan which are known to be secreted by reactive astrocytes and contribute to the glial scar formation. The topical treatment of histamine markedly inhibited expression of neurocan around the lesion site, but had no significant effect on expression of phosphacan (data not shown). This is similar to other studies both in vitro 21 and in vivo 31. We speculate that phosphacan may not be a sensitive indicator of glial scar. In addition, more NF‐200‐positive fibers and myelination were found at the lesion site after SCI (Figure 1A, 2D). Taken together, our results suggest that exogenous delivery of histamine impairs the physical and chemical barrier induced by glial scar which can subsequently support axonal regrowth beyond the glial scars. Recent studies have shown that reactive astrogliosis limited the inflammatory response 5, 32, and conditional ablation of scar‐forming astrocytes induced widespread infiltration of inflammatory cells 6. However, our result showed that histamine treatment reduced the area of Iba1+ (a marker of microglia/macrophages) cells (Figure S1). It suggested that here the inhibition of glial scar by histamine did not favor the spread of inflammation and even restricted the inflammatory area, although the detailed mechanism is still unclear.
In addition, histamine H1‐ and H2‐specific antagonists (diphenhydramine and cimetidine) were used to verify the participation of histamine in SCI. Diphenhydramine reversed the neuroprotective effect of histamine in BBB scores, while cimetidine had no effect on the protection of histamine. Pretreatment of diphenhydramine, but not cimetidine, can reverse the inhibitory effect of histamine on astrogliosis. It suggests that the suppression of astrogliosis and glial scar formation by H1 receptor stimulation may account for the alleviation of histological outcome. Although we focused on astrogliosis and glial scar formation in the current research, histamine may provide a multitude of neuroprotective effects relying on its different receptors. It has been reported that histamine could upregulate astrocytic GLT‐1 and GS expression via stimulating histamine H1 receptor in astrocytes, leading to neuroprotection against excitotoxicity 12, 13. On the other hand, a number of studies showed that the stimulation of H2 receptor in neurons may be protective. Treatment of H2 agonist alleviates the neuronal damage after MCAO, which is aggravated by the H2 antagonists 33, 34. In cultured neuron, histamine protects against NMDA‐induced excitotoxicity through stimulation of H2 receptors 35. Therefore, we raise a notion that the protection of histamine may be accounted for by different receptors in different cell types.
Histamine increases in the brain tissue, spinal cord lesion site, brain blood, and cerebrospinal fluid in various forms of CNS injury 15, 16. In our study, we found that histamine content increased in the lesion segment from 2 h, peaked at 24 h, and returned to normal at 3 dpo in the experimental female rats (data not shown). We speculated that the gradual decrease of histamine boosts the glial scar formation. An observation that astrocyte proliferation usually occurs within 7 dpo 5 indicates that first 7 day may be an appropriate therapeutic time window to intervene against glial scar formation. For this purpose, we administrated histamine topically using gelfoam on the lesion site after surgery. Gelfoam is an absorbable medical device used as a hemostatic and as a carrier to deliver some agents such as thrombin in the clinic 36. Topical treatment of gelfoam soaked with histamine makes it more convenient for administration. Also, a high concentration and long application term can be achieved without systemic side effect. Indeed, we found that this early topical treatment leads to a persistent increase of histamine content until 7 dpo (data not shown) and is sufficient to attenuate the astrogliosis and improve the functional recovery at 4 weeks after SCI.
In conclusion, our current data are the first to provide direct evidence that histamine significantly improved the chronic locomotor recovery via attenuating astrogliosis after SCI by stimulating histamine H1 receptor. This study highlights a therapeutic potential of histamine and its related drugs for SCI.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. Effect of histamine on reactive microglia and macrophages in the damaged spinal cord.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81030061, 81273490, 81273506, 81473186), the National Basic Research of China 973 Program (2011CB504403), and the Program for Zhejiang Leading Team of S&T Innovation (2011R50014).
References
- 1. Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 1996;76:319–370. [DOI] [PubMed] [Google Scholar]
- 2. Wu Q, Zhang YJ, Gao JY, et al. Aquaporin‐4 mitigates retrograde degeneration of rubrospinal neurons by facilitating edema clearance and glial scar formation after spinal cord injury in mice. Mol Neurobiol 2014;49:1327–1337. [DOI] [PubMed] [Google Scholar]
- 3. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 2004;5:146–156. [DOI] [PubMed] [Google Scholar]
- 4. Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia 2005;50:427–434. [DOI] [PubMed] [Google Scholar]
- 5. Okada S, Nakamura M, Katoh H, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 2006;12:829–834. [DOI] [PubMed] [Google Scholar]
- 6. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004;24:2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 2006;7:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu FT, Xu SM, Xiang ZH, et al. Molecular Hydrogen Suppresses Reactive Astrogliosis Related to Oxidative Injury during Spinal Cord Injury in Rats. CNS Neurosci Ther 2014;20:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ribotta MG, Menet V, Privat A. Glial scar and axonal regeneration in the CNS: Lessons from GFAP and vimentin transgenic mice. Acta Neurochir Suppl 2004;89:87–92. [DOI] [PubMed] [Google Scholar]
- 10. Hosli L, Hosli E, Schneider U, Wiget W. Evidence for the existence of histamine H1‐ and H2‐receptors on astrocytes of cultured rat central nervous system. Neurosci Lett 1984;48:287–291. [DOI] [PubMed] [Google Scholar]
- 11. Carman‐Krzan M, Lipnik‐Stangelj M. Molecular properties of central and peripheral histamine H1 and H2 receptors. Pflugers Arch 2000;439:R131–R132. [DOI] [PubMed] [Google Scholar]
- 12. Fang Q, Hu WW, Wang XF, et al. Histamine up‐regulates astrocytic glutamate transporter 1 and protects neurons against ischemic injury. Neuropharmacology 2014;77:156–166. [DOI] [PubMed] [Google Scholar]
- 13. Wang XF, Hu WW, Yan HJ, et al. Modulation of astrocytic glutamine synthetase expression and cell viability by histamine in cultured cortical astrocytes exposed to OGD insults. Neurosci Lett 2013;549:69–73. [DOI] [PubMed] [Google Scholar]
- 14. Hu WW, Chen Z. Role of histamine and its receptors in cerebral ischemia. ACS Chem Neurosci 2012;3:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naftchi NE, Demeny M, DeCrescito V, Tomasula JJ, Flamm ES, Campbell JB. Biogenic amine concentrations in traumatized spinal cords of cats. Effect of drug therapy. J Neurosurg 1974;40:52–57. [DOI] [PubMed] [Google Scholar]
- 16. Kuruvilla A, Theodore DR, Abraham J. Changes in norepinephrine and histamine in monkey spinal cords traumatized by weight drop and compression. Cent Nerv Syst Trauma 1985;2:61–71. [DOI] [PubMed] [Google Scholar]
- 17. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995;12:1–21. [DOI] [PubMed] [Google Scholar]
- 18. Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006;23:635–659. [DOI] [PubMed] [Google Scholar]
- 19. Karimi‐Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 2006;26:3377–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loers G, Cui YF, Neumaier I, Schachner M, Skerra A. A Fab fragment directed against the neural cell adhesion molecule L1 enhances functional recovery after injury of the adult mouse spinal cord. Biochem J 2014;460:437–446. [DOI] [PubMed] [Google Scholar]
- 21. Wang R, Zhang X, Zhang J, et al. Oxygen‐glucose deprivation induced glial scar‐like change in astrocytes. PLoS ONE 2012;7:e37574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuan Y, Su Z, Pu Y, et al. Ethyl pyruvate promotes spinal cord repair by ameliorating the glial microenvironment. Br J Pharmacol 2012;166:749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu R, Zhou J, Luo C, et al. Glial scar and neuroregeneration: Histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J Neurosurg Spine 2010;13:169–180. [DOI] [PubMed] [Google Scholar]
- 24. Rangasamy SB. Locomotor recovery after spinal cord hemisection/contusion injures in bonnet monkeys: Footprint testing–a minireview. Synapse 2013;67:427–453. [DOI] [PubMed] [Google Scholar]
- 25. Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin‐B2 and EphB2 regulation of astrocyte‐meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci 2003;23:7789–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Apostolova I, Irintchev A, Schachner M. Tenascin‐R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J Neurosci 2006;26:7849–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winkler T, Sharma HS, Stalberg E, Olsson Y, Nyberg F. Role of histamine in spinal cord evoked potentials and edema following spinal cord injury: Experimental observations in the rat. Inflamm Res 1995;44(Suppl 1):S44–S45. [DOI] [PubMed] [Google Scholar]
- 28. Sharma HS, Vannemreddy P, Patnaik R, Patnaik S, Mohanty S. Histamine receptors influence blood‐spinal cord barrier permeability, edema formation, and spinal cord blood flow following trauma to the rat spinal cord. Acta Neurochir Suppl 2006;96:316–321. [DOI] [PubMed] [Google Scholar]
- 29. Jefferson SC, Tester NJ, Howland DR. Chondroitinase ABC promotes recovery of adaptive limb movements and enhances axonal growth caudal to a spinal hemisection. J Neurosci 2011;31:5710–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian DS, Yu ZY, Xie MJ, Bu BT, Witte OW, Wang W. Suppression of astroglial scar formation and enhanced axonal regeneration associated with functional recovery in a spinal cord injury rat model by the cell cycle inhibitor olomoucine. J Neurosci Res 2006;84:1053–1063. [DOI] [PubMed] [Google Scholar]
- 31. Huang X, Kim JM, Kong TH, et al. GM‐CSF inhibits glial scar formation and shows long‐term protective effect after spinal cord injury. J Neurol Sci 2009;277:87–97. [DOI] [PubMed] [Google Scholar]
- 32. Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist 2005;11:400–407. [DOI] [PubMed] [Google Scholar]
- 33. Hamami G, Adachi N, Liu K, Arai T. Alleviation of ischemic neuronal damage by histamine H2 receptor stimulation in the rat striatum. Eur J Pharmacol 2004;484:167–173. [DOI] [PubMed] [Google Scholar]
- 34. Adachi N, Terao K, Otsuka R, Arai T. Histaminergic H(2) blockade facilitates ischemic release of dopamine in gerbil striatum. Brain Res 2002;926:172–175. [DOI] [PubMed] [Google Scholar]
- 35. Dai H, Zhang Z, Zhu Y, et al. Histamine protects against NMDA‐induced necrosis in cultured cortical neurons through H receptor/cyclic AMP/protein kinase A and H receptor/GABA release pathways. J Neurochem 2006;96:1390–1400. [DOI] [PubMed] [Google Scholar]
- 36. Cho SK, Yi JS, Park MS, et al. Hemostatic techniques reduce hospital stay following multilevel posterior cervical spine surgery. J Bone Joint Surg Am 2012;94:1952–1958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of histamine on reactive microglia and macrophages in the damaged spinal cord.


