Abstract
Abstract. Strategies to generate differentiated cells from haematopoetic progenitor cells will enhance potential use of adult stem cells for therapeutic transplantation or tissue engineering. Transplantation of undifferentiated stem cells into recipient tissue hinges on the hypothesis of a milieu dependent differentiation and it has been suggested that a clot‐equivalent scaffold is crucial for these circulating cells to anchor and multiply. Here a natural scaffold, fibrin along with fibronectin, gelatin and growth factors has been used to induce endothelial progenitor cells and smooth muscle progenitor cells to differentiate into endothelial cells and smooth muscle cells, respectively, from peripheral blood mononuclear cells. Characteristics of endothelial cells have been verified by the detection of mRNA for and immunostaining the cells for von Willebrand factor, uptake of acetylated low‐density lipoproteins and measurement of released nitric oxide in the culture medium, as nitrite. The specific molecules that characterized smooth muscle cells were alpha smooth muscle actin and calponin, besides deposition of collagen type I and elastin, onto the culture matrix. The adhesive proteins used for the fabrication of endothelial progenitor cells matrix and smooth muscle progenitor cells matrix were the same, but specific differentiation was brought about by modulating the growth factor composition in the matrix and in the culture medium. Both endothelial and smooth muscle cells were consistently developed from 20 ml of human blood.
INTRODUCTION
There is a bright future for cell transplantation for repair and/or regeneration of injured cardiovascular tissues. Direct injection or transplantation of cells may effectively restore small areas of damage, but to reconstruct severe damage to injured tissue, resulting from major coronary artery blockage, for example, will require extensive therapy with numerous differentiated cells. Other than cell‐based therapy, creating replacement structures and organs using tissue engineering offers an exciting alternative to existing technologies in the treatment of cardiovascular diseases (Rezai et al. 2003). The major limitation in these kinds of approach is the availability of appropriate cells.
It is now known that there are somatic stem cells that mobilize to remote damaged tissue sites, where they differentiate into required lineages and participate in organ repair and regeneration (Asahara 1997; McKay 2000; Orlic et al. 2001). Recent investigations have demonstrated that circulating bone marrow (BM)‐derived endothelial progenitor cells (EPC) play an important role in neoangiogenesis of ischaemic tissue and endothelial cell (EC) repair following the damage (Asahara et al. 1999; Crosby et al. 2000). Walter and co‐workers (Walter et al. 2002) demonstrated that circulating endothelial cells can adhere to denuded parts of an artery after balloon angioplasty. Data supporting a paradigm for circulating smooth muscle progenitor cells (SMPC) with the potential for differentiation, homing and proliferation at sites rich in extra cellular matrix proteins, such as fibronectin, were also reported (Walter et al. 2002). It has been observed that BM‐derived cells did not express markers of smooth muscle cells (SMCs) and ECs when they attached to the luminal side of an artery 1 week after mechanical injury (Sata et al. 2002). Furthermore, it was reported that certain blood stem cell populations harbour progenitors that have the potential to differentiate into either ECs or SMPCs in vitro, depending on the composition of the culture medium (Kaushal et al. 2001). Thus, it is likely that immature cells exhibiting plasticity may be caused to differentiate in vitro using mechanical and/or humoral stimuli, and eventually may be used for therapeutic applications. Establishment of a contractile SMC culture would facilitate studies of smooth muscle differentiation and control of SMC contraction. Proteins such as alpha smooth muscle actin (α‐SMA), tropomyosin, caldesmin and calponin are also important in determining SMC contractility (Sobue et al. 1998).
Un‐fractionated mononuclear cells (MNCs) may be cultured in medium enriched with endothelial‐specific growth factors such as vascular endothelial growth factor (VEGF) (Griese et al. 2003), yet Yoon et al. (2004) recently reported that injection of total BM cells into the heart of infarcted rats could potentially lead to severe intramyocardial calcification. In contrast, animals receiving the same number of clonally expanded BM cells have not shown myocardial calcification. Thus, this finding brings attention to the potential risks of transplanting unselected BM cells and cautions against their premature use in the clinical setting.
These findings provide the basis for the development of new therapeutic strategies for vascular diseases, by targeting mobilization, homing, differentiation and proliferation of BM‐derived vascular progenitor cells. In order to translate this principle into clinical use efficiently, it is crucial to build up competent in vitro methods that produce adequate numbers of differentiated cells. Apparently, homing signals for circulating stem or progenitor cells mostly result from local injury and guide them presumably to the target tissue (Werner et al. 2003). It is suggested that interaction of EPCs with growth and differentiation factors may finally lead to the activation of tissue‐specific genes, resulting in a cell phenotype of the host organ. In the body, fibrin forms a cohesive network of haemostatic plugs and thrombi at injured sites of blood vessels and provides the temporary matrix for initial support of healing and of neovascularization. Our group has already shown that in vitro use of a biomimetic composite that consists of fibrin, fibronectin, gelatin and various growth factors is effective for maintaining cell proliferation potential, and for reducing apoptosis and dedifferentiation of human umbilical vein endothelial cells (HUVEC) even after continuous passaging (Chennuzhy & Krishnan 2005).
The object of this study was to provide biomimetic mechanical and humoral homing signals to progenitor cells from circulating blood to promote their differentiation into ECs and SMCs in vitro. Matrix and medium compositions were altered appropriately to allow differentiation of adult stem cells into SMC and EC.
MATERIALS AND METHODS
Gelatin, gluteraldehyde, Histopaque 1077 and heparin were purchased from Sigma Chemicals (St. Louis, MO, USA); M199, MCDB 131 culture medium, trypsin‐ethylenediamenetetraacetic acid (EDTA), antibiotics and ascorbic acid were from Gibco BRL (Grand Isle, NY, USA). A mixture of fibroblast growth factor (FGF) and VEGF was prepared from bovine hypothalamus according to an established method (Maciag et al. 1979) denoted as endothelial cell growth factor (ECGF). Platelet growth factor (PGF) was prepared as described earlier (Resmi & Krishnan 2002). Foetal bovine serum (FBS) was from Gibco BRL, USA. The complete culture medium contained 20% FBS, 50 IU/ml heparin, 5 µg/ml ECGF, 1 µg/ml ascorbic acid, 0.8 µg/ml PGF and 40 µl/ml antibiotics.
Isolation of peripheral blood mononuclear cells
Twenty millilitres of blood was collected from volunteers (laboratory personnel) after obtaining informed consent, using plastic syringes containing 500 USP heparin. Peripheral blood mononuclear cells (PBMNs) were isolated by Histopaque‐1077 density gradient centrifugation. Briefly, red blood cells (RBCs) were settled by centrifugation at 280 g for 15 min in 15‐ml centrifuge tubes using a Hareus Stratos centrifuge (Hareus, Germany). Superficial plasma was discarded; PBMNCs with red blood cells (RBCs) at the interface were collected and diluted with equal volumes of M199 to make up the volume to 8 ml, mixed well and were layered over 7 ml Histopaque‐1077 and were centrifuged at 400 g for 30 min at 25 °C. The layer containing PBMNCs was carefully separated from the plasma‐Histopaque interface and was washed with serum‐free M199 by centrifugation at 250 g at 4 °C for 10 min. The washed PBMNCs were suspended in complete medium MCDB131.
Cell culture
NUNC (Raskilde, Denmark) culture plates were coated with fibrin composite as described earlier (Chennuzhy & Krishnan 2005) with some modification. For making the EPC matrix, cryoprecipitate was added with 50 µg/ml endothelial growth factor, whereas for making SMPC matrix, 40 µg/ml PGF was added. Both types of dish were simultaneously used for culture of PBMNCs from the same donor. The PBMNCs suspended in complete medium were initially seeded in an uncoated 10‐cm2 culture dish and were incubated for 1 h in a humidified incubator under 5% CO2 at 37 °C. After 1 h, the medium was removed gently and unattached cells were collected in freshly added complete medium. The contents were then divided into two parts; one part was added to the EPC matrix and the other was added to the SMPC matrix. After 24 h, the medium was mixed mildly and aspirated to remove any remaining contaminating RBCs and floating cells, the medium was replaced with fresh ones each day for 72 h; afterwards, the media were changed on alternate days. At confluence, cells were harvested with 0.25% trypsin‐EDTA (Gibco, BRL) from composite‐coated dishes and were subcultured into fresh coated dishes. From the second passage onwards, the medium used was M199 with 10% FCS and either ECGF or PGF.
Characterization of EPCs
The EC phenotype was confirmed by analysis of acetylated low‐density lipoprotein (Ac‐LDL) uptake, cellular expression of von Willebrand factor (vWF), and nitric oxide (NO) release.
Cellular uptake of Ac‐LDL
Uptake of Ac‐LDL labelled with the fluorescent probe DiI (Molecular Probes, Eugene, OR, USA) was detected using a fluorescence microscope (DM IRB, Leica, Wetzlar, Germany) to evaluate cell identity. Briefly, the cells were grown in four‐well composite‐coated plates, washed with PBS, and were incubated with 2 µg/ml DiI for 4 h at 37 °C. The cells were then washed with PBS once more and were viewed by fluorescence microscopy (Leica, DM IRB). The cells from the second and third passages were analysed.
Immunostaining for vWF
Endothelial progenitor cells from the second passage that had been grown in composite‐coated four‐well plates, were fixed with 3.7% paraformaldehyde in PBS, were washed with PBS, quenched for 20 min with 0.27% NH4Cl/0.38% glycine in PBS and were permeabilized with Triton X‐100 (0.1%) in PBS. vWF was developed by incubation with fluorescein isothiocyanate (FITC)‐conjugated mouse anti‐human vWF antibody (2 µg/ml). The cells were then washed once more with PBS and were viewed using a fluorescence microscope (Leica, DM IRB). The cells from the second and third passages were analysed.
vWF m‐RNA expression
RNA was isolated from the third and fifth passage EPCs using an isolation kit (Stratagene, USA) according to the manufacturer's instructions. After purification, 20 ng of RNA (quantified using HP Diode array spectrophotometer) was utilized for the reverse transcriptase‐polymerase chain reaction (RT‐PCR), using commercially available kits (Stratagene, USA). After reverse transcription, PCR was performed in the same tube (at 94 °C for 1 min, at 60 °C for 45 s, and at 72 °C for 1 min), for 40 cycles on a mini PCR system (Eppendorf, Germany). The following primers were used:
Forward – 5′‐CACCATTCAGCTAAGAGGAGG‐3′
Reverse – 5′‐GCCCTGGCAGTAGTGGATA‐3′
Assessment of NO synthesis
Nitrite, which is the stable metabolite of NO, was estimated by use of the Griess reagent (Sigma, USA). For this assay, EPCs grown on a composite‐coated four‐well plate, at confluence were used. To eliminate protein interference, complete medium from the culture wells was removed 24 h before estimation, and fresh serum‐free, phenol red‐free M199 medium (Sigma, USA) was added. After 24 h incubation, the medium was collected and centrifuged to remove particulates if there were any. Samples were then added to an equal volume of Griess reagent and were incubated for 15 min at room temperature; colour developed was read at 540 nm using a diode array spectrophotometer (Hewlett Packard 8053, USA). For quantification of nitrite, a standard curve was prepared from known concentrations of sodium nitrite (0–4.0 µmol/ml) with the help of the software chemstation (Hewlett Packard).
Characterization of SMPCs
Immunostaining for α‐SMA and calponin
SMPCs from the second passage that were grown in composite‐coated four‐well plates were fixed with 3.7% paraformaldehyde in PBS, and were washed with PBS. Cells were then permeabilized with 0.1% Triton X‐100 for 3 min then washed three times with PBS. Smooth muscle actin (SMA) and calponin were identified by incubation of fixed cells with antihuman antibodies (2 µg/ml) against each antigen, in separate wells. Anti–α‐SMA antibody was obtained from Dakocytomation (Denmark) and anticalponin antibodies were from Novo Castra (UK). For staining of both, the manufacturer's instructions were followed to develop the protocol. Anti–α‐SMA treated cells were then incubated with FITC‐conjugated secondary antibody and anticalponin‐treated cells were incubated with tetramethyl rhodamine isothiocyanate (TRITC)‐conjugated secondary antibody (Bangalore Genie, India). After immunostaining, the nuclei were developed using a dilute (5 µm) solution of Sytox Blue obtained from Molecular Probe, USA. Samples were viewed by confocal microscopy (Zeiss LSM 510 Meta). The excitation wavelength for FITC was 488 nm and the emission wavelength was 520 nm. For TRITC, the excitation wavelength was 543 nm and the emission wavelength was 572 nm. For Sytox Blue, the excitation used was 430 nm and emission 480 nm.
Recovery of matrices and staining
SMCs in the third passage were allowed to grow on fibrin‐composite matrix for 120 h then the culture matrix (ECM) was recovered by washing the monolayer with PBS and incubating with 0.1% Triton X‐100 in PBS for 20 min. The matrices were washed with PBS and then were fixed with 3.7% paraformaldehyde. Immunostaining was performed using antibodies against elastin and type I collagen. Monoclonal antibodies against human type I collagen (Sigma, USA), and against human elastin (Novocastra, UK) were used and horseradish peroxidase‐conjugated secondary antibodies (Bangalore Genie, India) bound to primary antibodies were detected using the substrate di‐aminobenzidine. Stained matrices were viewed using a conventional light microscope (Lieca DMIRB).
RESULTS
No progenitor cell identification nor sorting of PBMNCs obtained by Ficoll gradient centrifugation were performed before initial culture of the stem cells. In vivo, it would be anticipated that the adult stem cells would home to the injured site where the natural matrix would support their adhesion and the milieu would be perfect for their differentiation. Thus, from the mixed population of PBMNCs of this experiment, the progenitor cells were expected to selectively bind to the biomimetic matrix provided in the culture dishes. With CD34 as stem cell marker, sorting the PBMNCs using a cell sorter (FACS Aria, BD Science) provided a low yield, 0.05%, of CD34+ve cells from the complete population (data not shown). Therefore, we used the mixed‐cell population to culture ECs and SMCs.
For 2 days after seeding PBMNCs, they maintained a round morphology (Fig. 1a,b), on both EPC and SMPC matrices with several clusters of cells distributed throughout the culture area. However, by day 5, distinctions between the cells on the EPC and SMPC matrices were evident; EPCs that seemed to sprout from clusters of cells were much shorter (Fig. 1c) compared to sprouting SMPCs (Fig. 1d). Divergence between EPC and SMPC morphology became more apparent by day 9 (Fig. 1e,f). By day 14, culture areas were more or less covered with clusters of cells, grown into bundles, both on EPC and SMPC matrices, with distinct morphologies from each other (Fig. 1g,h). Thick bundles were seen at different locations of the culture areas; however, some cell‐free or partially filled areas were also seen in the dishes. At this time, the cells could be harvested and subcultured to a larger space. If cell populations were allowed to remain in the culture without splitting, the cells became closely packed into thick bundles (Fig. 1i,j). Once successfully sprouted, cell populations were harvested (using trypsin) and plated in a fresh dish coated with fibrin composite, single elongated cells were seen in 2 h and by a further 72 h they formed monolayers of cells with characteristic morphology for ECs and alternatively for SMPCs, as seen in Fig. 1(k,l), respectively. Evenly distributed monolayers were formed only after subculture, and sometimes it was possible to passage the initial culture as early as day 15. From passage 2 onwards, cells multiplied exponentially; a 1 : 3 split was possible after every 72 h and the cells showed a compressed cobblestone morphology in the case of EPCs and typical, elongated, hill‐and‐valley morphology for SMPCs.
Figure 1.


Light and phase contrast micrographs of PBMNS at various stages of growth in composite‐coated culture dishes. All figures in the left panel are cells on EPC matrix and those in the right panel are cells on SMPC matrix. a and b, day 2; c and d day 5; e and f day 9; g and h day 14; i and j day 20; k and l, subculture (second passage) 72 h after splitting. Magnification: 30×.
Isolation and culture of PBMNCs is likely to result in differentiation of progenitor cells into other cell types. ECs were found to express anti‐vWF, as discovered by immunostaining; Weibel‐Palade bodies appeared as small rod‐shaped structures in the cytoplasm; the nuclei were found as distinct unstained areas (Fig. 2a). Immunostained EPCs from the second passage and differentiated SMPCs from the fourth passage did not express vWF (Fig. 2b,c), their cytoplasm appearing with a light green background and without any granular structures. In addition, Ac‐LDL was taken up by cells from the third passage, into the cytoplasm, demarcating the negative nucleus (Fig. 2d). This is a positive indication of endothelial phenotype of the differentiated progenitor cells. Furthermore, RT‐PCR for vWF indicated its expression (Fig. 2e). Results shown are of third and fifth passages from replicate cell isolations. Expression of vWF in ECs from the second passage was very low, almost undetectable after 40 cycles PCR (Fig. 2f), whereas from passages 3 onwards, significant bands were visible after 30 cycles of amplification. In the fifth passage, the intensity of the bands was higher (Fig. 2e. lanes 1,2,3), compared to those in the third passage (Fig. 2e. lanes 5,6,7).
Figure 2.

Data showing the phenotypic characterization of ECs. (a) Third‐passage EPCs; (b) second‐passage EPCs; and (c) fourth‐passage SMPCs stained with FITC conjugated antivWF. (d) EPCs that took up Ac‐LDL (DiI‐labelled); (e) RT‐PCR (30 cycles) data showing bands corresponding to amplified vWF in PCR: lanes 1, 2 and 3 are of cells from fifth passage, lane 4, marker (arrow indicating 300 bp MW), lanes 5,6 and 7 are of cells from the third passage; (f) RT‐PCR(40 cycles) from the second passage. Lane 4, marker (arrow indicating 300 bp MW) lanes 1–3 and 5–7 are with RNA isolated from a different batch of cell isolation. Magnification of all micrographs is 40×.
The synthesis of nitric oxide by the growing ECs and its release into the culture medium was stronger evidence of the endothelial phenotype derived from these progenitor cells. Cells from different donors were used to study NO synthesis and an average of 1.26 ± 0.42 µm (n = 8) was detected in the medium that contained approximately 0.2 × 106 cells in each well.
Smooth muscle cell markers used for verification of the SMC phenotype were α‐SMA specific actin and calponin. Cells grown on SMPC matrix expressed SMC‐specific α‐SMA and calponin from the second passage of cells (Fig. 3a,b) onwards. α‐SMA was seen clearly as filaments through the cells. As α‐SMA is known to be expressed in other cell types, calponin staining was performed and appeared relatively faint compared to α‐SMA. ECs were used as controls for α‐SMA and calponin staining and were found to not express these proteins (data not shown).
Figure 3.
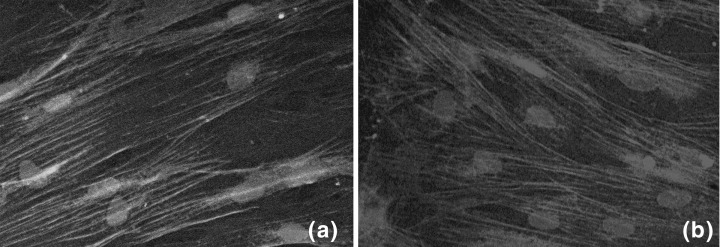
Data showing the phenotypic characterization of SMCs. (a) α‐SMA stained SMPC from the fourth passage; (b) calponin‐stained SMPC from the fourth passage and in both cases nuclei were developed with Sytox Blue. All images by confocal microscopy. Magnification, 60×.
Differentiated SMCs were found to synthesize both type I collagen and elastin (Fig. 4a,b), identifying the contractile phenotype of the cells. Collagen is seen as fibrils and elastin as aggregates on the matrix after cell digestion. Thus, the evidence is supportive of selective adhesion of these progenitor cell derivatives to the fibrin composite matrix and the differentiation of the attached cells to ECs and SMCs, depending on growth factors supplemented in the medium and on the matrix.
Figure 4.

Identification of elastin and type I collagen on matrix recovered after SMPC culture. (a) Immunostained elastin deposited on matrix recovered after 6 days of SMPC culture; (b) immunostained type I collagen on matrix recovered after 6 days of SMPC culture.
DISCUSSION
The objective of this study was to provide a biomimetic niche or milieu in vitro to support attachment of circulating EPCs and SMPCs, and to provide appropriate signals necessary for them to proliferate, survive and differentiate. The recent identification of putative progenitor cells in peripheral blood is expected to allow the design of autologous cell‐based strategies for neo‐vascularization of ischaemic tissues. Regenerative repair of injured blood vessels and bioengineering of vascular prostheses (Riese et al. 2003) will be important. Although isolation and characterization of EPCs and SMPCs from peripheral blood have already been reported (Asahara 1997; Simper et al. 2002), many of them relate inconsistent results and difficulties in obtaining the differentiated cells.
Thus, strategies need to be developed to standardize protocols for isolation, culture and therapeutic application for cell‐based therapy. Recruitment and incorporation of EPCs and SMPCs requires a co‐ordinated sequence of multistep adhesive and signalling events including chemoattraction, adhesion, transmigration and finally differentiation to endothelial and SMC phenotypes. To switch from a quiescent to a ‘sprouting’ phenotype, ECs require angiogenic growth factors, such as basic FGF (bFGF), as well as interactions with ECM molecules (Juliano & Haskill 1993; Clark & Brugge 1995; Sahni et al. 1999). Several growth factors have been implicated in embryonic SMC differentiation, including transforming growth factors β1, β3, and platelet‐derived growth factor BB (PDGF‐BB) (Hellstrom et al. 1999).
In normal adult artery wall, the basement membrane is the primary ECM compartment that interacts with vascular SMC, and its components are believed to be important in maintaining stable and well‐differentiated SMCs (Thyberg & Hultgardh‐Nilsson 1994). It has been demonstrated that SMCs require de novo production of collagen to sustain normal migration in vitro. It has been proposed that this biosynthetic response is necessary to ensure that the collagenous ECM is appropriately structured to maintain the trans‐cellular traction system required for locomotion (Rocnik et al. 1998). Cell growth control in non‐transformed cells depends in part on adhesive interactions with the ECM. Following injury, excess or altered fibronectin deposition into the extracellular matrix may contribute to pathogenesis of fibrosis and atherosclerosis, by triggering changes in specific cell functions associated with wound repair, including cell proliferation and migration (Sottile et al. 1998).
Primary cultures of neonatal rat aortic SMCs after 10 days produce insoluble elastin, which increases rapidly to become large irregular refractile aggregates and later coalesces to form even larger aggregates and small fibres (Oakes et al. 1982). Elastin aggregates and newly formed elastic fibres were abundant in our matrices. Quantitative analysis of insoluble elastin formation in the cell layer during the culture period indicated continuous biosynthesis and deposition that paralleled that of desmosome formation. Results of this study demonstrate that SMCs developed from PBMNCs synthesize both collagen and elastin.
The notion of microenvironments affecting stem cell division and function is not new. Schofield (1978) dubbed these ‘niches’ with respect to haematopoietic stem cells and subsequent reports have described their presence in numerous tissues including neural, germline, skin, intestinal and others (Spradling et al. 2001). The forces driving stem cell differentiation, or maintaining them in a state of suspended dedifferentiation, include secreted and bound messengers or homing signals. It has been shown that a change in the microenvironment can potentially effect a transient or permanent change in the differentiation process in the case of primary culture of HUVECs (Chennuzhy & Krishnan 2005).
It is well recognized that blood platelets are a major source of VEGF, and activated platelets release VEGF (Mohle et al. 1997). Furthermore, thrombin‐activated platelets have been shown to release intact VEGF/FN complexes, which stimulate EC migration and can be inhibited by soluble high‐affinity VEGF receptor 1 and α5β1 integrin antibodies (Wijelath et al. 2002). This is a significant finding with regard to the process of neo‐vascularization in wound healing and tumour angiogenesis. Other than VEGF, PDGF‐BB can contribute to angiogenesis in vitro, PDGFR‐beta is specific for cord/tube‐forming ECs and mediates endothelial proliferation and cord/tube formation, in angiogenic and non‐angiogenic ECs (Battegay et al. 1994). In this study, the use of platelet release has been found to have induced EPC differentiation and SMPC differentiation, whereas Shirota et al. (2003) used insulin growth factor, and epidermal growth factor treatment to obtain EPC differentiation. In contrast with their success rate of 4 out of 13 attempts, we were successful in obtaining 8 EPC cultures and 10 SMPC cultures out of each 10 blood samples collected, the initial failure mainly being caused by low yield of EPC. Use of an appropriate matrix for initial cell adhesion seemed to prevail over the low initial cell adhesion noticed by Shirota et al. (2003).
In summary, elective adhesion in vitro and subsequent differentiation of circulating blood progenitor cells, isolated from human blood, to biomimetic extracellular matrix coating on tissue culture plastic is demonstrated. ECs thus obtained expressed vWF, endocytosis of Ac‐LDL and release of NO. α‐SMA and calponin were identified in SMCs, and in addition, deposition of elastin and type I collagen by SMCs confirmed the contractile SMC phenotype.
ACKNOWLEDGEMENTS
We appreciate the support and encouragement of Dr K. Mohandas, Director of SCTIMST and Dr G.S. Bhuvaneshwar, Head of BMT Wing. Also, we would like to acknowledge the financial support from the Department of Science and Technology (DST), Government of India. We acknowledge our colleague, Krishna Prasad for his help with confocal microscopy and further colleagues who have donated blood for PBMNC isolation.
REFERENCES
- Asahara T (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 85, 221–228. [DOI] [PubMed] [Google Scholar]
- Battegay EJ, Rupp J, Iruela‐Arispe L, Sage EH, Pech M (1994) PDGF‐BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta‐receptors. Cell Biol. 125, 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennuzhy KP, Krishnan LK (2005) Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 26, 5658–5667. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS (1995) Integrins and signal transduction pathways: the road taken. Science 268, 233–239. [DOI] [PubMed] [Google Scholar]
- Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen‐Pope DF (2000) Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ. Res. 87, 728–730. [DOI] [PubMed] [Google Scholar]
- Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ (2003) Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell‐based vascular therapy. Circulation 108, 2710–2715. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kaln M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF‐B and PDGFR‐β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Haskill S (1993) Signal transduction from the extracellular matrix. J. Cell Biol. 120, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S, Amiel GEFJ, Atala A, Soker S, Bischoff J (2001) Functional small‐diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 7, 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T, Cerundolo J, Ilsley S, Kelly PR (1979) An endothelial cell growth factor from bovine hypothalamus: Identification and partial characterization. Proc. Natl Acad. Sci. 76, 5674–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R (2000) Stem cells – hype and hope. Nature 406, 361–364. [DOI] [PubMed] [Google Scholar]
- Mohle R, Green D, Moore MA, Nachman RL, Rafii S (1997) Constitutive production and thrombin‐induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. USA 94, 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes BW, Batty AC, Handley CJ, Sandberg LB (1982) The synthesis of elastin, collagen, and glycosaminoglycans by high‐density primary cultures of neonatal rat aortic smooth muscle. An ultrastructural and biochemical study. Eur. J. Cell Biol. 27, 34–46. [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal‐Ginard B, Bodine DM, Leri A, Anversa P (2001) Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. USA 98, 10344–10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resmi KR, Krishnan LK (2002) Protease action and generation of β‐thromboglobulin‐like protein on platelet activation. Thromb. Res. 107, 23–29. [DOI] [PubMed] [Google Scholar]
- Rezai N, Podor TJ, McManus BM (2003) Bone marrow cells in the repair and modulation of blood vessels: emerging opportunities in native and engineered tissue and biomechanical materials. Artif. Organs 28, 142–151. [DOI] [PubMed] [Google Scholar]
- Riese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ (2003) Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell‐based vascular therapy. Circulation 108, 2710–2715. [DOI] [PubMed] [Google Scholar]
- Rocnik EF, Chan BMC, Pickering GJ (1998) Evidence for a role of collagen synthesis in arterial smooth muscle cell migration. J. Clin. Invest. 101, 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni A, Sporn LA, Francis CW (1999) Potentiation of endothelial cell proliferation by fibrin (ogen)‐bound fibroblast growth factor‐2. J. Biol. Chem. 274, 14936–14941. [DOI] [PubMed] [Google Scholar]
- Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R (2002) Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat. Med. 8, 403–409. [DOI] [PubMed] [Google Scholar]
- Schofield R (1978) The relationship between the spleen colony‐forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25. [PubMed] [Google Scholar]
- Shirota T, He H, Yasui H, Matsuda T (2003) Human endothelial progenitor cell seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 9, 127–136. [DOI] [PubMed] [Google Scholar]
- Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM (2002) Smooth muscle progenitor cells in human blood. Circulation 106, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Sobue K, Hayashi K, Nishida W (1998) Molecular mechanism of phenotypic modulation of smooth muscle cells. Horm. Res. 50, 15–24. [DOI] [PubMed] [Google Scholar]
- Sottile J, Hocking DC, Swiatek PJ (1998) Fibronectin matrix assembly enhances adhesion‐dependent cell growth. J. Cell Sci. 111, 2933–2943. [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond‐Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414, 98–104. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Hultgardh‐Nilsson A (1994) Fibronectin and the basement membrane components laminin and collagen type IV influence the phenotype properties of subcultured rat aortic smooth muscle cells differently. Cell Tissue Res. 276, 263–271. [DOI] [PubMed] [Google Scholar]
- Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM (2002) Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow‐derived endothelial progenitor cells. Circulation 105, 3017–3024. [DOI] [PubMed] [Google Scholar]
- Werner N, Junk S, Laufs U, Link A, Walenta K, Michael B, Nickenig G (2003) Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ. Res. 93, e17–e24. [DOI] [PubMed] [Google Scholar]
- Wijelath ES, Murray J, Rahman S, Patel Y, Ishida A, Strand K, Aziz S, Cardona C, Hammond WP, Savidge GF, Rafii S, Sobel M (2002) Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ. Res. 91, 25–37. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW (2004) Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation 109, 3154–3157. [DOI] [PubMed] [Google Scholar]


