Abstract
Abstract. Many mouse models of neoplasia and pre‐neoplasia require the examination of whole mounts of the gastrointestinal tract. A simple device has been produced to facilitate the rapid preparation of mouse intestines for subsequent quantification of tumours and pre‐neoplastic lesions such as aberrant crypt foci. The device greatly speeds up the production of whole mounts and also provides far more consistent and better‐quality preparations.
INTRODUCTION
Nearly a million people worldwide develop cancer of the colon and rectum and half a million patients die of the disease each year (Weitz et al. 2005). While there have been great successes in understanding ways of preventing colorectal cancer, especially by removing pre‐neoplastic polyps during endoscopic screening, there is still a great need for improvements in the treatment of established tumours. Recent advances have shown that many tumours are affected by genetic changes, blood‐borne and local factors, which can be selectively targeted with various agents (Ferrara et al. 2004; Gschwind et al. 2004). In addition, there is great potential to reduce colorectal cancer by dietary modification (Goodlad 2001; Bashir et al. 2004; Johnson 2004).
There are several rodent models of cancer (Boivin et al. 2003), and we have used the Apc Min/+ (multiple intestinal neoplasia, Min mouse) (Moser et al. 1990) to study the action of some of the above factors. The mice are heterozygous at the adenomatous polyposis coli (Apc) locus and carry one wild‐type and one mutated, functionally inactive allele. Loss of the wild‐type allele leads to the development of multiple adenomas, as occurs in familial adenomatous polyposis (FAP) in man, where thousands of polyps develop in the colon in adolescence. In the Apc Min/+ mouse, the polyps number between 60 and 100 in the small intestine and 2 to 6 in the colon. Scoring the number and diameter of polyps in the various segments of the gut can then be used to determine the effects of various interventions and treatments.
To best quantify these tissues, the intestine needs to be flushed, opened and spread out flat for scoring under a dissection microscope. Opening the gut using scissors is time consuming and requires a great deal of skill to obtain a good preparation. If the preparation is less than optimal, it can be difficult to see all the polyps, especially when there are many present.
In other genetic and chemically induced models of intestinal cancer there tend not to be as many polyps as are seen in the Apc Min/+ mouse, so that the scoring of polyps is usually easier. However, many models, especially of chemically induced cancer, give rise to many pre‐neoplastic lesions such as aberrant crypt foci and mucin‐depleted foci that are best scored in en face whole mount preparations.
A device using stainless steel rods threaded through the intestine and a cutting guide with narrow slots to pass a razor blade through was used successfully by Bass Hassan (personal communication) to prepare intestines from Apc Min/+ mice. We constructed a copy of his device but found it difficult to use, as one cannot see the tissue and the blade could slide off the rods. In addition, our cell proliferation measures (Goodlad et al. 1991; Goodlad 1994) require that we process our mice within a relatively short time frame, thus an easier and faster device was required.
Here, we describe a new design of gut cutting device, constructed in our laboratory mechanical workshop, which enables us to process over 30 mice in one morning. With two prosectors we can harvest several non‐gastrointestinal tissues, weigh the main regions of the gut and obtain a preparation of the opened small bowel and colon on a single piece of filter paper within 6 min per mouse. The resultant preparations are more uniform than manually opened ones, which greatly facilitates quantification. The preparations also allow polyps to be scored even if they are at the cut edge of the tissue, as with hand‐opened tissue we often found that the edge of the tissue could curl under the paper. If microscopic preparations are required, the resultant flat and even tissues can easily be rolled into a ‘Swiss roll’ for histological embedding.
Design of the device
The main portion of the base of the engine was machined from a solid block of high‐density acetal resin (Delrin®; Ryan Plastics, Earls Barton, UK). At each end there were 3 mm‐high elevated strips, which were channelled (2.5 mm) to take the rods (1, 2). The ends of the channels were capped off with the end pieces shown in white. Rods were made of stainless steel 2.4 mm in diameter with the ends rounded off. The main frame of the lid was machined from 6 mm thick duralumin plate and 1 × 15 mm stainless steel plates inset into the top of the lid. The duralumin cutting guides were angle machined and were attached.
Figure 1.
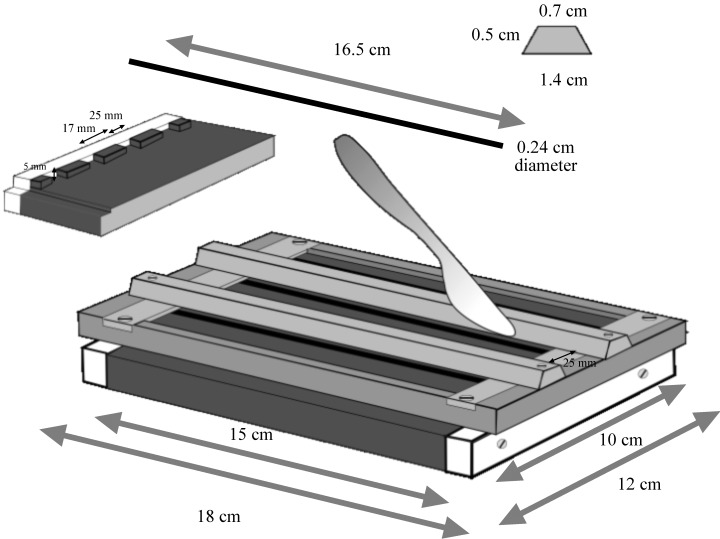
Diagram of the gut‐cutting engine. The segments of intestine are threaded over rods that sit in the grooves shown in the insert on the left. The two shaped sections in the centre of the ‘lid’ (shown at the right) are used to guide a knife and longitudinally dissect the intestines.
Figure 2.

The gut‐cutting device. Shown with the lid on or off. Note the rods placed in their grooves.
Use of the device
After mice were killed, small intestine and colon were isolated and rinsed using a Gilson‐type pipette tip, cut at the wide end to fit on the Luer connection of a 10‐ or 20‐ml syringe. The stomach and caecum can also be rinsed with cold phosphate buffered saline (PBS), blotted and weighed. The small bowel (SB) is divided into three equal sections (proximal, SB1; middle, SB2; distal, SB3); the colon is left uncut. The stainless steel rods are then wet in PBS, threaded through the segments and then placed over a piece of filter paper in the device. (Precut paper can be obtained from Hollingsworth and Vose Co. Ltd., Postlip Mills, Winchcombe, Cheltenham, Gloucestershire, GL54 5BB). The lid of the device is then fitted and its angled bars used to guide a scalpel blade to longitudinally dissect the tissues. The tissue can be gently held to ensure that it does not move with the knife.
The lid is then removed. Filter paper and tissues (still on their rods) are carefully removed (a piece of stiff card can be placed beneath the filter paper to aid lifting). The tissue is then wet slightly with a gloved fingertip, dipped into PBS and rubbed along the segments. Rods are then rolled side to side to open up the gut and spread it flat. At this point, the tissue should adhere to the filter paper. Typically, preparations from Apc Min/+ mice prepared using the engine, are shown in Fig. 3(a); preparations using scissors are shown in Fig. 3(b).
Figure 3.
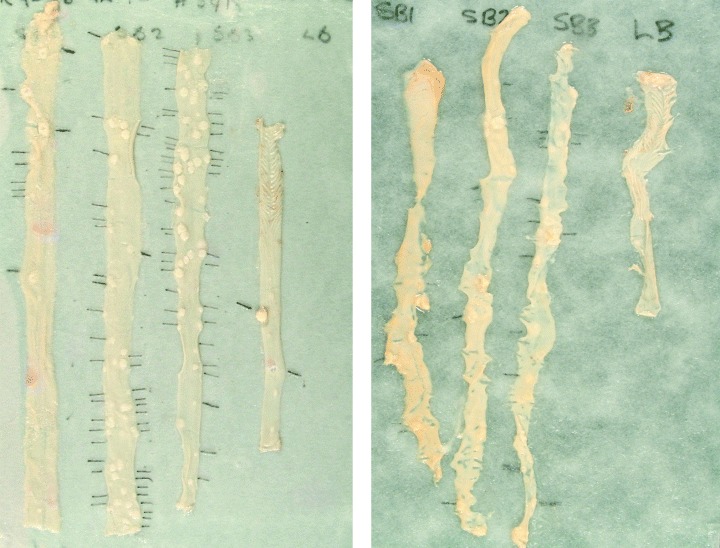
(a) Typical preparation of an Apc Min/+ mouse prepared using the gut engine. (b) Preparation of a typical preparation Apc Min/+ mouse prepared using a pair of offset scissors.
Whereas the tissue features can be scored fresh, it is usually more convenient to fix the preparation first. This is also desirable as well‐preserved tissue may be used for subsequent procedures, such as histological and immunohistochemical analysis. For fixation in Carnoy's solution, tissues are immersed for 3 h and are then stored in 70% ethanol (Bashir et al. 2004); alternatively, they may be fixed in neutral buffered formalin. Practically, it has been found that formalin‐fixed tissues do not stick well to filter paper. Using index card has been found to be preferable to filter paper for this fixative. However, Carnoy's fixed tissue is best for scoring polyps; they are more prominent and Carnoy's fixed tissue is required for some ‘microdissection’ methods of cell proliferation and crypt fission used in our laboratory. It is also better for nuclear preservation and some immunohistochemical techniques.
Macroscopic polyp scoring
Fixed tissues can be assessed under a stereomicroscope ( × 20 magnification) for polyp number and diameter (measured with digital callipers). Polyp volume can be derived from polyp diameter, assuming a hemispherical shape (common in the small intestine) and assuming a spherical shape in the colon.
Other measures
Tissue can be bulk‐stained with 0.1% methylene blue to score the number (and multiplicity) of aberrant crypt foci (ACFs) (Pretlow et al. 2003; Raju & Bird 2003). Location of ACFs within the colon can easily be performed by generating a series of 10 square grids of different sizes on overhead transparency paper, so that a grid can be found to fit under the tissue and divide it into tenths. Tissue can also be re‐stained with high iron diamine Alcian blue stain to detect mucin‐depleted foci (Caderni et al. 2003); this stain however, is permanent, which needs to be taken into consideration if further procedures are required.
Sampling a preparation several times can result in loss of some tissue adhesion, in which case it is advisable to secure it using fine etymological pins (Watkins & Doncaster, Kent, UK).
Micropolyp counts
For further histological evaluation tissue may be rolled up in a ‘Swiss roll’ configuration and processed to paraffin blocks in the usual manner. Five 4‐µm thick ‘step sections’ at least 100 µm apart are cut and stained with haematoxylin and eosin. The micropolyps are assigned to a category ranging from 1 to 4; adenomas that remain within the limits of a single villus are classified as category 1, those that occupy the space of 2–5 villi are classified as category 2, 6–10 villi as category 3 and more than 10 villi are category 4 (Kongkanuntn et al. 1999).
REFERENCES
- Bashir O, Fitzgerald AJ, Goodlad RA (2004) Both suboptimal and elevated vitamin intake increase intestinal neoplasia and alter crypt fission in the Apc Min/+ mouse. Carcinogenesis 25, 1507–1515. [DOI] [PubMed] [Google Scholar]
- Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz. SH, Groden J, Coffey RJ (2003) Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124, 762–777. [DOI] [PubMed] [Google Scholar]
- Caderni G, Femia AP, Giannini A, Favuzza A, Luceri C, Salvadori M, Dolara P (2003) Identification of mucin‐depleted foci in the unsectioned colon of azoxymethane‐treated rats: correlation with carcinogenesis. Cancer Res. 63, 2388–2392. [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti‐VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 3, 391–400. [DOI] [PubMed] [Google Scholar]
- Goodlad RA (1994) Microdissection‐based techniques for the determination of cell proliferation in gastrointestinal epithelium: application to animal and human studies In: Celis JE, ed. Cell Biology: a Laboratory Handbook, pp. 205–216. San Diego and London: Academic Press. [Google Scholar]
- Goodlad RA (2001) Dietary fibre and the risk of colorectal cancer. Gut 48, 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad RA, Levi S, Lee CY, Mandir N, Hodgeson H, Wright NA (1991) Morphometry and cell proliferation in endoscopic biopsies: evaluation of a technique. Gastroenterology 101, 1235–1241. [DOI] [PubMed] [Google Scholar]
- Gschwind A, Fischer OM, Ullrich A (2004) The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat. Rev. Cancer 4, 361–370. [DOI] [PubMed] [Google Scholar]
- Johnson IT (2004) New approaches to the role of diet in the prevention of cancers of the alimentary tract. Mutat Res. 551, 9–28. [DOI] [PubMed] [Google Scholar]
- Kongkanuntn R, Bubb VJ, Sansom OJ, Wyllie AH, Harrison DJ, Clarke AR (1999) Dysregulated expression of beta‐catenin marks early neoplastic change in Apc mutant mice, but not all lesions arising in Msh2‐deficient mice. Oncogene 18, 7219–7225. [DOI] [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF (1990) A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324. [DOI] [PubMed] [Google Scholar]
- Pretlow TP, Edelmann W, Kucherlapati R, Pretlow TG, Augenlicht LH (2003) Spontaneous aberrant crypt foci in Apc1638N mice with a mutant Apc allele. Am. J. Pathol. 163, 1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju J, Bird RP (2003) Energy restriction reduces the number of advanced aberrant crypt foci and attenuates the expression of colonic transforming growth factor beta and cyclooxygenase isoforms in Zucker obese (fa/fa) rats. Cancer Res. 63, 6595–6601. [PubMed] [Google Scholar]
- Weitz. J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW (2005) Colorectal cancer. Lancet 365, 153–156. [DOI] [PubMed] [Google Scholar]


