Summary
Aims
Amino acids are important body metabolites and seem to be helpful for understanding pathogenesis and predicting therapeutic response in major depressive disorder (MDD). We performed amino acid profiling to discover potential biomarkers in major depressive patients treated with selective serotonin reuptake inhibitors (SSRIs).
Methods
Amino acid profiling using aTRAQ ™ kits for Amino Acid Analysis in Physiological Fluids on a liquid chromatography–tandem mass spectrometry (LC‐MS/MS) system was performed on 158 specimens at baseline and at 6 weeks after the initiation of SSRI treatment for 68 patients with MDD and from 22 healthy controls.
Results
Baseline alpha‐aminobutyric acid (ABA) discriminated the patients according to the therapeutic response. Plasma glutamic acid concentration and glutamine/glutamic acid ratio were different between before and after SSRI treatment only in the response group. Comparing patients with MDD with healthy controls, alterations of ten amino acids, including alanine, beta‐alanine, beta‐aminoisobutyric acid, cystathionine, ethanolamine, glutamic acid, homocystine, methionine, O‐phospho‐L‐serine, and sarcosine, were observed in MDD.
Conclusion
Metabolism of amino acids, including ABA and glutamic acid, has the potential to contribute to understandings of pathogenesis and predictions of therapeutic response in MDD.
Keywords: Alpha‐aminobutyric acid, Ethanolamine, Glutamic acid, Metabolomics, Selective serotonin reuptake inhibitor
Introduction
Major depressive disorder (MDD) is a common mental disorder that affects approximately 5–20% of the world population 1, 2. MDD has multifactored etiologies including environmental and genetic factors; however, a clear pathogenesis remains unrevealed 3, 4. The most frequently used antidepressants are selective serotonin reuptake inhibitors (SSRIs) due to their safety and efficacy 5, 6. They have been used for many years as first‐line therapeutic agents as the first SSRI, fluoxetine, was introduced in the 1980s 7. However, about half of patients with MDD show failure to respond to SSRIs and approximately 10–20% or higher of patients treated with first‐ and second‐generation antidepressants do not achieve complete recovery 8, 9, 10, 11, 12, 13.
To understand the pathogenesis and to predict the prognosis of MDD, numerous studies have been conducted in diverse research fields, including genomics, transcriptomics, proteomics, and metabolomics 14, 15, 16. In particular, the application of metabolomics has increased for the detection of changes according to the development of disease and treatment 17, 18, 19, 20. Metabolomics is on the terminal part of a series of processes from DNA to physical change; thus, it directly reflects alterations of body responses. Amino acids are a group of metabolites that may be an important part of human metabolome. Amino acids play a role as brain neurotransmitters and intermediates in the biosynthesis and metabolism of body compounds. Amino acids have been studied as candidates for biomarkers of diverse diseases, including metabolic syndrome, cancer, and psychiatric disorders including Alzheimer's disease, schizophrenia, and MDD 21, 22, 23, 24, 25, 26.
While methodologies and results are not strictly consistent, findings of the disturbance of amino acids in MDD compared with healthy individuals were replicated in previous studies 21, 26, 27. However, there is scant data on the changes and differences of amino acids according to the treatment including pharmacotherapy with SSRIs and therapeutic response in MDD 28, 29. Discovering alterations of amino acids in MDD would not only improve understandings of the disease but also help the selection of therapeutic agents and lead to the improvement of treatment efficiency. Recently, with the development of technology and medical knowledge, more‐precise evaluations have been possible of the disturbance of amino acids in MDD through amino acid profiling using liquid chromatography–tandem mass spectrometry (LC‐MS/MS) and isotope‐labeled internal standards 30, 31. We performed profiling of 40 amino acids to identify differences according to the presence of disease in 68 patients with MDD and in 22 healthy individuals and according to the response of SSRI treatment in patients. We also assessed the significance for the changes of amino acids after SSRI treatment in total and subset (response and nonresponse) of patients and the association between the response of SSRI treatment and the changes of amino acids.
Experimental Procedures
Patients
Sixty‐eight patients with MDD who were treated with SSRIs at Samsung Medical Center were included. Patients fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for major depressive disorder, single episode or recurrent. Diagnoses were confirmed by a board‐certified psychiatrist on the basis of the Samsung Psychiatric Evaluation Schedule, case review notes, and SCID (structured clinical interview for DSM‐IV) to diagnose depression. A minimum baseline 17‐item Hamilton Rating Scale for Depression (HAM‐D) score of 15 was required 32. Study participants were excluded for pregnancy, significant medical conditions, abnormal laboratory baseline values, unstable psychiatric features (e.g., suicidal attempt in current episode), history of alcohol or drug dependence, seizures, head trauma with loss of consciousness, or neurological illness. We also excluded patients with concomitant Axis I psychiatric disorder; however, patients with concomitant Axis II psychiatric disorder were not excluded in this study. All patients were treated with SSRIs, 10–30 mg/day for escitalopram, 20–40 mg/day for fluoxetine, 10–40 mg/day for paroxetine, and 100–125 mg/day for sertraline. Therapeutic response was defined as a 50% or more reduction of HAM‐D score by 6 weeks after the initiation of antidepressant treatment. Twenty‐two healthy individuals without family history of MDD were included as controls. This study was approved by the Samsung Medical Center institutional review board. Informed consent was obtained by all patients.
Amino Acid Analysis
We performed amino acid profiling using morning, overnight fasting plasma samples from patients at baseline and at 6 weeks after the initiation of antidepressant therapy as well as from healthy individuals. Protein precipitation using 200 μL of plasma was performed, and 40 amino acids in specimens using the aTRAQ™ kits for Amino Acid Analysis in Physiological Fluids (AB Sciex, Foster City, CA, USA) were labeled with isobaric tags that have distinguishable report ions to internal standards according to the manufacturer's instructions. The Agilent 1260 Infinity LC system (Agilent Technologies Inc., Santa Clara, CA, USA) with a reverse‐phase C18 (5 μm, 4.6 mm×150 mm) column at 50°C was used to separate amino acids by a gradient mobile phase of 0.1% formic acid in water and 0.01% heptafluorobutyric acids in methanol at a flow rate of 0.45 mL/min. The amino acids were monitored using Agilent 6460 Triple Quadrupole MS/MS (Agilent Technologies Inc.) with positive electrospray ionization (ESI) in multiple‐reaction monitoring (MRM) mode. Each amino acid was analyzed using a single transition. The analytical cycle time was 28 min. The amounts of amino acids were calculated by comparing the peak intensities of amino acids between specimens and internal standards at one to one.
Statistical Analysis
The comparisons of clinical variables between response and nonresponse groups were performed by the Wilcoxon rank‐sum test or t‐test for continuous variables and the Fisher's exact test for categorical variables. To discover amino acids at baseline that can be associated with therapeutic response, we analyzed using logistic regression model in univariable and multivariable analyses. For each amino acid, age, sex, antidepressant, and HAM‐D score at baseline were included as covariates in the multivariable analysis. We also performed receiver operating characteristic (ROC) curve analysis for each amino acid at baseline to discriminate between response and nonresponse groups and obtained values of area under the curve (AUC). The association between the changes of amino acid concentrations and the therapeutic response after adjustment of covariates (age, sex, antidepressant, and HAM‐D score at baseline) was investigated using partial Spearman correlation analysis. The comparisons of amino acid concentrations between before and after SSRI treatment in total and subset of patients were performed by the Wilcoxon rank‐sum test or t‐test. Bonferroni's correction was applied to the results from subset analysis of response and nonresponse group due to multiple testing. Results were considered statistically significant with P‐value less than 0.05. SAS version 9.3 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Comparison between patients with MDD and controls
We analyzed 40 amino acids in 158 specimens at baseline and at 6 weeks after the initiation of SSRI treatment, from 68 patients with MDD and from 22 healthy controls. Twenty male (22.2%) and 70 females (77.8%) with a median age of 64.5 years (range, 50–86 years) were included in this study. Forty‐eight (70.6%) patients responded to the SSRI treatment, and median HAM‐D scores were decreased from 18.5 at baseline to 7 at 6 weeks after treatment in the response group. Baseline plasma concentrations of alanine, beta‐alanine, beta‐aminoisobutyric acid, cystathionine, ethanolamine, glutamic acid, homocystine, methionine, O‐phospho‐L‐serine, and sarcosine in patients with MDD showed differences from healthy controls (Table 1).
Table 1.
The characteristics and plasma concentrations of amino acids between healthy controls (n = 22) and major depressive patients (n = 68) at baseline
| Characteristics; Amino acid (μmol/L) | Controls | Patients | P‐valuea | ||
|---|---|---|---|---|---|
| Median | Q1–Q3 | Median | Q1–Q3 | ||
| Age | 68.0 | 65.3–73.3 | 65.0 | 60.8–70.0 | 0.118b |
| Sex, M:F | 4:18 | 16:52 | 0.771c | ||
| Alanine | 406 | 345–412 | 446 | 351–540 | 0.043 |
| Beta‐alanine | 23.5 | 9.00–62.2 | 11.0 | 7.80–17.5 | 0.024 |
| Anserine | 0.03 | 0.00–0.16 | 0.00 | 0.00–4.73 | 0.364 |
| Aminoadipic acid | 0.78 | 0.67–0.85 | 0.85 | 0.58–1.12 | 0.615 |
| Alpha‐aminobutyric acid | 12.5 | 11.1–17.6 | 16.2 | 12.7–21.1 | 0.144 |
| Gamma‐aminobutyric acid | 0.19 | 0.12–0.74 | 1.16 | 0.00–2.01 | 0.050 |
| Beta‐aminoisobutyric acid | 3.89 | 1.02–6.58 | 1.12 | 0.27–1.92 | <0.001 |
| Arginine | 58.7 | 39.1–75.2 | 69.7 | 49.0–94.1 | 0.099 |
| Asparagine | 53.5 | 47.1–64.6 | 50.9 | 41.3–70.3 | 0.662 |
| Aspartic acid | 4.30 | 3.14–5.49 | 3.51 | 2.60–5.73 | 0.411 |
| Carnosine | 0.02 | 0.00–0.08 | 0.00 | 0.00–0.00 | 0.055 |
| Citrulline | 30.3 | 25.7–36.6 | 34.0 | 25.2–45.6 | 0.194 |
| Cystathionine | 0.08 | 0.00–0.16 | 0.00 | 0.00–0.00 | <0.001 |
| Cystine | 97.5 | 54.4–126 | 59.0 | 36.3–90.6 | 0.083 |
| Ethanolamine | 5.49 | 4.92–6.23 | 35.4 | 23.6–36.8 | <0.001 |
| Glutamic acid | 51.8 | 35.1–61.6 | 59.6 | 39.1–76.9 | 0.047 |
| Glutamine | 600 | 554–686 | 621 | 495–794 | 0.796 |
| Glycine | 232 | 182–289 | 251 | 186–298 | 0.455 |
| Histidine | 82.4 | 74.7–92.3 | 73.8 | 63.1–96.5 | 0.304 |
| Homocystine | 0.02 | 0.00–0.06 | 0.00 | 0.00–0.00 | <0.001 |
| 5‐hydroxylysine | 0.53 | 0.00–1.89 | 0.00 | 0.00–0.75 | 0.057 |
| Hydroxyproline | 6.12 | 4.14–8.79 | 7.94 | 5.08–14.3 | 0.088 |
| Isoleucine | 55.6 | 46.1–63.7 | 64.4 | 48.8–81.5 | 0.061 |
| Leucine | 114 | 88.6–121 | 117 | 89.4–148 | 0.478 |
| Lysine | 189 | 158–224 | 182 | 152–232 | 0.884 |
| Methionine | 21.0 | 19.3–25.6 | 27.3 | 21.6–40.6 | 0.004 |
| 1‐methylhistidine | 0.54 | 0.09–0.99 | 0.68 | 0.00–1.49 | 0.882 |
| 3‐methylhistidine | 4.86 | 3.78–5.52 | 4.23 | 2.48–5.49 | 0.109 |
| Ornithine | 109 | 88.2–132 | 98.1 | 70.5–129 | 0.242 |
| Phenylalanine | 53.0 | 49.0–57.1 | 59.3 | 47.7–74.9 | 0.072 |
| O‐phosphoethanolamine | 1.49 | 0.93–1.94 | 1.64 | 1.10–2.27 | 0.242 |
| O‐phospho‐L‐serine | 5.45 | 4.42–6.29 | 0.00 | 0.00–0.61 | <0.001 |
| Proline | 142 | 127–185 | 162 | 123–231 | 0.258 |
| Sarcosine | 5.75 | 4.44–10.8 | 2.67 | 2.20–4.40 | <0.001 |
| Serine | 119 | 103–143 | 114 | 92.8–150 | 0.870 |
| Taurine | 52.5 | 41.1–60.9 | 55.6 | 42.2–79.1 | 0.300 |
| Threonine | 118 | 102–150 | 111 | 88.3–150 | 0.450 |
| Tryptophan | 43.2 | 39.4–46.1 | 43.6 | 35.7–52.7 | 0.662 |
| Tyrosine | 56.6 | 50.1–61.6 | 61.4 | 49.6–74.6 | 0.258 |
| Valine | 215 | 196–251 | 237 | 206–311 | 0.144 |
| Tyrosine/LNAA | 0.13 | 0.12–0.15 | 0.12 | 0.11–0.14 | 0.270 |
| Tryptophan/LNAA | 0.10 | 0.09–0.11 | 0.09 | 0.08–0.10 | 0.176 |
| Glutamine/glutamic acid | 11.8 | 8.87–16.7 | 10.9 | 8.10–15.0 | 0.228 |
| Serine/glycine | 0.52 | 0.48–0.56 | 0.50 | 0.41–0.57 | 0.313 |
Q1, lower quartile; Q3, upper quartile; LNAA, large neutral amino acid. a P‐values from Wilcoxon rank‐sum test. b P‐values from t‐test. c P‐values from Fisher's exact test.
Comparison Between Response and Nonresponse Groups at Baseline
The demographic and clinical characteristics according to the therapeutic response are summarized in Table 2. Baseline alpha‐aminobutyric acid (ABA) was higher in response group than in nonresponse group after adjustment of covariates (P = 0.017; odds ratio (OR), 1.12, 95% confidence interval (CI), 1.02–1.23; Figure 1). In ROC curve analysis of amino acids at baseline on the response, ABA (0.697) and aspartic acid (0.663) showed relatively high AUC of above 0.650 (Figure 1). In a combined ROC curve analysis of ABA and aspartic acid, the AUC value was 0.714.
Table 2.
Characteristics of response (n = 48) and nonresponse (n = 20) depressive patients to antidepressant treatment
| Characteristics | Patients (%) | P‐value | |
|---|---|---|---|
| Response | Nonresponse | ||
| Age, median (Q1–Q3), years | 65.0 (62.0–69.3) | 64.0 (58.5–71.0) | 0.439b |
| Sex, M:F | 12:36 | 4:16 | 0.762b |
| BMI, median (Q1–Q3) | 23.8 (22.3–26.0) | 24.4 (22.7–27.3) | 0.369c |
| Onset age, median (Q1–Q3), years | 59.5 (50.0–65.3) | 57.5 (50.0–61.5) | 0.839c |
| Episodes of MDD, median (Q1–Q3) | 2 (1–3) | 2 (1–2) | 0.390c |
| Antidepressant used | |||
| Fluoxetine | 25 (52.1) | 12 (60.0) | 0.602b |
| Othersa | 23 (47.9) | 8 (40.0) | |
| HAM–D score at baseline, median (Q1–Q3) | 18.5 (17.0–21.0) | 21.0 (19.0–22.3) | 0.033c |
| Family history | 11 (22.9) | 2 (10.0) | 0.317c |
| Comorbid conditions | |||
| Hypertension | 17 (35.4) | 7 (35.0) | 1.000b |
| Diabetes | 9 (18.8) | 4 (20.0) | 1.000b |
| Hyperlipidemia | 2 (4.2) | 0 (0.0) | 1.000b |
Q1, lower quartile; Q3, upper quartile; BMI, body mass index; MDD, major depressive disorder; HAM‐D, Hamilton rating scale for depression. aSSRIs including paroxetine, escitalopram, and sertraline. b P‐values from Fisher's exact test. c P‐values from Wilcoxon rank‐sum test.
Figure 1.
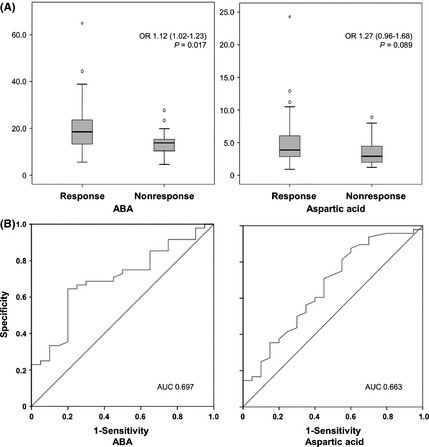
Differences of baseline amino acids between response and nonresponse groups in logistic regression analysis. (A) Alpha‐aminobutyric acid (ABA) and aspartic acid are higher in the response group than in the nonresponse group. P‐values are presented from multivariable analysis including clinical variables. (B) The receiver operating characteristic (ROC) curve analysis demonstrates the discrimination of the ABA and aspartic acid between response and nonresponse groups at baseline.
Changes of Amino Acid Concentrations and the Therapeutic Response
The ABA concentration was also decreased after antidepressant treatment in the response group, but not in the nonresponse group (median differences between before and after treatment: −3.8 vs. 2.2; P = 0.010). The association between the change of ABA concentration and the therapeutic response was significant when the effect of covariates (age, sex, antidepressant, and HAM‐D score) is controlled (P = 0.010), and the change was larger in the negative direction for the response group than the nonresponse group (ρ = −0.321). The changes of isoleucine (P = 0.024, ρ = −0.282), 1‐methylhistidine (P = 0.033, ρ = −0.267) and proline (P = 0.014, ρ = −0.306) concentrations, and tryptophan/large neutral amino acid (LNAA) ratio (P = 0.025, ρ = 0.281) showed the significant correlation with the therapeutic response according to antidepressant treatment after adjusting covariates.
Comparison between before and after SSRI treatment
Plasma concentrations of arginine (P = 0.039), asparagine (P = 0.017), cystine (P = 0.039), and glutamine/glutamic acid (P = 0.039) were increased at 6 weeks after the treatment, compared to the baseline in all patients (Table 3 and Figure 2). On the other hand, the median value of glutamic acid (59.81 vs. 48.02, P = 0.045 in response group; 57.30 vs. 55.52, P = 1.000 in nonresponse group) and the glutamine/glutamic acid ratio (10.5 vs. 12.8, P = 0.014 in response group; 13.1 vs. 13.2, P = 1.000 in nonresponse group) were significantly different between before and after treatment only in the response group.
Table 3.
Plasma concentrations of amino acids at baseline and at 6 weeks after the antidepressant treatment in depressive patients (n = 68)
| Amino acid (μmol/L) | At baseline | At 6 weeks | P‐valuea | ||
|---|---|---|---|---|---|
| Median | Q1–Q3 | Median | Q1–Q3 | ||
| Alanine | 446 | 351–540 | 434 | 357–599 | 0.851 |
| Beta‐alanine | 11.0 | 7.80–17.5 | 11.9 | 8.32–17.7 | 0.383 |
| Anserine | 0.00 | 0.00–4.73 | 0.00 | 0.00–4.77 | 0.760 |
| Aminoadipic acid | 0.85 | 0.58–1.12 | 0.74 | 0.57–1.16 | 0.829 |
| Alpha‐aminobutyric acid | 16.2 | 12.7–21.1 | 16.0 | 12.6–23.0 | 0.553 |
| Gamma‐aminobutyric acid | 1.16 | 0.00–2.01 | 1.15 | 0.00–1.89 | 0.194 |
| Beta‐aminoisobutyric acid | 1.12 | 0.27–1.92 | 1.07 | 0.00–1.96 | 0.795 |
| Arginine | 69.7 | 49.0–94.1 | 70.0 | 58.3–103 | 0.039 |
| Asparagine | 50.9 | 41.3–70.3 | 56.7 | 44.6–75.0 | 0.017 |
| Aspartic acid | 3.51 | 2.60–5.73 | 3.26 | 2.34–4.31 | 0.261 |
| Carnosine | 0.00 | 0.00–0.00 | 0.00 | 0.00–0.00 | 0.644 |
| Citrulline | 34.0 | 25.2–45.6 | 35.5 | 23.4–49.5 | 0.909 |
| Cystathionine | 0.00 | 0.00–0.00 | 0.00 | 0.00–0.00 | 0.570 |
| Cystine | 59.0 | 36.3–90.6 | 67.4 | 47.3–102 | 0.039 |
| Ethanolamine | 35.4 | 23.6–36.8 | 34.8 | 18.4–36.7 | 0.479 |
| Glutamic acid | 59.6 | 39.1–76.9 | 50.6 | 36.6–67.0 | 0.071 |
| Glutamine | 621 | 495–794 | 616 | 490–861 | 0.485b |
| Glycine | 251 | 186–298 | 241 | 176–383 | 0.231 |
| Histidine | 73.8 | 63.1–96.5 | 72.8 | 58.1–106 | 0.467 |
| Homocystine | 0.00 | 0.00–0.00 | 0.00 | 0.00–0.00 | 0.500 |
| 5‐hydroxylysine | 0.00 | 0.00–0.75 | 0.25 | 0.00–0.77 | 0.222 |
| Hydroxyproline | 7.94 | 5.08–14.3 | 7.65 | 5.01–12.5 | 0.995 |
| Isoleucine | 64.4 | 48.8–81.5 | 62.3 | 53.5–80.1 | 0.483 |
| Leucine | 117 | 89.4–148 | 114 | 92.4–136 | 0.590 |
| Lysine | 182 | 153–232 | 188 | 135–251 | 0.926b |
| Methionine | 27.3 | 21.6–40.6 | 29.0 | 23.1–42.8 | 0.832 |
| 1‐methylhistidine | 0.68 | 0.00–1.49 | 0.58 | 0.00–1.24 | 0.317 |
| 3‐methylhistidine | 4.23 | 2.48–5.49 | 3.49 | 2.50–5.20 | 0.718b |
| Ornithine | 98.1 | 70.5–129 | 95.9 | 67.5–136 | 0.672 |
| Phenylalanine | 59.3 | 47.7–74.9 | 58.9 | 45.9–77.8 | 0.541 |
| O‐phosphoethanolamine | 1.64 | 1.10–2.27 | 1.57 | 0.98–2.46 | 0.911 |
| O‐phospho‐L‐serine | 0.00 | 0.00–0.61 | 0.00 | 0.00–0.71 | 0.666 |
| Proline | 162 | 123–231 | 174 | 133–242 | 0.229 |
| Sarcosine | 2.67 | 2.20–4.40 | 2.93 | 2.10–5.53 | 0.204 |
| Serine | 114 | 92.8–150 | 122 | 90.6–172 | 0.545 |
| Taurine | 55.6 | 42.2–79.1 | 54.3 | 35.1–77.0 | 0.376 |
| Threonine | 111 | 88.3–150 | 113 | 91.7–160 | 0.467b |
| Tryptophan | 43.6 | 35.7–52.7 | 43.6 | 33.7–55.0 | 0.762 |
| Tyrosine | 61.4 | 49.6–74.6 | 61.2 | 47.6–80.7 | 0.431 |
| Valine | 237 | 206–311 | 245 | 196–296 | 0.957 |
| Tyrosine/LNAA | 0.12 | 0.11–0.14 | 0.13 | 0.11–0.14 | 0.400 |
| Tryptophan/LNAA | 0.09 | 0.08–0.10 | 0.09 | 0.08–0.10 | 0.603 |
| Glutamine/glutamic acid | 10.9 | 8.10–15.0 | 12.8 | 9.42–16.9 | 0.039 |
| Serine/glycine | 0.50 | 0.41–0.57 | 0.51 | 0.41–0.56 | 0.725b |
Q1, lower quartile; Q3, upper quartile; LNAA, large neutral amino acid. a P‐values from Wilcoxon rank‐sum test. b P‐values from t‐test.
Figure 2.
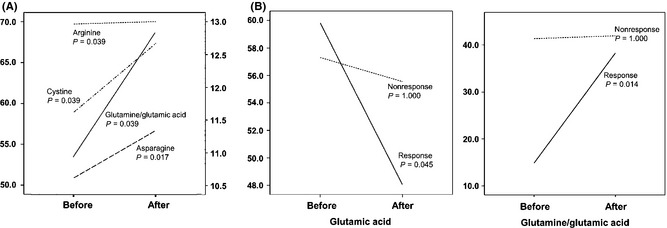
Comparison of amino acid concentrations between before and after SSRI treatment. (A) Arginine, asparagine, cystine (median value, left scale), and glutamine/glutamic acid ratio (median value, right scale) were increased after antidepressant treatment in Wilcoxon signed rank test. (B) Glutamic acid and glutamine/glutamic acid ratio were significantly changed after antidepressant treatment only in the response group, and not in the nonresponse group.
Discussion
In this study, we performed amino acid profiling to identify the alteration of plasma amino acid concentrations in patients with MDD. ABA was differentially expressed at baseline between response and nonresponse groups. In comparisons between patients and healthy individuals, ten amino acids showed differences. Plasma glutamic acid concentration and glutamine/glutamic acid ratio were different between before and after SSRI treatment only in the response group.
Alpha‐aminobutyric acid is a catabolic product derived from 2‐ketobutyrate (2KB) which is a metabolite from the metabolism of methionine, threonine, serine, and glycine 1, 33, 34. The increase of ABA has been considered as a general marker of various conditions including malnutrition, protein, catabolic status, sepsis, liver disease, and multiple organ failure 33, 35. In our study, the high ABA in patients with MDD compared to healthy individuals and the decreased concentration after the antidepressant treatment in the response group compared to nonresponse group may reflect the chronic catabolic status in MDD caused by the poor appetite which is a common symptom of MDD. Another possibility is that the alteration of ABA may be caused by the impairment and restoration of the entrance of 2KB to tricarboxylic acid cycle, which would imply a disturbance and a normalization of energy metabolism, and the hypometabolic state. This hypothesis was previously suggested for pathogenesis in MDD 4, 33, 36, 37, 38. An interesting finding is the differences in ABA concentrations at baseline between response and nonresponse groups. Although the discrimination power of ABA was modest, ROC curve analyses show the potential of ABA to discriminate responsive patients from nonresponsive patients. The significance of the effect of ABA on the therapeutic response was maintained after adjustment with clinical variables, including HAM‐D score at baseline; thus, this finding is not caused by differences in severity of MDD. To clarify that ABA could be an indicator to predict therapeutic response in patients with MDD treated with SSRI, further study including patients treated with other types of antidepressants would be needed.
The phospholipid is an important constituent of mitochondrial membrane; it is a product from phosphoethanolamine that is produced by the phosphorylation of ethanolamine by ethanolamine kinase 38, 39, 40. The increase of ethanolamine and phosphoethanolamine has been reported in psychiatric disorders including MDD, and the increase of these metabolites can be caused by enzyme defects such as ethanolamine‐phosphate phosphorylase 38, 40, 41, 42. The mitochondrial dysfunction due to the disturbance of phospholipid metabolism might result in an alteration in energy production 38, which is correlated with the finding of elevated ABA in the present study. Brain is a highly energy‐dependent tissue, in the same context, several studies have been reported that patients with mitochondrial disorders present with neuropsychiatric manifestations 43, 44. These findings and our study results support the previous hypothesis that the disturbance of energy metabolism caused by various etiologies, including mitochondrial dysfunction, may be involved in the development of MDD 38, 45, 46, 47. Alteration of excitatory neurotransmitter amino acids, including glutamic acid and aspartic acid, was also observed in the present study. Previous studies showed the increase of these excitatory amino acids in MDD and a positive correlation between glutamic acid concentration and HAM‐D score 21, 29, 48, 49. Glutamatergic abnormalities in MDD have been reported to have a role in pathogenesis of MDD, and the efficacy of glutamatergic antidepressants including N‐methyl‐d‐aspartate (NMDA) receptor antagonists has been demonstrated in MDD 50, 51, 52. The alteration of glutamic acid in our study could reflect the glutamatergic abnormalities in MDD. Consistent with previous studies, we observed elevated concentration of glutamic acid in MDD and decreases after treatment in the present study. The glutamic acid and glutamine/glutamic acid ratio were recovered after antidepressant treatment only in the response group. Although these amino acids are not primary targets of SSRIs, these findings would imply that the metabolic disturbance in patients with MDD could be recovered by SSRIs treatment and support previous studies that SSRIs also influence other systems including glutamatergic neurons 53, 54. The disturbances of other amino acids were also observed in patients with MDD. Among these findings, we observed a low concentration of beta‐aminoisobutyric acid in MDD, which was previously reported to have an inverse correlation with the severity of MDD 26. We also observed a difference of alanine between patients and healthy individuals, which previously showed a positive correlation with HAM‐D scores in patients with MDD and alterations in the brain in stressed rats 27, 49.
We precisely measured 40 amino acids using internal standards for each compound in a relatively large number of patients comparing to previous studies. Our results showed alterations of amino acids on various pathways in patients with MDD compared to healthy individuals. This finding corresponds with previous literature reporting that MDD is a multifactorial disorder, and various etiologies, including energy metabolism and mitochondrial dysfunction, seem to be involved in the development of MDD 3, 38, 45, 55. However, we should acknowledge the limitations in this study. We could not identify amino acids that are consistently altered in all of the comparison and prediction analyses. Most findings correspond with previous results, but some were discordant. These inconsistencies could be caused by the limited sample size, and the differences of the analytic methods and the antidepressants. The high proportion of females among the participants in our study could act as a confounding factor. And identical to previous studies, it was not possible to define whether each metabolic disturbance is the cause or result of MDD. Although our study presents the disturbance of amino acid metabolism, we did not measure some of the metabolites and enzymes on interesting pathways because our study was designed to perform general amino acid profiling. Unfortunately, we did not measure the plasma or serum concentration of serotonin which is a key neurotransmitter on the pharmacological action of SSRI. Previous studies reported that the peripheral serotonin concentration was lower in patients with MDD than in healthy individuals, and it was drastically decreased after SSRI treatment 56, 57, 58. Further in‐depth analysis is needed of the metabolites and enzymes on the pathways on which alterations of amino acids are found. The measurement of serotonin concentration will contribute in a comprehensive understanding of the changes in amino acids including tryptophan which has a direct relationship with serotonin.
In conclusion, the significance of this study is that we not only have identified alterations of amino acids between patients with MDD and healthy individuals, but we also have demonstrated changes of amino acids after SSRI treatment and differences at baseline associated with responsiveness to antidepressant treatment. These findings indicate that disarrangement and restoration of systemic metabolic status are involved in the development and improvement of MDD. In particular, metabolites on various pathways of amino acids metabolism, including ABA and glutamic acid, have potential to increase understandings of pathogenesis and predictions of therapeutic response in MDD.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A110339).
Drs. Kim and Lee contributed equally to this work.
References
- 1. Ferrari AJ, Somerville AJ, Baxter AJ, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med 2013;43:471–481. [DOI] [PubMed] [Google Scholar]
- 2. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
- 3. Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008;358:55–68. [DOI] [PubMed] [Google Scholar]
- 4. Leonard B, Maes M. Mechanistic explanations how cell‐mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012;36:764–785. [DOI] [PubMed] [Google Scholar]
- 5. Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM, National Birth Defects Prevention S . Use of selective serotonin‐reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007;356:2684–2692. [DOI] [PubMed] [Google Scholar]
- 6. Mann JJ. The medical management of depression. N Engl J Med 2005;353:1819–1834. [DOI] [PubMed] [Google Scholar]
- 7. Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov 2005;4:764–774. [DOI] [PubMed] [Google Scholar]
- 8. Nierenberg AA, Farabaugh AH, Alpert JE, et al. Timing of onset of antidepressant response with fluoxetine treatment. Am J Psychiatry 2000;157:1423–1428. [DOI] [PubMed] [Google Scholar]
- 9. Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta‐analysis of studies of newer agents. Biol Psychiatry 2007;62:1217–1227. [DOI] [PubMed] [Google Scholar]
- 10. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163:28–40. [DOI] [PubMed] [Google Scholar]
- 11. Gartlehner G, Hansen RA, Morgan LC, et al. Second‐Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality, 2011. [PubMed] [Google Scholar]
- 12. Mulder RT, Frampton CM, Luty SE, Joyce PR. Eighteen months of drug treatment for depression: predicting relapse and recovery. J Affect Disord 2009;114:263–270. [DOI] [PubMed] [Google Scholar]
- 13. Dunlop BW, Holland P, Bao W, Ninan PT, Keller MB. Recovery and subsequent recurrence in patients with recurrent major depressive disorder. J Psychiatr Res 2012;46:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Machado‐Vieira R, Ibrahim L, Zarate CA Jr. Histone deacetylases and mood disorders: epigenetic programming in gene‐environment interactions. CNS Neurosci Ther 2011;17:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wingenfeld K, Wolf OT. HPA axis alterations in mental disorders: impact on memory and its relevance for therapeutic interventions. CNS Neurosci Ther 2011;17:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cattaneo A, Gennarelli M, Uher R, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology 2013;38:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaddurah‐Daouk R, Krishnan KR. Metabolomics: a global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology 2009;34:173–186. [DOI] [PubMed] [Google Scholar]
- 18. Kaddurah‐Daouk R, Weinshilboum RM. Pharmacometabolomics: implications for clinical pharmacology and systems pharmacology. Clin Pharmacol Ther 2014;95:154–167. [DOI] [PubMed] [Google Scholar]
- 19. Quinones MP, Kaddurah‐Daouk R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol Dis 2009;35:165–176. [DOI] [PubMed] [Google Scholar]
- 20. Rhee EP, Gerszten RE. Metabolomics and cardiovascular biomarker discovery. Clin Chem 2012;58:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol 1995;5(Suppl):71–75. [DOI] [PubMed] [Google Scholar]
- 22. Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Do KQ, Lauer CJ, Schreiber W, et al. gamma‐Glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug‐naive patients with schizophrenic disorders. J Neurochem 1995;65:2652–2662. [DOI] [PubMed] [Google Scholar]
- 24. Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG. Free amino acid and dipeptide changes in the body fluids from Alzheimer's disease subjects. Amino Acids 2007;32:213–224. [DOI] [PubMed] [Google Scholar]
- 25. Leichtle AB, Nuoffer JM, Ceglarek U, et al. Serum amino acid profiles and their alterations in colorectal cancer. Metabolomics 2012;8:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu HB, Fang L, Hu ZC, et al. Potential clinical utility of plasma amino acid profiling in the detection of major depressive disorder. Psychiatry Res 2012;200:1054–1057. [DOI] [PubMed] [Google Scholar]
- 27. Ni Y, Su M, Lin J, et al. Metabolic profiling reveals disorder of amino acid metabolism in four brain regions from a rat model of chronic unpredictable mild stress. FEBS Lett 2008;582:2627–2636. [DOI] [PubMed] [Google Scholar]
- 28. Mauri MC, Boscati L, Volonteri LS, et al. Predictive value of amino acids in the treatment of major depression with fluvoxamine. Neuropsychobiology 2001;44:134–138. [DOI] [PubMed] [Google Scholar]
- 29. Palmio J, Huuhka M, Saransaari P, et al. Changes in plasma amino acids after electroconvulsive therapy of depressed patients. Psychiatry Res 2005;137:183–190. [DOI] [PubMed] [Google Scholar]
- 30. Held PK, White L, Pasquali M. Quantitative urine amino acid analysis using liquid chromatography tandem mass spectrometry and aTRAQ reagents. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:2695–2703. [DOI] [PubMed] [Google Scholar]
- 31. Johnson DW. Free amino acid quantification by LC‐MS/MS using derivatization generated isotope‐labelled standards. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:1345–1352. [DOI] [PubMed] [Google Scholar]
- 32. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chiarla C, Giovannini I, Siegel JH. Characterization of alpha‐amino‐n‐butyric acid correlations in sepsis. Transl Res 2011;158:328–333. [DOI] [PubMed] [Google Scholar]
- 34. Yudkoff M, Blazer‐Yost B, Cohn R, Segal S. On the clinical significance of the plasma alpha‐amino‐n‐butyric acid:leucine ratio. Am J Clin Nutr 1979;32:282–285. [DOI] [PubMed] [Google Scholar]
- 35. Effros RM. Alpha aminobutyric acid, an alternative measure of hepatic injury in sepsis? Transl Res 2011;158:326–327. [DOI] [PubMed] [Google Scholar]
- 36. Behr GA, Moreira JC, Frey BN. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid Med Cell Longev 2012;2012:609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martins‐de‐Souza D, Guest PC, Harris LW, et al. Identification of proteomic signatures associated with depression and psychotic depression in post‐mortem brains from major depression patients. Transl Psychiatry 2012;2:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Modica‐Napolitano JS, Renshaw PF. Ethanolamine and phosphoethanolamine inhibit mitochondrial function in vitro: implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol Psychiatry 2004;55:273–277. [DOI] [PubMed] [Google Scholar]
- 39. Lykidis A, Wang J, Karim MA, Jackowski S. Overexpression of a mammalian ethanolamine‐specific kinase accelerates the CDP‐ethanolamine pathway. J Biol Chem 2001;276:2174–2179. [DOI] [PubMed] [Google Scholar]
- 40. Veiga‐da‐Cunha M, Hadi F, Balligand T, Stroobant V, Van Schaftingen E. Molecular identification of hydroxylysine kinase and of ammoniophospholyases acting on 5‐phosphohydroxy‐L‐lysine and phosphoethanolamine. J Biol Chem 2012;287:7246–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim S, Choi KH, Baykiz AF, Gershenfeld HK. Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post‐mortem prefrontal cortex. BMC Genom 2007;8:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sugden C. One‐carbon metabolism in psychiatric illness. Nutr Res Rev 2006;19:117–136. [DOI] [PubMed] [Google Scholar]
- 43. Anglin RE, Garside SL, Tarnopolsky MA, Mazurek MF, Rosebush PI. The psychiatric manifestations of mitochondrial disorders: a case and review of the literature. J Clin Psychiatry 2012;73:506–512. [DOI] [PubMed] [Google Scholar]
- 44. Marazziti D, Baroni S, Picchetti M, et al. Mitochondrial alterations and neuropsychiatric disorders. Curr Med Chem 2011;18:4715–4721. [DOI] [PubMed] [Google Scholar]
- 45. Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. J Affect Disord 2013;148:12–27. [DOI] [PubMed] [Google Scholar]
- 46. Zubenko GS, Hughes HB 3rd, Jordan RM, Lyons‐Weiler J, Cohen BM. Differential hippocampal gene expression and pathway analysis in an etiology‐based mouse model of major depressive disorder. Am J Med Genet B Neuropsychiatr Genet 2014;165B:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:730–743. [DOI] [PubMed] [Google Scholar]
- 48. Mauri MC, Ferrara A, Boscati L, et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 1998;37:124–129. [DOI] [PubMed] [Google Scholar]
- 49. Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:1155–1158. [DOI] [PubMed] [Google Scholar]
- 50. Krystal JH, Sanacora G, Blumberg H, et al. Glutamate and GABA systems as targets for novel antidepressant and mood‐stabilizing treatments. Mol Psychiatry 2002;7(Suppl 1):S71–S80. [DOI] [PubMed] [Google Scholar]
- 51. McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta‐analysis of randomized, double‐blind, placebo‐controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 2014;10:1–12. [DOI] [PubMed] [Google Scholar]
- 52. Serafini G, Pompili M, Innamorati M, Dwivedi Y, Brahmachari G, Girardi P. Pharmacological properties of glutamatergic drugs targeting NMDA receptors and their application in major depression. Curr Pharm Des 2013;19:1898–1922. [DOI] [PubMed] [Google Scholar]
- 53. Wang SJ, Su CF, Kuo YH. Fluoxetine depresses glutamate exocytosis in the rat cerebrocortical nerve terminals (synaptosomes) via inhibition of P/Q‐type Ca2 + channels. Synapse 2003;48:170–177. [DOI] [PubMed] [Google Scholar]
- 54. Schipke CG, Heuser I, Peters O. Antidepressants act on glial cells: SSRIs and serotonin elicit astrocyte calcium signaling in the mouse prefrontal cortex. J Psychiatr Res 2011;45:242–248. [DOI] [PubMed] [Google Scholar]
- 55. Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:676–692. [DOI] [PubMed] [Google Scholar]
- 56. Karege F, Widmer J, Bovier P, Gaillard JM. Platelet serotonin and plasma tryptophan in depressed patients: effect of drug treatment and clinical outcome. Neuropsychopharmacology 1994;10:207–214. [DOI] [PubMed] [Google Scholar]
- 57. Urbina M, Pineda S, Piñango L, Carreira I, Lima L. [3H]Paroxetine binding to human peripheral lymphocyte membranes of patients with major depression before and after treatment with fluoxetine. Int J Immunopharmacol 1999;21:631–646. [DOI] [PubMed] [Google Scholar]
- 58. Zoga M, Oulis P, Chatzipanagiotou S, et al. Indoleamine 2,3‐dioxygenase and immune changes under antidepressive treatment in major depression in females. In Vivo 2014;28:633–638. [PubMed] [Google Scholar]


