Abstract
Objective
The aim of this study was to investigate effects of mechanical compressive force on differentiation of adipose‐derived stem cells (ASCs) in vitro.
Materials and methods
Mice ASCs were treated with compressive force (2000 με, 1 Hz) for 2 or 6 h after adipogenic induction for 3 days, then oil red O staining was used to examine oil droplet‐filled cells. Adipogenic genes, PPAR‐γ1 and APN, were examined by real‐time PCR and immunofluorescence (IF) staining was performed to test expression of de‐PPAR‐γ and ph‐PPAR‐γ at the protein level.
Results
Our data showed that mechanical compressive force reduced numbers of oil droplet‐filled cells, and down‐regulated mRNA levels of both PPAR‐γ1 and APN and protein level of PPAR‐γ, in ASCs.
Conclusions
In culture medium containing adipogenic stimuli, mechanical compressive force inhibited adipogenesis of ASCs.
Introduction
Obesity is widely recognized as a major public health problem owing to its rising prevalence and deleterious impact on many chronic diseases, such as dyslipidemia, atherosclerosis, hypertension and diabetes 1; it is characterized by increased mass of adipose tissue 2. Hypertrophy of adipocytes is an apparent cause of obese adipose tissue, but increase in numbers of adipocytes is also observed in obese adipose tissue 2. There are three main mechanisms for increase in numbers of adipocytes: (i) differentiation from mesenchymal stem cells (MSCs) and preadipocytes; (ii) cell division of normal adipocytes then increase in size of divided cells; and (iii) cell division of adipocytes with large lipid droplets 3, 4. Although exact mechanisms functioning in vivo are not known, adipogenic induction of MSCs and preadipocytes are considered to be important causes of obesity.
To achieve and maintain healthier body weight, the mainstay treatment for obesity is energy‐limited diet and increased exercise 1. Weight loss by exercise may result from not only increase in caloric expenditure but also from the influence of mechanical stimulation. Mechanical stimuli such as pressing fat, through gymnastic exercises or massage, rubbing, stretching and whole body vibration are believed to reduce or prevent obesity 5, 6. There are many current studies investigating effects of mechanical stimuli on differentiation of cell lineages and MSCs 7, 8, 9, but reports concerning effects of mechanical stimuli on adipogenesis are rare. In recent years, several investigations have demonstrated that mechanical strain inhibits adipogenesis of C3H10T1/2 MSCs 10, 11, mouse preadipocyte 3T3‐L1 cells 12 and human umbilical cord progenitor cells 13. Also, in our previous studies, exposure of adipose stem cells (ASCs) to cyclic mechanical stretch (2000 με, 1 Hz) in the presence of adipogenic medium was shown to reduce mRNA and protein level of PPAR‐γ (peroxisome proliferator‐activated receptor‐γ), indicating that mechanical stretch inhibited adipogenesis of ASCs in the presence of adipogenic medium 14. Yen et al. found that low magnitude mechanical signals on differentiation potential of MSCs in bone marrow was biased towards osteoblastic and against adipogenic differentiation 15. In contrast, our previous study showed that in culture medium containing adipogenic stimuli, low‐intensity pulsed ultrasound enhanced adipogenesis of ASCs through up‐regulating both mRNA level of PPAR‐γ1 and APN, and protein level of PPAR‐γ 16.
Despite mechanical stimuli mentioned above, compressive force is also an important research area. Many studies have demonstrated that cyclic mechanical compression can enhance expression of chondrogenic markers in rabbit bone marrow‐derived mesenchymal stem cells (BMSCs) 17, 18, 19 and mesenchymal progenitor cells 20, but research on effects of compression in adipogenic induction has been ignored. Hossain et al. applied compressive force of 226 Pa for 12 h to a human preadipocyte cell line, SGBS, before adipogenic induction; they found that compressive force inhibited adipogenesis by suppressing expression of PPAR‐γ2 and C/EBP‐α in a COX‐2‐dependent manner 21. In this study, the effect of mechanical stimulation by compressive force on adipogenesis in ASCs was investigated.
Materials and methods
Cell culture
Adipose‐derived stem cells were isolated as described by Zuk et al. 22 with minor modifications. Four‐week‐old Kunming mice were prepared, using standard sterile techniques, to excise inguinal fat pads, following protocols approved by the Institutional Animal Care and Use Committee at Sichuan University. Tissue obtained was washed twice in phosphate‐buffered saline supplemented with streptomycin sulphate and penicillin, incubated in alpha‐modified Eagle's medium (α‐MEM) and finely minced into small pieces of around 0.5 cm3. Tissue was then digested using 0.05% type 1 collagenase, with vigorous shaking, for 40 min at 37 °C. Floating cell populations were removed by centrifugation at 250 g for 8 min and cells were pelleted. Single‐cell suspension was obtained and re‐suspended in culture medium of α‐MEM supplemented with antibiotics (penicillin–streptomycin solution), sodium bicarbonate and 10% foetal bovine serum (Gibco, Paisley, UK), and finally cells were seeded in plastic flasks for the final isolation step to select plastic adherent cell populations. Cells were cultured at 37 °C in 90% humidified atmosphere and 5% CO2. Third passage cells were used for the following test: phenotypic profile of ASCs was investigated by immunofluorescence staining for STRO‐1, and immunocytochemical staining for CD34, CD44 and CD90; cells were characterized as adipose‐derived stem cells, as noted in our previous investigations 9.
Induction of adipogenic differentiation
The mouse ASCs were transferred into adipogenic medium (1 μm dexamethasone, 10 μm insulin, 200 μm indomethacin, and 0.5 mm isobutyl‐methylxanthine and 10% FBS), 1 × 105⁄ml density. After 72 h induction, cells reached 70% confluence and differentiated ones stained with oil red O thus assessing adipogenic differentiation.
Application of cyclic compressive force
As previous studies have shown, application of cyclic stretching only during the late phase of induction, inhibits adipose‐differentiation of preadipocytes 12; thus we also applied mechanical compressive force after 3 days adipogenic induction. Force‐loading plates were made from bottoms of BD Falcon™ 75 cm2 cell culture flasks (BD Bioscience, CA, USA), 7.8 × 3.8 cm sides, 1.2 mm thick. Passage 3 adipose‐derived stem cells were seeded on the loading plates 2 × 105 cell density, per plate. When cells were totally attached to plates 6 h later, culture medium was replaced by adipogenic medium. A four‐point bending mechanical loading device (Fig. 1) 14 was then used. Cells were randomly divided into three groups and loading groups were exposed to uniaxial cyclic compressive force for 2000 με, 1 Hz for 6 and 2 h, respectively, after ASCs were adipose‐induced for 72 h. ASCs retained in adipogenic medium without being loaded were used as control. All measurements began 2 h after the final compression cycle.
Figure 1.
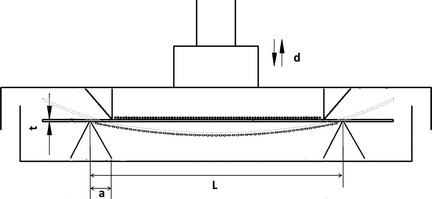
Four‐point bending mechanical loading device. L, distance between two outer pressure points; a, distance between outer and inner pressure points; t, thickness of loading plate; d, distance of pressure point movement; strain (ε) of cells attached to upper side of loading plate was calculated according the following formula: ε = td⁄a(L − 1.33a). ASCs exposed to uniaxial cyclic compressive force for 2000 le, 1 Hz, for 6 and 2 h.
Oil red O staining
Cells on each plate were fixed in 10% paraformaldehyde solution for 20 min, washed in PBS, and stained with oil red O (ORO) (Amresco, Solon, OH, USA) in 60% isopropanol, for 20 min. To quantify adipogenic progress of ASCs, 10 random microscopic fields (amplification time: 10 × 10) were observed on each plate, using an Olympus IX 710 microscope (Olympus, Tokyo, Japan). Images were captured for each field and image analysis was carried out using Image‐Pro Plus 6.0.0.260 (Media Cybernetics, Silver Spring, MD, USA).
Analysis of mRNA by real‐time PCR
Total RNA of ASCs (mASCs) was extracted using Total Tissue⁄Cell RNA Extraction Kits (Watson, Beijing, China) according to the manufacturer's protocol. Total RNA integrity was verified by UV‐spectroscopy, 2% agarose gel electrophoresis, and yield and purity were confirmed by ratio of A (260)⁄A (280). cDNA synthesis was performed using transcriptor reverse transcriptase (Takara Biotechnology, Shiga, Japan). To establish the standard curve of a certain gene, cDNA samples were amplified using an RT‐PCR kit (Tiangen Biotech, Beijing, China); specifically designed primers are shown in Table 1. Real‐time PCR was run in the ABI PRI SM 7300 Sequence Detection system (Applied Biosystems, Foster City, CA, USA) using Hot Start DNA Master SYBR Green I Kit (Takara Biotechnology, Co., Ltd) with the following programme: 95 °C for 10 min; 40 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by melting curve analysis. Specificities of PCR products were verified by melting curve analyses between 60 °C and 95 °C. For each reaction, a melting curve was generated to test primer dimer formation and false priming. Values of relative gene expression were normalized by house‐keeping gene glyceraldehyde 3‐phosphate dehydrogenase (GAPDH).
Table 1.
Primer sequences of target genes and GAPDH for real‐time PCR
| Target gene | Primer sequence (5′–3′) |
|---|---|
| PPAR‐γ1 | F: CCAACTTCGGAATCAGCTCT |
| R: CAACCATTGGGTCAGCTCTT | |
| Adiponectin (APN) | F: GCAGAGATGCACTCCTGGA |
| R: CCCTTCAGCTCCTGTCATTCC | |
| GAPDH | F: TATGACTCTACCCACGGCAAGT |
| R: ATACTCAGCACCAGCATCACC |
Immunofluorescence staining
Adipose‐derived stem cells were seeded on loading plates, cultured with adipogenic medium and exposed to mechanical loading or kept static, as described above. Before staining, cells were washed briefly in PBS, fixed in 4% buffered paraformaldehyde for 30 min at room temperature and blocked in 0.5% bovine serum albumin (BSA) for 15 min. They were subsequently incubated overnight at 4 °C with rabbit anti‐PPAR‐γ polyclonal antibody (1:100) (Abcam, Cambridge, UK) or rabbit anti‐PPAR‐γ monoclonal antibody with phosphor serine at residue 82 (1:100) (Up State, Lake Placid, NY, USA). Sequentially, specimens were incubated in secondary antibodies conjugated to Rhodamine (Pierce Biotechnology, Rockford, IL, USA) for 1 h at room temperature, and nuclei were stained with DAPI (Molecular Probes, Eugene, OH, USA) for 1 min. After rinsing in PBS, cells were observed and imaged using the Olympus IX 710 microscope (Olympus). Images were captured for each field and image analysis was carried out using Image‐Pro Plus 6.0.0.260 (Media Cybernetics); IOD (mean) was used instead of IOD (sum) in this study.
Statistical analysis
We performed three or more independent sets of experiments. Data are presented as mean ± SD and analysed by ANOVA. P < 0.05 was considered statistically significant.
Results
Cyclic compressive force inhibited accumulation of lipid droplets
Two hours after the final compression cycle, oil red O staining was performed to analyse adipogenesis (Fig. 2a). In control groups, large numbers of small lipid droplets appeared throughout the cytoplasm of cells. However, in compressive force groups, time of loading was longer and lipid droplets were fewer. Oil red O staining results suggest that cyclic compressive force could significantly inhibit differentiation and the process of adipogenesis (Fig. 2b).
Figure 2.
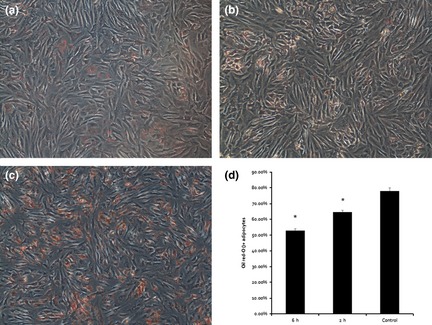
Mechanical compressive force significantly reduced adipogenesis of adipose‐derived stem cells. Three days after adipogenic induction, lipid‐filled cells, positive after oil red O staining, were observed by light microscopy in 6‐h groups (a), 2‐h groups (b) and controls (c). Six hours and 2 h mechanical compressive force significantly reduced numbers of oil droplet‐filled cells from 80% to 53% and 62%, respectively (d). *Represents P < 0.05.
Cyclic compressive force inhibited PPAR‐γ and APN transcription after adipogenic induction
PPAR‐γ is one of the most critical transactivators in initiation of adipogenesis, and APN is one of the adipocyte‐predominant proteins. Thus, effects of mechanical compressive force on expression of these factors were assessed by quantitative real‐time polymerase chain reaction. Results indicate that mRNA levels of PPAR‐γ reduced significantly to 50% after loading for 2 h (P < 0.001) and 3% after loading for 6 h (P < 0.001) compared to static control groups (Fig. 3a). Similarly, mRNA levels of APN reduced significantly to 80% after loading for 2 h (P < 0.001) and 20% after loading for 6 h (P < 0.001) compared to static control groups (Fig. 3b). mRNA levels of PPAR‐γ and APN were different between the two cyclic compressive force groups. Compared to the 2 h group, mRNA levels of PPAR‐γ and APN reduced significantly to 6% and 25%, respectively, after loading for 6 h (P < 0.001).
Figure 3.
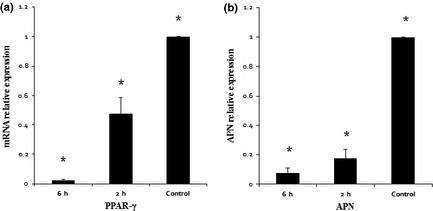
Mechanical compressive force inhibited PPAR‐γ and Adiponectin (APN) transcription after adipogenic induction. (a) In all groups, PPAR‐γ1 mRNA was detected by real‐time PCR which indicated that mRNA level of PPAR‐γ1 of control groups was significantly higher than compressive force groups, and mRNA level of 2 h groups was significantly higher than 6 h groups. (b) In all groups, APN mRNA was detected by real‐time PCR. Similar to PPAR‐γ1 results, data of real‐time PCR indicated that mRNA level of APN of control groups was significantly higher than compressive force groups, and mRNA level of 2 h groups was significantly higher than 6 h groups. *Represents P < 0.05.
Cyclic compressive force inhibited expression of de‐PPAR‐γ and ph‐PPAR‐γ after adipogenic induction
PPAR‐γ is commonly activated in the cytoplasm by dephosphorylation; dephosphorylated PPAR‐γ (de‐PPAR‐γ) moves into nuclei and functions as an intranuclear transcriptional factor. Here, after the final compression cycle, the ASCs remained in the incubator for 2 h then IF was performed using anti‐de‐PPAR‐γ (Fig. 4a) and anti‐ph‐PPAR‐γ (Fig. 5a) antibodies, respectively. We observed that expression of de‐PPAR‐γ was significantly higher in the control group (3.03 ± 0.21%) than that in 2 h (2.82 ± 0.11%) and 6 h groups (2.20 ± 0.15%) (Fig. 4b). Similarly, ph‐PPAR‐γ was expressed significantly more highly in the control group (3.08 ± 0.27%) than in 2 h (2.55 ± 0.19%) and 6 h groups (2.27 ± 0.22%) (Fig. 5b). Also we found that expressions of de‐PPAR‐γ and ph‐PPAR‐γ were higher in the 2 h group than the 6 h group.
Figure 4.
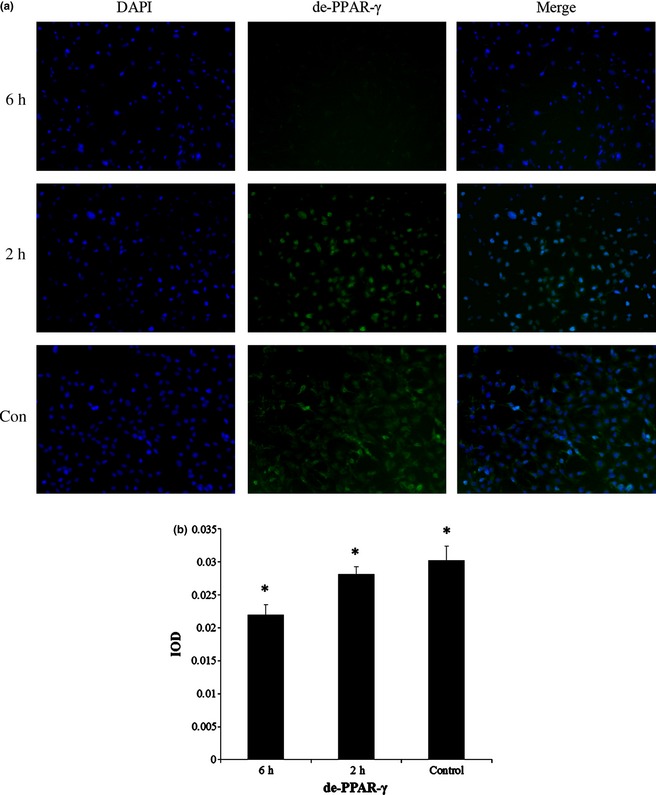
Mechanical compressive force inhibited expression of de‐ PPAR ‐γ after adipogenic induction. (a) Immunofluorescence staining was performed with anti‐de‐PPAR‐γ (green). Cell nuclei were counterstained with 4, 6‐diamidino‐2‐phenylindole (blue). (b) IOD (mean) of de‐PPAR‐γ was significantly higher in control groups than 2 and 6 h groups, and IOD (mean) of 2 h groups was significantly higher than 6 h groups. *Represents P < 0.05. Magnification ×100.
Figure 5.
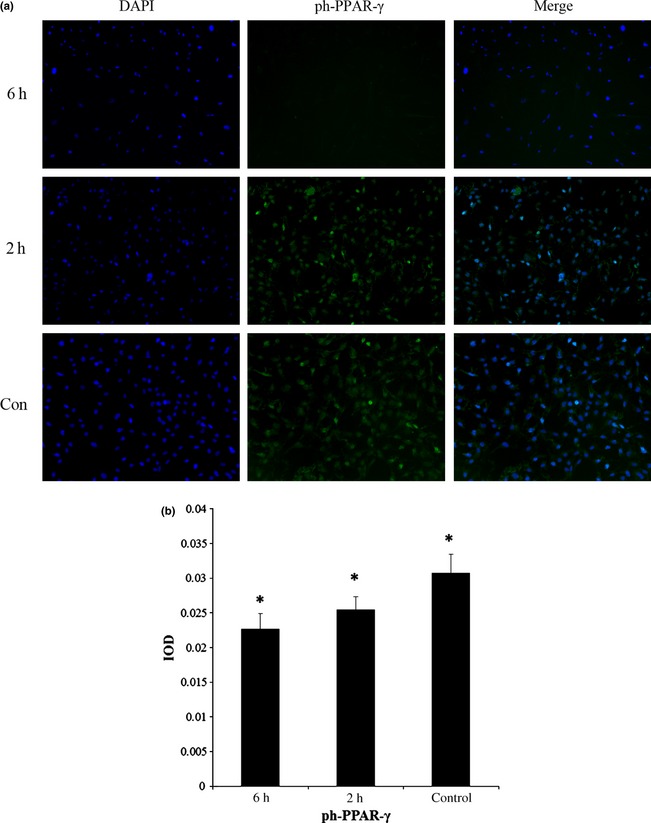
Mechanical compressive force inhibited expression of ph‐ PPAR ‐γ after adipogenic induction. (a) Immunofluorescence staining was performed with anti‐ph‐PPAR‐γ (green). Cell nuclei were counterstained with 4, 6‐diamidino‐2‐phenylindole (blue). (b) IOD (mean) of ph‐PPAR‐γ was significantly higher in control groups than 2 and 6 h groups, and IOD (mean) of 2 h groups was significantly higher than 6 h groups. *Represents P < 0.05. Magnification ×100.
Discussion
Mechanical forces modulate various cell functions such as adhesion, proliferation and differentiation, which are crucial for development, growth and regeneration of various tissues in mammals 23. Effects of mechanical stimuli including stretch, shear force and compression, on differentiation in mesoderm‐derived cells, have been particularly well studied. Regarding effects of mechanical stimuli on adipogenesis, adipogenic differentiation was attenuated by cyclic stretching in C3H10T1/2 cells 10, 11 and cyclic compressive force in SGBS 21. Recently, low‐magnitude mechanical signals have been reported to suppress differentiation of BMSCs into adipocytes in mice 15. In our group, we have been paying more attention to stem cells derived from adipose tissue. Compared to BMSCs, ASCs have an abundant and possible autologous cell source, carry relatively lower donor site morbidity, grow fast and are available in large numbers of stem cells at harvest, from small volumes of adipose tissue. ASCs can be differentiated into adipocytes using a traditional differentiation medium cocktail containing dexamethasone, 3‐isobutyl‐1‐methylxanthine, insulin and indomethacin 24. Thus, differentiation of ASCs provides an excellent in vitro model to uncover mechanisms of adipogenic differentiation and insulin sensitivity of mature adipocytes – vital for treatment of obesity, insulin resistance and fat tissue regeneration. Some functional studies on adipogenic differentiation have been performed on preadipogenic progenitor cell lines such as a mouse preadipocyte cell line, 3T3‐L1 12 and human preadipocyte cell line, SGBS 21. Research based on the ASCs model is more beneficial for clinical purposes, as translation of knowledge from primary cells to clinical practice would be easier than that from immortalized cells. In our previous studies, we have found that mechanical stretch inhibited adipogenesis of ASCs in the presence of adipogenic medium 14, and here we have also demonstrated that mechanical compressive force inhibited adipogenesis of ASCs under adipogenic‐induction. Thus, all results mentioned above suggest that mechanical stimuli are likely to inhibit adipogenesis. However, a recent investigation of ours has shown that the LIPUS system up‐regulated both mRNA level of PPAR‐γ1 and APN and protein level of PPAR‐γ 16, which is the reverse of results of some other groups. The reason may be that mechanical force loading on cells of LIPUS was in the form of a mechanical wave, which included more than one kind of mechanism, and was more complicated than a single mechanical stress, as used elsewhere.
In this study, we assessed transcriptional levels of PPAR‐γ and APN in mASCs by real‐time PCR assay. PPAR‐γ is the most important regulator in the transcriptional control of adipogenesis; PPAR‐γ expression in the adipogenic process is activated by peroxisome proliferators (of the thiazolidinedione class of anti‐diabetic agents) and long‐chain fatty acids. It has already been shown that embryonic stem cells from mice lacking PPAR‐γ were unable to differentiate into adipocytes and overexpression of PPAR‐γ in fibroblast cell lines can initiate adipogenesis 25. According to these studies, PPAR‐γ plays a requisite and important role in regulation of adipogenesis. Adiponectin, also called GBP‐28, apM1, Adipo Q and Acrp30, is a novel adipose tissue‐specific protein that has some structural homology to collagens VIII and X and complement factor C1q. It is one of the physiologically active polypeptides secreted by adipose tissue, and modulates a number of metabolic processes, including glucose regulation and fatty acid catabolism 26. Although all results of this study have demonstrated that compressive force inhibited adipogenesis of adipose stem cells, effects of inhibition of PPAR‐γ and APN genes appear much more significant than protein levels and oil red O staining. We conjecture that this may be due to time needed for transcription from mRNA to protein incurs a time delay of alteration in protein levels, that is, changes in protein levels appear after changes in gene levels. Also, proteins levels are not decided by mRNA presence alone, but also can be affected by post‐transcriptional controls. Thus in our results, the trend of protein levels and oil red O staining is in conformity with gene levels, but effects of inhibition are different.
Work of Hossain and colleague compared effects of different initiation times of compressive force loading on cells, before adipogenic induction or immediately after induction, by oil red O staining and mRNA levels of an adipocyte fatty acid‐binding protein, aP2. Their results showed that triglyceride and mRNA levels of aP2 were unaffected by compressive force after initiation of induction. So compressive force was applied only before adipogenic induction in their subsequent experiments 21; here however, mechanical stress was applied after adipogenic induction. The reason why compressive force after initiation of induction failed to influenced adipogenesis may be that time of mechanical stress loading on cells was less than induction time, and any effect caused by compressive force had disappeared before testing. According to these studies, we can suggest that mechanical stress can be loaded on cells before and after the induction, but not during its period.
The mechanism of influence of mechanical stimuli on adipogenic differentiation is still controversial. Choy et al. have demonstrated the involvement of both Smad2 and Smad3 in inhibition of adipogenesis in the 3T3‐F442A cell line, with Smad2 and Smad3 suggested to be having distinct functions in control of differentiation 27. Smad3 inhibits adipogenic differentiation by interaction with C/EBPs, but not PPAR‐γ 28. Neill et al. have found that constant cyclic stretching inhibits adipogenic differentiation of human umbilical cord progenitor cells via autocrine/paracrine stimulation of the TGFβ1/Smad2 signalling pathway although no change in ERK 1/2 phosphorylation was detected 13. However, Tanabe et al. demonstrated that stretching induced phosphorylation of ERK1/2 in preadipocytes, and that blockade of ERK signalling reversed stretch‐induced reduction in PPAR‐γ expression and cytoplasmic lipid accumulation 12. Our previous study showed that mechanical stretch inhibited adipogenesis and stimulated osteogenesis of ASCs in the presence of adipogenic medium, and that ERK1/2 activation may be involved in mechanical stress‐induced trans‐differentiation. Interestingly, some studies have shown that transient activation of the ERK pathway is essential for early stages of adipogenic differentiation 29, 30, but sustained ERK signalling reduces adipogenic differentiation 31, 32. Hossain et al. found that compressive force inhibited adipogenesis of SGBS through COX‐2 mediated downregulation of PPARγ2 and C/EBPα. However, the mechanism of influence of compressive force on adipogenesis differentiation was unclear and is still an area for our future research.
In conclusion, conversion of ASCs into adipocytes driven by adipogenic conditions can be inhibited by mechanical compressive force, by reduction in expression of specific adipogenic genes.
Acknowledgements
This work was funded by National Natural Science Foundation of China (81071273, 31170929, 81201211, 81200810), Foundation for the Author of National Excellent Doctoral Dissertation of China (FANEDD 200977), Funding for Distinguished Young Scientists in Sichuan (2010JQ0010).
Competing interests: The authors declare that no competing interests exist.
References
- 1. Spiegelman BM, Flier JS (1996) Adipogenesis and obesity: rounding out the big picture. Cell 87, 377–389. [DOI] [PubMed] [Google Scholar]
- 2. Coppack SW (2005) Adipose tissue changes in obesity. Biochem. Soc. Trans. 33, 1049–1052. [DOI] [PubMed] [Google Scholar]
- 3. Dicker A, Le Blanc K, Åström G, van Harmelen V, Götherström C, Blomqvist L et al (2005) Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp. Cell Res. 308, 283–290. [DOI] [PubMed] [Google Scholar]
- 4. Nagayama M, Uchida T, Gohara K (2007) Temporal and spatial variations of lipid droplets during adipocyte division and differentiation. J. Lipid Res. 48, 9–18. [DOI] [PubMed] [Google Scholar]
- 5. Bei Y, Fang X, Yao Z (2004) Sixty‐two cases of simple obesity treated by acupuncture combined with massage. J. Tradit. Chin. Med. 24, 36–39. [PubMed] [Google Scholar]
- 6. Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ et al (2007) Adipogenesis is inhibited by brief, daily exposure to high‐frequency, extremely low‐magnitude mechanical signals. Proc. Natl. Acad. Sci. USA 104, 17879–17884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuscik MJ, Hilton MJ, Zhang X, Chen D, O'Keefe RJ (2008) Regulation of chondrogenesis and chondrocyte differentiation by stress. J. Clin. Invest. 118, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang XM, Gong P, Lin YF, Zhang LR, Li XY, Yuan Q et al (2010) Cyclic tensile stretch modulates osteogenic differentiation of adipose‐derived stem cells via the BMP‐2 pathway. Arch. Med. Sci. 2, 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J (2008) Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable β‐catenin signal. Endocrinology 149, 6065–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. David V, Martin A, Lafage‐Proust MH, Malaval L, Peyroche S, David BJ et al (2007) Mechanical loading down‐regulates peroxisome proliferator‐activated receptor in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148, 2553–2562. [DOI] [PubMed] [Google Scholar]
- 12. Tanabe Y, Koga M, Saito M, Matsunaga Y, Nakayama K (2004) Inhibition of adipocyte differentiation by mechanical stretching through ERK‐mediated downregulation of PPAR‐γ2. J. Cell Sci. 117, 3605–3614. [DOI] [PubMed] [Google Scholar]
- 13. Neill JT, Helen SJ, Jone ED, Ann EC (2008) Cyclic stretch‐induced TGFβ1/Smad signaling inhibits adipogenesis in umbilical cord progenitor cells. Biochem. Biophys. Res. Commun. 377, 1147–1151. [DOI] [PubMed] [Google Scholar]
- 14. Yang XM, Cai XX, Wang J, Tang H, Yuan Q, Gong P et al (2012) Mechanical stretch inhibits adipogenesis and stimulates osteogenesis of adipose stem cells. Cell Prolif. 45, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yen KL, Encarnacion C, Clifford JR, Vicente G, Jeffrey EP, Stefan J et al (2009) Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary‐induced obesity. J. Bone Miner. Res. 24, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu N, Yang XM, Ba K, Fu Y, Wei XQ, Yue Y et al (2013) Low‐intensity pulsed ultrasound (LIPUS) induced adipogenesis of adipose‐derived stem cells. Cell Prolif. 46, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park SH, Sim WY, Park SW, Yang SS, Choi BH, Park SR et al (2006) An electromagnetic compressive force by cell exciter stimulates chondrogenic differentiation of bone marrow–derived mesenchymal stem cells. Tissue Eng. 12, 3107–3117. [DOI] [PubMed] [Google Scholar]
- 18. Huang CCY, Hagar KL, Frost LE, Sun Y, Cheung HS (2004) Effects of cyclic compressive force loading on chondrogenesis of rabbit bone‐marrow derived mesenchymal stem cells. Stem Cells 22, 313–323. [DOI] [PubMed] [Google Scholar]
- 19. Huang CCY, Reuben PM, Cheung HS (2005) Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow–derived mesenchymal stem cells under cyclic compressive loading. Stem Cells 23, 1113–1121. [DOI] [PubMed] [Google Scholar]
- 20. Peter A, Detlef S, Martin A, Bernd K, Carsten E, Reiner H et al (2004) Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology 41, 335–346. [PubMed] [Google Scholar]
- 21. Hossain G, Iwata T, Mizusawa N, Shima SWN, Okutsu T, Ishimoto K et al (2010) Compressive force inhibits adipogenesis through COX‐2‐mediated down‐regulation of PPAR‐γ2 and C/EBP α. J. Biosci. Bioeng. 109, 297–303. [DOI] [PubMed] [Google Scholar]
- 22. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al (2001) Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng. 7, 211–228. [DOI] [PubMed] [Google Scholar]
- 23. Jaalouk DE, Lammerding J (2009) Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jing W, Lin Y, Wu L, Li X, Nie X, Liu L et al (2007) Ectopic adipogenesis of preconditioned adipose‐derived stromal cells in an alginate system. Cell Tissue Res. 330, 567–572. [DOI] [PubMed] [Google Scholar]
- 25. Duque G, Macoritto M, Kremer R (2004) 1,25 (OH) 2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM‐P/6) by decreasing the expression of peroxisome proliferator‐activated receptor gamma 2 (PPARgamma2). Exp. Gerontol. 39, 333–338. [DOI] [PubMed] [Google Scholar]
- 26. Diez JJ, Iglesias P (2003) The role of the novel adipocyte‐derived hormone adiponectin in human disease. Eur. J. Endocrinol. 148, 293–300. [DOI] [PubMed] [Google Scholar]
- 27. Choy L, Skillington J, Derynck R (2000) Roles of autocrine TGF‐beta receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 149, 667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choy L, Derynck R (2003) Transforming growth factor‐beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer‐binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 278, 9609–9619. [DOI] [PubMed] [Google Scholar]
- 29. Bost F, Aouadi M, Caron L, Even P, Belmonte N, Prot M et al (2005) The extracellular signal‐regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes 54, 402–411. [DOI] [PubMed] [Google Scholar]
- 30. Prusty D, Park BH, Davis KE, Farmer SR (2002) Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator‐activated receptor gamma (PPAR gamma) and C/EBP alpha gene expression during the differentiation of 3T3–L1 preadipocytes. J. Biol. Chem. 277, 46226–46232. [DOI] [PubMed] [Google Scholar]
- 31. Hu E (1996) Inhibition of adipogenesis through MAP kinase‐mediated phosphorylation of PPARgamma. Science 274, 2100–2103. [DOI] [PubMed] [Google Scholar]
- 32. Font JM, Porras A, Ahn N, Santos E (1997) Mitogen‐activated protein kinase activation is not necessary for but antagonizes, 3T3‐L1 adipocytic differentiation. Mol. Cell. Biol. 17, 6068–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]


