Abstract
Objectives
Storkhead box 1 (STOX1) belongs to the forkhead family of transcription factors, and is reported to be involved in apoptosis of Caenorhabditis elegans. However, up to now the precise role of STOX1 in mammalian epithelial development has not been established. Here, we report that it plays an important role in regulation of proliferation of inner ear epithelial cells.
Materials and methods
Immunohistochemistry and reverse transcription‐PCR assays were used to determine expression pattern of STOX1 in the mouse inner ear. Furthermore, its overexpression and knockdown effects on mouse inner ear epithelial cells were studied using RT‐PCR, immunofluorescence, MTT assay, BrdU labelling and western blotting.
Results
Storkhead box 1 was selectively expressed in epithelial cells, but not in stromal cells of the inner ear. Its over‐expression enhanced cell proliferation and sphere formation, however, STOX1 knockdown inhibited cell proliferation and sphere formation in purified utricular epithelial cells in culture. Consistently, several cell cycle regulatory genes such as for PCNA, cyclin A and cyclin E, were up‐regulated by STOX1 over‐expression. Furthermore, biochemical analyses indicated that proliferation‐promoting effects induced by STOX1 were mediated via phosphorylation of AKT in these cells.
Conclusions
Taken together, we demonstrate that STOX1 is a novel stimulatory factor for inner ear epithelial cell proliferation and might be an important target to be considered in regeneration or repair of inner ear epithelium.
Introduction
Prevalence of loss of hearing after damage, in mammals, has been thought to be due to lack of spontaneous regeneration of mechanosensory receptor hair cells 1, 2. Normally, stimuli of sound and movements of the head, are transduced by hair cells into electrical signals, in the inner ear. These are further relayed to the brain by spiral ganglion neurons or vestibular ganglion neurons, respectively. Hair cells can be damaged by disease, ototoxic drugs and processes associated with ageing. It has been suggested that mature mammals may also have a limited capacity for hair cell regeneration of the vestibular sensory epithelium 3, 4, 5, 6, 7. Several lines of evidence indicate that supporting cells of the sensory epithelium can act as precursors of regenerating hair cells. In the chicken or some lower vertebrates, following hair cell loss, adjacent supporting cells proliferate prior to generation of new hair cells 1, 4, 8, 9. Thus, proliferation of supporting cells seems to be a first major step during the hair cell regeneration process 10, 11, 12, 13. Identification of factors that regulate proliferation of inner ear hair cell precursors will be helpful for stimulating hair cell regeneration and may provide novel targets for clinical treatment of hearing and balance impairment.
Storkhead box 1 (STOX1), a transcription factor structurally and functionally related to the forkhead family of transcription factors, has been shown to be expressed abundantly in the Caenorhabditis elegans central nervous system 14, 15. In general, they play a fundamental role during cell proliferation and differentiation 16. HAM‐1, homologous to STOX1 in non‐mammals, has been reported to prevent neurons undergoing apoptosis and to regulate survival and fate of neural precursors 15. However, functions of STOX1 have not been determined in mammalian epithelial cells.
To examine this role of STOX1, we used the mouse utricular epithelium as a working system, in this study. First we determined expression pattern of STOX1 during inner ear epithelial development. Then we studied its functions in inner ear epithelial cells by over‐expression and gene silencing approaches. In addition, we performed CCK‐8 and western blot analyses to discover signalling pathways that mediate effects of STOX1. Collectively, our study shows that STOX1 is selectively expressed in epithelial cells, but not in stromal cells of the inner ear, and that it regulates proliferation of inner ear epithelial cells via the AKT signalling pathway.
Materials and methods
Materials
Antibodies to STOX1, ZO‐1, vimentin, GFAP and NF were purchased from Abcam Co. (St. Louis, MO, USA) and antibodies to PCNA, cyclin E, cyclin A, AKT, p‐AKT, p‐38, p‐p38, ERK and p‐ERK,β‐actin were obtained from Santa Cruz Biotechnology, Inc (California, USA). Thermolysin and DNase were purchased from Sigma (St. Louis, MO, USA). Enhanced chemiluminesence (ECL) reagents and BrdU cell proliferation kit were bought from Amersham International (Amersham, UK) and Cell Counting Kit‐8 from Dojindo Laboratories (Tokyo, Japan). MK‐2206 was purchased from Selleck Chemicals (Houston, USA). All reagents for cell culture were obtained from Invitrogen Life Technologies, Inc. (Burlington, Canada).
Animals
Newborn ICR mice were from the Slack Laboratory Animal Center in Shanghai, fully accredited by the Institutional Animal Care and Use Committee (IACUC). Our experimental work was in full compliance with the Guide for Care and Use of Laboratory Animals, published by US National Institutes of Health (NIH Publication No. 85‐23, revised 1996) and approved by the Animal Ethics Committee of Shanghai Jiaotong University.
Epithelial cell cultures
Preparation of adult mouse utricle cultures has been described previously 13, 17, 18, 19. Utricles were dissected under sterile conditions and epithelial sheets were separated from sacrificed post‐natal day 0 (P0) ICR mice with 0.5 mg/ml thermolysin. A 30‐gauge needle was used to gently separate sensory epithelium from basement membranes and associated connective tissue. Utricular epithelial sheets were then transferred to calcium‐ and magnesium‐free HBSS, for 30 min at 37 °C. Epithelial sheets were incubated in a mixture of 0.125% trypsin and 0.125% collagenase with 0.005% soybean trypsin inhibitor and 0.005% DNase for 5 min at 37 °C, before using a 1 ml pipette tip 10 times to shake and depress the tissue pieces. In this way, the epithelial sheets were partially dissociated into small pieces containing ~10–80 cells; 5% foetal bovine serum‐supplemented medium was used. Finally, the cell suspension was plated in poly‐d‐lysine (500 mg/ml) coated 96‐well plates (for CCK‐8 assay) or 24‐well Lab‐Tek slides (for BrdU labelling and other immunocytochemistry), in 200 μl serum containing medium (DMEM plus 5% foetal bovine serum, 4.5 mg/ml glucose, 2 mm glutamine, 25 ng/ml fungizone and 10 U/ml penicillin) at ~70 cells/mm2. Cells were then incubated at 37 °C in 5% CO2 and 95% air.
Plasmids, transfection and lentivirus production
Storkhead box 1 shRNA was cloned into lentiviral vector pUCKP. STOX1 cDNA was cloned into lentiviral expression vector pLV.Des2d.P/puro. Sequence of STOX1 shRNA was 3′‐CTAGGCTCCCACTTGATATAT‐5′. Lentiviruses were produced by co‐transfection of 293T cells with lentiviral vector and packaging vectors pCMV‐DR8 and pMD2.2 using the calcium phosphate transfection method. After 48 h transfection, lentiviral supernatant was collected, filtered and mixed with polybrene (8 g/ml), then added directly to target cell lines for infection. Starting 48 h post‐infection, infected cells were then selected with 5 g/ml puromycin, for around 14 days, to generate stable transfectants.
Cell proliferation assays in vitro
In each well of a 96‐well culture plate, 1 × 104 un‐transfected, lentivirus‐targeting shSTOX1 or over‐expressing STOX1, and paired vector controls stably transfected utricular sensory epithelial cells, were seeded. Cell numbers from three wells were counted every 48 h; viable ones on a Cell Counting Kit‐8 (Dojindo) according to the manufacturer's instructions. Three independent experiments were performed, and means were used to depict the growth curve.
RT‐PCR
Tissues or cells were subjected to RT‐PCR to determine STOX1 mRNA expression. Total RNA was extracted using an RNA isolation kit (Watson, Shanghai, China) according to the manufacturer's instructions, and DNase I was used to remove potential genomic DNA contamination. RNA was reverse transcribed into cDNA using a reverse transcription kit (Takara, Dalian, China). Resultant cDNA was amplified using a specific primer pair for mouse STOX1: forward, 3′‐TTACCCAGGTATTGCTGTTCCC‐5′; and reverse: 3′‐TGAGCACCCTTCGTTTGACA‐5′. Beta‐actin was used as internal standard, and its mRNA was amplified with the following primers: forward, 5′‐ACTATCGGCAATGAGCG‐3′; and reverse, 5′‐GAGCCAGGGCAGTAATCT‐3′. The cycling program was performed as follows: denaturing 94 °C for 2 min, 30 cycles at 94 °C for 40 s, 52 °C for 30 s, 72 °C for 1 min and final extension at 72 °C for 5 min. RT‐PCR amplicons were electrophoresed through a 2% agarose gel containing GelRed 20.
BrdU labelling
BrdU labelling was performed according to a previously reported method 18. Briefly, 30 μg/ml BrdU was added to cells in culture medium and incubated for 24 h. Cells were fixed in 4% paraformaldehyde for 15 min, treated with 2 N HCl for 1 h, then incubated with anti‐BrdU monoclonal antibody (1:100 in PBS containing 0.2% Triton X‐100 in 5% normal donkey serum) overnight at 4 °C. After diaminobenzidine‐peroxidase reaction, cells were washed in PBS and mounted with mounting solution containing DAPI, for DNA counterstaining 13.
Western blotting
Protein lysates from transfected cells were obtained by directly scraping cells into loading buffer including β‐mercaptoethanol. Lysates were separated by SDS‐polyacrylamide gel electrophoresis and electroblotted on to a PVDF‐membrane. Antibodies to AKT, p‐AKT, p‐38, p‐p38, ERK, p‐ERK, β‐actin, PCNA, cyclin A or cyclin E were used. Protein bands were detected by enhanced‐chemiluminescence assay, using LAS3000 technology.
Immunofluorescence
For immunofluorescence, stably transfected sensory epithelial cells were grown on glass coverslips. Samples were fixed in 4% paraformaldehyde for 15 min at room temperature. After fixation, they were rinsed in PBS, 0.2% Triton X‐100 and incubated with 1% Triton X‐100 in PBS for 15 min at room temperature for permeabilization. Coverslips were washed in washing buffer (donkey serum albumin) blocked for 1 h and incubated with antibodies against STOX1, SOX‐2 and myosinVIIa at 4 °C overnight. After washing, cells were incubated for 1 h with anti‐rabbit or anti‐mouse secondary antibodies conjugated with Alexa Fluor 594 and 488 (Invitrogen), washed in washing buffer and mounted with mounting solution containing DAPI 13.
Cell counting and quantification
After 120 h in culture, spheres were counted from entire areas of 10 or more culture wells, for each of the experimental groups. Data were expressed as means ± SEM. Two‐tailed, unpaired t‐testing was used for statistical analysis.
Data analysis
Composite data are expressed as means ± SEM. Statistical analysis was performed with one‐way ANOVA followed by Dunnett's test. Differences were considered to be significant at P ≤ 0.05. Analysis of obtained data was carried out using GraphPad Prism program (San Diego, California, USA).
Results
Expression of STOX1 was selectively high in sensory epithelial cells, but low in stromal cells
To determine the role of STOX1 in development of sensory epithelium in the inner ear, we first studied localization of STOX1 within the otic vesicle or developing ear, at different stages, when mechanosensory hair cells are gradually generated. Immunofluorescence results showed that STOX1 was specifically expressed in the otic vesicle at E14, and was subsequently selectively expressed in sensory epithelium at E18 and P3 (Fig. 1a), but not in adjacent surrounding tissue. In addition, expression of STOX1 was highest at E14, then reduced gradually (Fig. 1b,d). These findings suggest that STOX1 was involved in regulation of inner ear sensory epithelial development.
Figure 1.
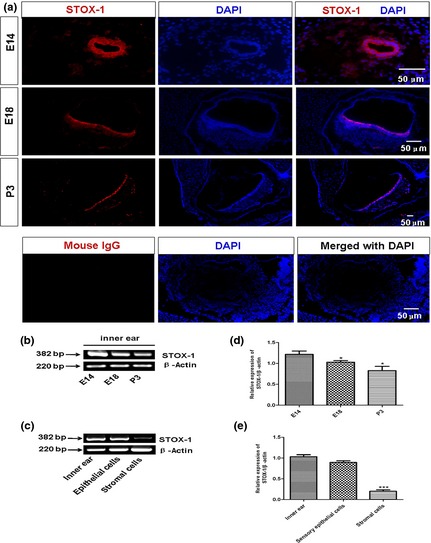
Storkhead box 1 (STOX1) expression patterns in the developing inner ear. (a) STOX1 was localized to specialized cells surrounding the utricle and macula utriculi in 12 μm adjacent sections of inner ear tissues, at stages embryonic day 14 (E14), E18 and postnatal day 3 (P3); antibody against mouse IgG was served as negative control for primary antibody. (b, d) RT‐TCR assays for mRNA expression of STOX1 in developing inner ears. Note that expression of STOX1 was significantly higher at E14, but gradually decreased as time proceeded. (c, e) STOX1 was expressed highly in epithelial cells, but was low in stromal cells; detected by RT‐PCR. Scale bar = 50 μm.
To provide clear evidence for selective expression patterns of STOX1 in inner ear epithelium, but not in the stroma, we adopted mouse utricular epithelial cell cultures that had previously been established in our laboratory 18. Characterization of these indicated that, as expected, these cells expressed epithelial progenitor marker Sox2 and hair cell maker myosin VII a, but not stromal cell marker vimentin, glial cell marker GFAP or neuronal cell marker neurofilaments (see Fig. S1 and Table S1). RT‐PCR results further confirmed that STOX1 was expressed mainly in sensory epithelial cells, but not in stromal cells when purified utricular epithelial cells versus the lower stromal cells were examined (Fig. 1c,e). Of the utricular epithelial cells, some STOX1‐positive ones had overlapping patterns with myosinVIIa (marker for hair cells) and SOX2 (early marker for sensory epithelial progenitors in the inner ear) 21 (Fig. 2a). In contrast, cultured stromal cells expressed vimentin (fibroblast antigen) and very weak STOX1 signal, but virtually no SOX‐2 and myosinVIIa (Fig. 2b). Labelling for STOX 1 in inner ear sections was observed in epithelial cell nuclei as shown in Fig. 1a, but diffuse labelling was also found in the cytoplasm, particularly in peri‐membrane cortical regions of the cultured epithelial cells (Fig. 2a). Such cytoplasmic expression including cortical distribution of HAM1 (homolog of STOX1), in C. elegans cells has previously been reported 22.
Figure 2.
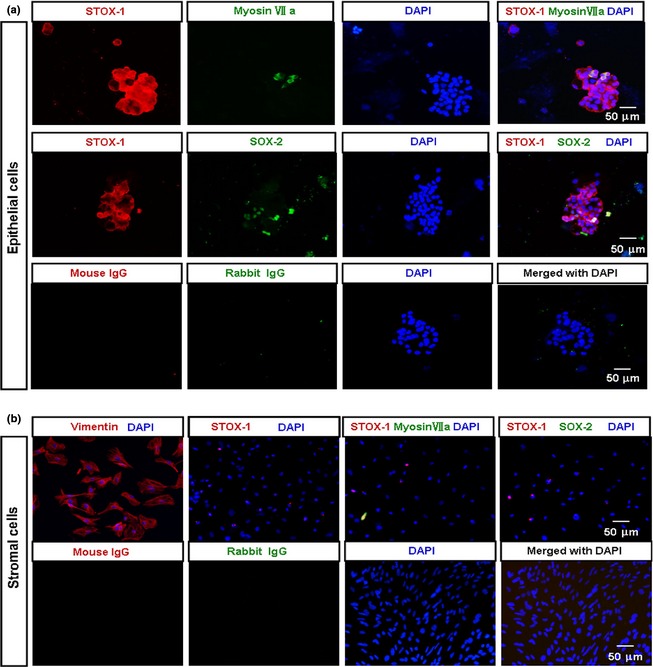
Gene expression patterns of storkhead box 1 (STOX1), myosinVIIa and SOX2 proteins? in cultured mouse utricular sensory epithelial cells by immunocytochemistry. (a) Fluorescence images of 2d epithelial cell cultures with antibodies against STOX1, myosinVIIa and SOX2. STOX1 and myosinVIIa were co‐expressed in hair cells. SOX2 gene protein expression overlapped with that of STOX1 in the epithelial cells. Note that although STOX1 is a transcription factor, diffuse labelling was present in cultured epithelial cell cytoplasm, particularly in the cortical region of the cells. (b) Immunostaining of stromal cells with antibodies as (a). Stromal cells expressed vimentin (fibroblast antigen) and only few expressed STOX1, but virtually did not express SOX‐2 and myosinVIIa. Normal mouse IgG and rabbit IgG served as negative controls for primary antibodies. Scale bar = 50 μm.
STOX1 stimulated proliferation of cultured utricular epithelial cells
To examine whether STOX1 influenced proliferation of the utricular epithelial cells, we performed BrdU labelling immunocytochemistry. As shown in Fig. 3, under control culture conditions, moderate numbers of BrdU‐positive cells were detected (Fig. 3a,b), while much lower numbers of BrdU‐positive cells were seen after STOX1 shRNA lentivirus knockdown. In contrast, much higher numbers of BrdU‐positive cells were detected, by over‐expression of STOX1 (Fig. 3a). Both NC and vector‐control of over‐expressed STOX1 did not show any significant effect (Fig. 3a). Efficiency of STOX1 over‐expression and knockdown was confirmed by western blotting (Fig S2). Cell counts performed on cultures infected with STOX1 over‐expression lentivirus confirmed that STOX1 significantly enhanced proliferation of the utricular epithelial cells (Fig. 3c). Furthermore, CCK‐8 assays indicated that treatment of STOX1 knockdown lentivirus reduced total number of cells (Fig. 4a). On the contrary, addition of STOX1 over‐expression lentivirus increased total number of cells in the cultures (Fig. 4b). These data clearly indicate that STOX1 regulated utricular epithelial cell proliferation.
Figure 3.
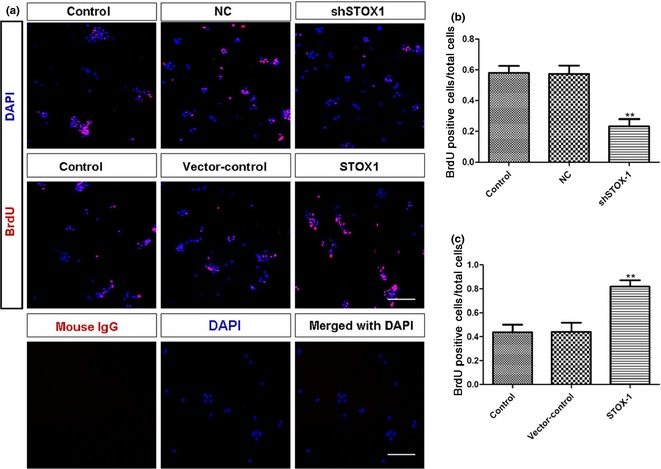
BrdU immunocytochemistry of utricular epithelial cell cultures. (a) Control cultures and cultures infected with lentivirus (20 multiplicity of infection) targeting NC, shSTOX1, vector‐control, STOX1 over‐expression lentivirus, respectively. BrdU was added to cultures at 24 h, and cultures were fixed at 48 h for immunocytochemistry. Antibody against mouse IgG served as negative control for primary antibody. (b, c) Bar graphs indicating proportion of BrdU‐positive cells relative to total cells. Note that numbers of BrdU‐positive cells were greatly reduced by shSTOX1 and significantly increased by over‐expression of STOX1. Data shown as means ± SD (n = 3, **P < 0.01 versus pairing negative control, **P < 0.01 versus pairing vector‐control). Scale bar = 100 μm.
Figure 4.
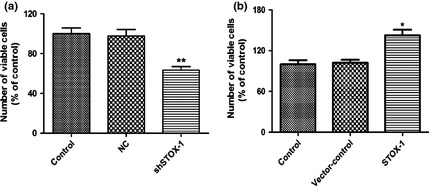
Lentivirus‐delivered storkhead box 1 (STOX1)‐shRNA induced epithelial cell growth arrest, while over‐expression of STOX1 stimulated epithelial cell proliferation. (a) Total of 1 × 104 epithelial cells per well were plated in 96‐well plates for 24 h and then were transfected with lentivirus (20 multiplicity of infection) targeting STOX1‐shRNA along with paired negative control. (b) 1 × 104 epithelial cells per well were plated in 96‐well plates for 24 h as (a), then were transfected with (20 multiplicity of infection) STOX1 over‐expression lentivirus along with the paired vector‐control. Cells were incubated at 37 °C for 48 h and then cell proliferation was assessed using Cell Counting Kit 8. Data shown as means ± SD (n = 3, **P < 0.01 versus pairing negative control, *P < 0.05 versus pairing vector‐control).
ShSTOX1 inhibited sphere‐forming capability of sensory epithelial cells
The auditory sensory epithelium has recently been shown to harbour cells with stem cell features. Such cells have proliferative capacity and the ability to form floating colonies, so‐called spheres 23, 24. Inner ear development is accompanied by enormous increase in numbers of sensory epithelial cells. ShSTOX1 significantly reduced the number of propagated spheres from P3 onward, and also reduced overall size of each sphere (Fig. 5a,b), suggesting that STOX1 plays an important role in proliferation of inner ear sensory epithelium stem cells.
Figure 5.
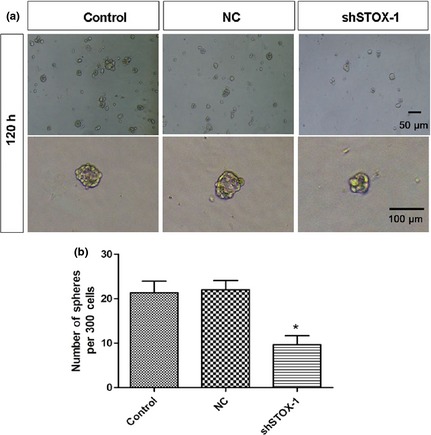
Numbers of spheres formed by epithelial cells were significantly reduced by shRNA‐mediated gene silencing of storkhead box 1 (STOX1). Utricular epithelial cells were transfected with lentivirus (20 multiplicity of infection) targeting STOX1‐shRNA and paired negative control. After 120 h, spheres were harvested and analyzed. Sphere counts were performed as described in the Materials and methods section. To compare numbers of spheres in control and STOX1 shRNA‐treated epithelial cells, the same numbers of cells per culture stage were used for accurate quantification (n = 3, *P < 0.05 versus pairing negative control).
STOX‐1 significantly up‐regulated cell cycle regulatory genes in utricular sensory epithelial cells
To investigate the molecular basis for this function of STOX1 in utricular sensory epithelial cells, we measured levels of several cell cycle‐related proteins including PCNA 25, cyclin A 26 and cyclin E 27. PCNA performs critical functions during DNA replication in cell proliferation. Cyclin A preferentially stimulates microtubule‐nucleating activity of centrosomes, and cyclin E is a key regulator of G1‐S transition. They are thus cell proliferation‐related proteins, coded for by their specific genes. As shown in Fig. 6a,c, PCNA, cyclin A and cyclin E expression levels were markedly reduced by shSTOX1 but significantly increased by STOX1 over‐expression in utricular sensory epithelial cells compared to vehicle‐treated samples (Fig. 6b,d).
Figure 6.
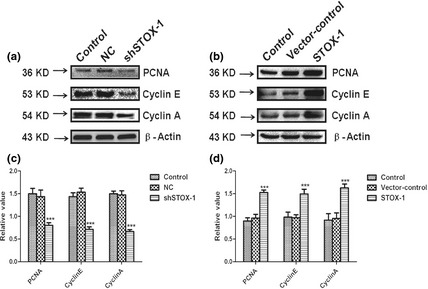
Expression of PCNA, cyclin E and cyclin A under normal culture conditions and after transfection with shSTOX1 or STOX1 over‐expression lentivirus. Protein levels of PCNA, cyclin E, cyclin A and β‐actin were analyzed by western‐blotting. (a, c) Densitometric quantification of protein bands showed significant reduction in PCNA, cyclin E, cyclin A after 48 h transfection with STOX1 shRNA lentivirus, whereas mismatch shRNA with only one nucleotide exchange (NC) showed no significant effect. (b, d) Densitometric quantification of protein bands showed significant increase in PCNA, cyclin E, cyclin A expression after 48 h transfection with STOX1 over‐expression lentivirus, whereas paired vector control showed no significant effect. Densitometric quantification was performed with at least three western‐blots from three different experiments. Epithelial cells transducted by over‐expression lentivirus, exhibited over 60% higher level of PCNA, cyclin E and cyclin A than the normal group, while in RNAi transfected cells, expression of PCNA, cyclin E and cyclin A was reduced by around 50%. Data are shown as means ± SD (n = 3, ***P < 0.001).
STOX1 regulated expression of phospho‐AKT, but not expression of phospho‐ p38 and phospho‐ERK
To determine potential involvement of protein kinase pathways in STOX1‐induced sensory epithelial cell proliferation, we examined phosphorylation status (indicative of activation states) of the three major protein kinases after cells were exposed to STOX1 shRNA or STOX1 over‐expression lentivirus, for 48 h. Exposure to STOX1 shRNA lentivirus led to reduction in levels of phospho‐AKT (Fig. 7a,c), whereas over‐expression of STOX1 reversed inactivation of AKT caused by shSTOX1 (Fig. 7b,d). In contrast, STOX1 over‐expression or knockdown did not affect phospho‐ERK1/2, p38 MAPK phosphorylation status. Observed alterations in phosphorylation status in response to shSTOX1 or over‐expression of STOX1, were not caused by changes in total protein content of the respective protein kinases, as total AKT, ERK1/2 and p38 levels remained the same (Fig. 7a,b). These data suggest that STOX1 induced proliferation of the utricular epithelial cells via the AKT pathway.
Figure 7.
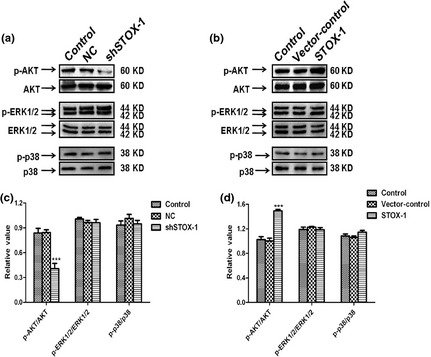
Expression of p‐AKT, p‐ERK, p‐p38 after transfection with STOX 1 knockdown and over‐expression lentivirus. Protein levels of p‐AKT, p‐ERK, p‐P38 and total AKT, ERK and P38 were determined by western blotting. Western blotting analysis was performed with cell lysates from utricular epithelial cells after transfection with shSTOX1 or STOX1 over‐expression lentivirus and paired controls respectively. Knockdown of STOX1 reduced phosphorylation of AKT expression, while STOX1 over‐expression induced phospho‐AKT activation relative to total AKT expression. In contrast, STOX1 had no significant effect on p‐ERK and p‐p38 expression. Three independent experiments were performed in triplicate for each sample. Data are shown as means ± SD. Asterisk indicates significant difference as determined by one‐way ANOVA (n = 3, ***P < 0.001).
Inhibition of AKT suppressed effects of STOX‐1 on epithelial cell proliferation
To provide more evidence that the AKT pathway mediated proliferation‐promoting effects by STOX1, we added 10 μmol/l MK‐2206 (an AKT inhibitor) prior to addition of STOX1 over‐expression lentivirus, to the cultures (Fig. 8). CCK‐8 assays showed that MK‐2206 treatment completely abolished the stimulatory effect of STOX‐1 on total numbers of epithelial cells. Total cell numbers were significantly lower in cultures co‐treated with MK‐2206 and STOX‐1 expressing lentivirus, compared to cultures stimulated with STOX‐1 expressing lentivirus alone (Fig. 8a). Furthermore, expression of PCNA, cyclin A and cyclin E was also significantly reduced in cultures co‐treated with MK‐2206 and STOX‐1 expressing lentivirus, than those treated only with STOX1 over‐expression lentivirus (Fig. 8b).
Figure 8.
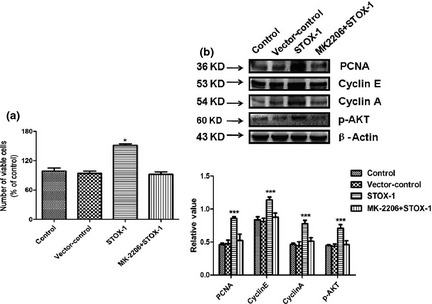
Effects of STOX ‐1 on cell numbers and PCNA , cyclin E, cyclin A expression are dependent upon the AKT pathway. (a) Total cell numbers were determined by CCK‐8 assay after utricular epithelial cells were transfected with STOX1 over‐expression lentivirus or paired vector control, and exposed to STOX1 over‐expression lentivirus, in the presence of AKT inhibitor MK2206 (10 μm). (b) Protein levels of PCNA, cyclin E, cyclin A, p‐AKT and β‐actin were determined by western blotting. Western blotting analysis was performed with cell lysates from utricular epithelial cells after transfection with STOX1 over‐expression lentivirus or paired vector control, and exposed to STOX1 over‐expression lentivirus in the presence or absence of AKT inhibitor MK2206 (10 μm), respectively. Note that STOX1 over‐expression induced PCNA, cyclin E, cyclin A, p‐AKT expression relative to β‐actin. In contrast, STOX1 had no significant effect on PCNA, cyclin E, cyclin A, p‐AKT expression in the presence of MK2206. Three independent experiments were performed in triplicate for each sample. Results are expressed as means ± SD (n = 3). Asterisk indicates significant difference as determined by one‐way ANOVA (*P < 0.05, ***P < 0.001).
Discussion
In this study, we have reported a novel function for STOX1 in the mammalian inner ear epithelium. We observed that STOX1 was highly expressed in sensory epithelial cells, but not in neighbouring stromal cells. We have demonstrated that STOX1, as a transcriptional activator, regulated sensory epithelial cell proliferation and enhanced their numbers. Since STOX1 is a regulatory factor for cell proliferation in the mammals, similar mechanisms might also occur in inner ears of non‐mammalian vertebrates, in which supporting cells retain the capacity to proliferate, differentiate and replace hair cells after damage. Consistent with this notion, a further recent study has also shown that STOX1 was a gene up‐regulated during avian inner ear regeneration 28.
This epithelium is uniquely vulnerable to permanent auditory and vestibular deficits in mature mammals, as cell proliferation that produces regenerated hair cells in other vertebrates, is limited in mammals 6, 29. The supporting cells of the inner ear epithelium are most likely to be the progenitors of hair cells, and thus, supporting cell proliferation is critical for replacement of lost hair cells, and supporting cells that convert into new hair cells 19, 30, 31, 32, 33, 34. In birds, after damage to the sensory epithelium, supporting cells dedifferentiate, re‐enter the cell cycle, and produce regenerated hair cells that restore the functions of hearing and balance 2. After damage to mature mammalian vestibular epithelium, some supporting cells proliferate 7, 35. Although non‐sensory epithelial cells may use a different mechanism for their proliferation, they are able to spread into lesions, to become supporting cells 32. These non‐sensory epithelial cells have also a capability to convert directly into hair cells when they are forced to express Math1 36, 37, 38. Stimulating proliferation of supporting cells and non‐sensory epithelial cells appears to enhance the possibility of mammalian inner ear epithelial repair; thus targeting up‐regulation of STOX1 may be a viable strategy, in combination with others, for stimulating cell replacement in the mammalian inner ear sensory epithelium.
The mechanism by which STOX1 promotes proliferation of hair cell precursors could be similar to Foxl1 39, 40, 41. We found that after STOX1 knockdown in cultured epithelial cells, expression of markers for proliferation (PCNA and cyclin A, cyclin E) 26, 42 was significantly reduced, but up‐regulated following STOX1 over‐expression. During mammalian organogenesis, the AKT pathway regulates both cell proliferation and programmed cell death 11, 43. Our results further show that shSTOX1 inhibited phosphorylation of AKT in the inner ear utricular epithelial cells, while over‐expression of STOX1 activated phosphorylation of AKT. On the other hand, either STOX1 knockdown or over‐expression affected phosphorylation of p‐38 and p‐ERK. Moreover, proliferation‐enhancing effects by STOX1 could be blocked by AKT inhibitor MK‐2206. Together, these data indicate that AKT, rather than p‐p38 and p‐ERK, is likely to be a possible mechanism through which STOX1 regulates cell‐cycle progression.
Storkhead box 1 localizes in cell nuclei and immunochemical localization of STOX1 in inner ear cryostat sections confirmed this, as shown in Fig. 1. However, diffuse labelling of STOX1 seemed to also appear in the cytoplasm, in particular, cortex of cultured inner ear epithelial cells, which is consistent with a previous study indicating that HAM1, a STOX1 homolog, has been observed in the cortex of most nematode cells 22.
Our results demonstrate that in the mouse, STOX1 is involved in controlling cell proliferation of utricular sensory epithelial cells during inner ear development; however, it is unknown whether STOX1 interacts with other genetic pathways implicated in this process. For example, Math1 has been shown to be sufficient for generation of ectopic hair cells in cochlear sensory epithelium 44, 45. There might be possible interactions between Math1 and STOX1 during hair cell differentiation. It is possible that STOX1 interacts with Math1 in the pro‐sensory region, and in the absence of STOX1, increase in binding of Math1 to its target genes/proteins might promote cells to choose the hair cell fate. In addition, findings that expression patterns of STOX1 and SOX2 overlap in the inner ear epithelium and STOX1 over‐expression promotes numbers and size of otospheres, implies that STOX1 may function in specification and maintenance of this stem cell type, in the mammalian inner ear. Future studies remain to be performed to address these possibilities.
Taken together, our study demonstrates that STOX1 transcriptional regulatory pathway functions in proliferation of inner ear epithelial cells. Additional studies on how STOX1 controls cell proliferation and differentiation, and interacts with other genes in the inner ear will further advance our understanding of inner ear development and hair cell differentiation, and will be helpful for identifying new strategies for stimulating hair cell regeneration.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Table S1 Immunocytochemical characterization of the cultured utricular sensory epithelial cells.
Fig. S1 Utricular epithelial cell cultures and immunostainings.
Fig. S2 Assessment of transfection efficiency.
Acknowledgements
The study was supported by funds to WQG from the Chinese Ministry of Science and Technology (2012CB966800, 2013CB945600 and 2012CB967903), the National Natural Science Foundation of China (81130038 and 81372189), Science and Technology Commission of Shanghai Municipality (Pujiang program), Shanghai Education Committee Key Discipline and Specialty Foundation (J50208), Shanghai Health Bureau Key Discipline and Specialty Foundation and KC Wong Foundation.
References
- 1. Corwin JT, Cotanche DA (1988) Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772–1774. [DOI] [PubMed] [Google Scholar]
- 2. Corwin JT, Jones JE, Katayama A, Kelley MW, Warchol ME (1991) Hair cell regeneration: the identities of progenitor cells, potential triggers and instructive cues. Ciba Found. Symp. 160, 103–120; discussion 20–30. [DOI] [PubMed] [Google Scholar]
- 3. Bormuth V, Barral J, Joanny JF, Julicher F, Martin P (2014) Transduction channels’ gating can control friction on vibrating hair‐cell bundles in the ear. Proc. Natl. Acad. Sci. USA 111, 7185–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burns JC, Corwin JT (2014) Responses to cell loss become restricted as the supporting cells in mammalian vestibular organs grow thick junctional actin bands that develop high stability. J. Neurosci. 34, 1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fekete DM, Muthukumar S, Karagogeos D (1998) Hair cells and supporting cells share a common progenitor in the avian inner ear. J. Neurosci. 18, 7811–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forge A, Li L, Corwin JT, Nevill G (1993) Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 259, 1616–1619. [DOI] [PubMed] [Google Scholar]
- 7. Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT (1993) Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 259, 1619–1622. [DOI] [PubMed] [Google Scholar]
- 8. Cotanche DA (1987) Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear. Res. 30, 181–195. [DOI] [PubMed] [Google Scholar]
- 9. Jones JE, Corwin JT (1996) Regeneration of sensory cells after laser ablation in the lateral line system: hair cell lineage and macrophage behavior revealed by time‐lapse video microscopy. J. Neurosci. 16, 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balak KJ, Corwin JT, Jones JE (1990) Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J. Neurosci. 10, 2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bell TJ, Oberholtzer JC (2010) cAMP‐induced auditory supporting cell proliferation is mediated by ERK MAPK signaling pathway. J. Assoc. Res. Otolaryngol. 11, 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng JL, Frantz G, Lewis AK, Sliwkowski M, Gao WQ (1999) Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage. J. Neurocytol. 28, 901–912. [DOI] [PubMed] [Google Scholar]
- 13. Zheng JL, Gao WQ (1997) Analysis of rat vestibular hair cell development and regeneration using calretinin as an early marker. J. Neurosci. 17, 8270–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abel D, Abdul‐Hamid O, Dijk M, Oudejans CB (2012) Transcription factor STOX1A promotes mitotic entry by binding to the CCNB1 promotor. PLoS One 7, e29769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng G, Yi P, Yang Y, Chai Y, Tian D, Zhu Z, et al (2013) Developmental stage‐dependent transcriptional regulatory pathways control neuroblast lineage progression. Development 140, 3838–3847. [DOI] [PubMed] [Google Scholar]
- 16. Blixt A, Mahlapuu M, Aitola M, Pelto‐Huikko M, Enerback S, Carlsson P (2000) A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 14, 245–254. [PMC free article] [PubMed] [Google Scholar]
- 17. Saffer LD, Gu R, Corwin JT (1996) An RT‐PCR analysis of mRNA for growth factor receptors in damaged and control sensory epithelia of rat utricles. Hear. Res. 94, 14–23. [DOI] [PubMed] [Google Scholar]
- 18. Zheng JL, Helbig C, Gao WQ (1997) Induction of cell proliferation by fibroblast and insulin‐like growth factors in pure rat inner ear epithelial cell cultures. J. Neurosci. 17, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng JL, Keller G, Gao WQ (1999) Immunocytochemical and morphological evidence for intracellular self‐repair as an important contributor to mammalian hair cell recovery. J. Neurosci. 19, 2161–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leow CC, Romero MS, Ross S, Polakis P, Gao WQ (2004) Hath1, down‐regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 64, 6050–6057. [DOI] [PubMed] [Google Scholar]
- 21. Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, et al (2005) Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031–1035. [DOI] [PubMed] [Google Scholar]
- 22. Frank CA, Hawkins NC, Guenther C, Horvitz HR, Garriga G (2005) C. elegans HAM‐1 positions the cleavage plane and regulates apoptosis in asymmetric neuroblast divisions. Dev. Biol. 284, 301–310. [DOI] [PubMed] [Google Scholar]
- 23. Diensthuber M, Oshima K, Heller S (2009) Stem/progenitor cells derived from the cochlear sensory epithelium give rise to spheres with distinct morphologies and features. J. Assoc. Res. Otolaryngol. 10, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhai S, Shi L, Wang BE, Zheng G, Song W, Hu Y, et al (2005) Isolation and culture of hair cell progenitors from postnatal rat cochleae. J. Neurobiol. 65, 282–293. [DOI] [PubMed] [Google Scholar]
- 25. Weisgerber UM, Boeing H, Nemitz R, Raedsch R, Waldherr R (1993) Proliferation cell nuclear antigen (clone 19A2) correlates with 5‐bromo‐2‐deoxyuridine labelling in human colonic epithelium. Gut 34, 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Minnebo N, Gornemann J, O'Connell N, Van Dessel N, Derua R, Vermunt MW, et al (2013) NIPP1 maintains EZH2 phosphorylation and promoter occupancy at proliferation‐related target genes. Nucleic Acids Res. 41, 842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Belachew S, Aguirre AA, Wang H, Vautier F, Yuan X, Anderson S, et al (2002) Cyclin‐dependent kinase‐2 controls oligodendrocyte progenitor cell cycle progression and is downregulated in adult oligodendrocyte progenitors. J. Neurosci. 22, 8553–8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ku YC, Renaud NA, Veile RA, Helms C, Voelker CC, Warchol ME, et al (2014) The transcriptome of utricle hair cell regeneration in the avian inner ear. J. Neurosci. 34, 3523–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Groves AK (2010) The challenge of hair cell regeneration. Exp. Biol. Med. 235, 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burns JC, Cox BC, Thiede BR, Zuo J, Corwin JT (2012) In vivo proliferative regeneration of balance hair cells in newborn mice. J. Neurosci. 32, 6570–6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelley MW, Talreja DR, Corwin JT (1995) Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J. Neurosci. 15, 3013–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyers JR, Corwin JT (2007) Shape change controls supporting cell proliferation in lesioned mammalian balance epithelium. J. Neurosci. 27, 4313–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morsli H, Choo D, Ryan A, Johnson R, Wu DK (1998) Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 18, 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsue TT, Watling DL, Weisleder P, Coltrera MD, Rubel EW (1994) Identification of hair cell progenitors and intermitotic migration of their nuclei in the normal and regenerating avian inner ear. J. Neurosci. 14, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawlor P, Marcotti W, Rivolta MN, Kros CJ, Holley MC (1999) Differentiation of mammalian vestibular hair cells from conditionally immortal, postnatal supporting cells. J. Neurosci. 19, 9445–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shou J, Zheng JL, Gao WQ (2003) Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol. Cell Neurosci. 23, 169–179. [DOI] [PubMed] [Google Scholar]
- 37. Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al (2005) Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 11, 271–276. [DOI] [PubMed] [Google Scholar]
- 38. Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y (2003) Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J. Neurosci. 23, 4395–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, et al (2006) The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 66, 2153–2161. [DOI] [PubMed] [Google Scholar]
- 40. Shi C, Sakuma M, Mooroka T, Liscoe A, Gao H, Croce KJ, et al (2008) Down‐regulation of the forkhead transcription factor Foxp1 is required for monocyte differentiation and macrophage function. Blood 112, 4699–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yan J, Xu L, Crawford G, Wang Z, Burgess SM (2006) The forkhead transcription factor FoxI1 remains bound to condensed mitotic chromosomes and stably remodels chromatin structure. Mol. Cell. Biol. 26, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M (1995) Human cyclin E, a nuclear protein essential for the G1‐to‐S phase transition. Mol. Cell. Biol. 15, 2612–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rahmani A, Kheradmand D, Keyhanvar P, Shoae‐Hassani A, Darbandi‐Azar A (2013) Neurogenesis and increase in differentiated neural cell survival via phosphorylation of Akt1 after fluoxetine treatment of stem cells. Biomed. Res. Int. 2013, 582526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng JL, Gao WQ (2000) Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 3, 580–586. [DOI] [PubMed] [Google Scholar]
- 45. Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ (2000) Hes1 is a negative regulator of inner ear hair cell differentiation. Development 127, 4551–4560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Immunocytochemical characterization of the cultured utricular sensory epithelial cells.
Fig. S1 Utricular epithelial cell cultures and immunostainings.
Fig. S2 Assessment of transfection efficiency.


