Summary
Aims
To validate whether the optimal magnetic resonance perfusion (MRP) thresholds for ischemic penumbra and infarct core, between voxel and volume‐based analysis, are varied greatly among Chinese acute ischemic stroke patients.
Materials and methods
Acute ischemic stroke patients receiving intravenous thrombolysis within 6 h of onset that obtained acute and 24‐h MRP were reviewed. Patients with either no reperfusion (<30% reperfusion at 24 h) or successful reperfusion (>70% reperfusion at 24 h) were enrolled to investigate the ischemic penumbra and infarct core, respectively. The final infarct was assessed on 24‐h diffusion‐weighted imaging (DWI), which was retrospectively matched to the baseline perfusion‐weighted imaging (PWI) images by volume or voxel‐based analysis. The optimal thresholds that determined by each approach were compared.
Results
From June 2009 to Jan 2014, of 50 patients enrolled, 19 patients achieved no reperfusion, and 20 patients reperfused at 24 h. In patients with no reperfusion, Tmax > 6 seconds was proved of the best agreement with the final infarct in both volumetric analysis (ratio: 1.05, 95% limits of agreement:−0.23 to 2.33, P < 0.001) and voxel‐by‐voxel analysis (sensitivity: 72.3%, specificity: 74.3%). In patients with reperfusion, rMTT>225% (ratio:2.4, 95% limits of agreement: −6.5 to 11.4, P < 0.001) was found of the best volumetric agreement with the final infarct, while Tmax > 5.6 seconds (sensitivity: 76.8%, specificity: 70.3%) performed most accurately in voxel‐based analysis.
Conclusion
Among Chinese acute stroke patients, volume of Tmax >6 seconds may precisely target ischemic penumbra tissue as good as voxel‐based analysis performed, albeit no concordant MRP parameter is found to accurately predict infarct core because reperfusion occurred within 24 h after thrombolysis fails to restrain the infarct growth.
Keywords: Acute ischemic stroke, Magnetic resonance imaging, Reperfusion, Threshold
Introduction
To date, thrombolysis is the only proven pharmacological therapy for acute ischemic stroke. However, only about 1.6% of patients presenting with acute ischemic stroke in China received thrombolysis 1. Many stroke patients are currently ineligible for intravenous thrombolysis owing to the narrow therapeutic time window and the overemphasis of adverse effect of thrombolysis, such as symptomatic hemorrhagic transformation. A more rational selection in identifying potential responders of thrombolysis is promptly required to cast off the rigid time frame. With the advent of advanced imaging, the mismatch model that identified by diffusion and perfusion‐weighted imaging (DWI and PWI) was consistently proved as the criteria to select candidates eligible to get benefit from reperfusion. However, DWI was doubt to represent the permanent infarct as diffusion lesion reversal was observed in patients achieved early reperfusion 2. And some other studies have questioned the accuracy of mismatch in distinguishing ischemic penumbra, because the volumetric method identified model that applied in clinical trials was supposed to oversimplify the real composition of the ischemic tissue 3, 4.
Coregistration and voxel‐by‐voxel analysis are widely accepted as means to settle this issue. A retrospective analysis on the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) data set proved that, using coregistration of the baseline DWI/PWI images, infarct growth was significantly attenuated in comparison of initial analysis without coregistration 5. Additionally, the voxel‐based analysis may precisely position the optimal perfusion thresholds of ischemic penumbra and infarct core by receiver operating characteristic (ROC) curve analysis 6, 7 . In spite of these, for being lack of consensus of how perfusion data should be acquired and processed, thresholds are still varied and there is no study, as yet, yield robust result based on the improved approach.
The important challenge at present is whether ischemic penumbra and infarct core have been estimated more accurately by this improved approach. The specific thresholds calculated by the coregistered voxel analysis have not been compared with that by traditional volume‐based analysis among the same cohort patients. It thus remains unknown if there is a big difference between them, which may greatly affect responders' selection.
In this study, to better identifying the ischemic penumbra and infarct core among Chinese acute ischemic stroke patients, two premises awaited to be verified: (1) in patients not being reperfused, the infarct would grow to encroach the entire penumbra to the benign oligemia border, while in patients get reperfused from thrombolysis, the infarct would be stabilized at the border of the infarct core without transgressing into penumbral territories. The values that best predict the final infarct, thus, should be the optimal thresholds of ischemic penumbra and infarct core, respectively. (2) The optimal threshold could best reflect the final infarct irrespective of any calculating method selected. The threshold determined by volume analysis would not be significantly different from that based on voxel analysis.
Methods
Ethics Statement
The consent was obtained from each patient or an appropriate family member. The protocol of this study had been approved by the human ethics committee of local hospital. All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki.
Patients Selection
We retrospectively reviewed our prospectively collected database for consecutive patients with acute ischemic stroke received intravenous thrombolytic therapy between June 2009 and September 2013. For this study, we enrolled patients who (1) had unilateral anterior circulation ischemic stroke confirmed by diffusion‐weighted imaging (DWI); (2) received IV recombinant tissue plasminogen activator (rtPA) within 6 h of symptom onset; (3) underwent PWI before rtPA infusion, with perfusion deficit volume on Tmax map no less than 10 mL 8; (4) no hemorrhagic transformation after thrombolysis which hinders the evaluation of image; (5) underwent follow‐up DWI and PWI 24 h after rtPA infusion.
Pretreatment demographic, clinical and imaging data, comorbid conditions including history of hypertension, diabetes, hyperlipidemia, atrial fibrillation (classified as first‐detected or chronic) etc., were prospectively entered into the stroke database by our stroke team. Intravenous rt‐PA was administered according to the international guidelines (0.9 mg/kg, 90 mg dose at maximum, 10% in a bolus in 1 min with the remaining dose in a 60‐min infusion) 9. Stroke severity was assessed with the National Institutes of Health Stroke Scale (NIHSS) score at admission, 24 h, 7 days, and 3 months after stroke onset. Functional outcome was defined by a modified Rankin Scale (mRS) score.
MR Acquisition
All subjects underwent MRI on a 3.0T system (Signa Excite HD, General Electric Medical System, WI, USA) equipped with an 8‐channel phased array head coil. Foam pads were inserted into the space between the subject's head and the MRI head coil to minimize head motion. The MRI protocol included an axial isotropic diffusion‐weighted echo‐planar spin‐echo sequence (DWI), enhanced 3D multiecho GRE T2*‐weighted angiography (ESWAN), and bolus‐tracking perfusion‐weighted imaging (PWI). DWI was performed with a spin echo‐planar sequence (field of view = 240 mm, slice thickness = 1 mm, number of slices = 18, slice gap = 1 mm, acquisition matrix = 160 × 160).
ESWAN was in an axial orientation parallel to the anterior commissure to posterior commissure (AC‐PC) line and covered the whole brain, using 11 equally spaced echoes: TE = 4.5 ms [first echo]; interecho spacing = 4.5 ms; TR = 58 ms; FOV = 24 × 24 cm²; matrix size = 256 × 256; flip angle = 20°; slice thickness = 2.0 mm with no gap between slices. PWI was performed with gradient echo‐planar imaging (field of view = 240 mm, repetitive time = 1500 ms, echo time = 30 ms, acquisition matrix = 128 × 128. Repetitive scanning times = 50, gadolinium dose = 15 mL, contrast speed = 4–5 mL/s,duration = l min 15 s).
Image Analysis
Initial and 24‐h PWI data were reprocessed and calculated with commercial software (MIStar; Apollo Medical Imaging Technology, Melbourne, Vic., Australia). Quantitative perfusion maps (cerebral blood volume, CBV; cerebral blood flow, CBF; mean transit time, MTT; and the time until the residue function reaches its peak, Tmax) were generated. Changes in lesion volume between initial and 24‐h MRP maps with Tmax > 6 seconds 10 less than 30% or more than 70% were set as no reperfusion or reperfusion, respectively. Patients without reperfusion were selected for assessment of ischemic penumbra, while patients achieved reperfusion were selected to assess infarct core. Patients achieved partial reperfusion (between 30% and 70% reperfusion) were excluded from this study.
The final infarct was defined by 24‐h DWI 11. Firstly, anatomic registration was applied to register 24‐h DWI to the PWI frame. Manual landmark‐based registration was used to initialize another automatic registration in cases in which automatic coregistration failed on the first attempt. The quality of registration was independently reviewed by one investigator (S.Z). Secondly, the maximal visual extent of the coregistered diffusion lesion with knowledge of ADC < 600·106 s/mm2 was manually outlined (by H.T.). These manual regions of interest were drawn without reference to other imaging over a 1‐month period and verified again with disagreements resolved by consensus. To provide balance in the number of voxels being measured and prevent a very large amount of negative value from overwhelming the ratio to positive findings in the calculation of specificity, only the ipsilateral hemispheric (ischemic side) brain voxels were analyzed (rather than the whole brain). Investigators (S.Z and H.T) were masked to all other imaging and clinical data.
Volume‐based Analysis
The area of interest was transferred to the coregistered initial MR perfusion maps for analysis. A range of relative (as a percentage of contralateral hemisphere) and absolute perfusion thresholds were then investigated at constant increments as shown in Table 1.
Table 1.
MR perfusion thresholds used to define penumbra
| Parameters | Range | Increment |
|---|---|---|
| Tmax, second | 2–10 | 2 |
| rMTT, % | 125–250 | 25 |
| rCBF, % | 20–80 | 10 |
| rCBV, % | 20–80 | 10 |
r, relative.
Voxel‐based Analysis
The 24‐h DWI was divided into two regions: the DWI lesions (ROI‐1) and the ipsilateral tissue surrounding DWI lesions (ROI‐2). Using coregistration techniques, the regions of interest of 24‐h DWI were then transferred to each coregistered initial MR perfusion map (area of the lateral ventricle was avoided). ROI‐1 indicates the area of initial perfusion deficit progressed to final infarct. ROI‐2 represents the areas having no risk of infarct, including the normal tissue and benign oligemia. All voxels of ROI‐1 and ROI‐2 were separately exported for further analysis.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 (SPSS, Inc, Chicago, USA). Group comparisons were made using the Mann–Whitney U‐test and Fisher's exact test. In analysis of patients with reperfusion or none, Wilcoxon paired signed‐rank test was firstly performed between volume of baseline and 24‐h DWI lesion, and it was then performed between volume of 24‐h DWI lesion and variable thresholds‐defined perfusion lesion. Pairs with significant difference (P < 0.05) were excluded from further analysis. Volumetric agreement with 24‐h DWI lesion was then assessed (Bland–Altman) among the remaining perfusion thresholds. The optimal threshold was defined as having best volumetric agreement with the 24‐h DWI lesion. In voxel‐based analysis, receiver operating characteristic (ROC) curve analysis was used to test the predictive performance of all the thresholds of perfusion parameters. The optimal threshold was determined by Youden index.
Results
Baseline Clinical Data
A total of 50 patients met study entry criteria, of which 19 (38%) had no reperfusion, 20 (40%) had reperfusion, and 11 (22%) had partial reperfusion which were excluded from this study. Among 39 patients available for this study, the mean age of patients was 71.1 years (SD, 11.2) and 64.1% were male. The average baseline NIHSS was 11.2 (SD, 6.3). The mean time from stroke onset to MR scans was 193.5 min (SD, 72.2), and the mean time from stroke onset to intravenous rt‐PA bolus injection was 236.0 min (SD, 77.5). 28 of 39 treated within 4.5 h after onset; 11 of 39 treated 4.5–6 h. Baseline variables between patients treated within or out of 4.5 h were shown of no significant difference (see more details in the Table S1). The baseline clinical and imaging characteristic of two groups with and without reperfusion did not differ (see Table 2). The 3‐month functional outcome (mRS 0‐2) differed between 2 groups (no reperfusion vs reperfusion: 21.1% vs. 70.0%, χ 2=9.393, P = 0.002).
Table 2.
Baseline clinical and imaging variables
| No reperfusion (n = 19) | Reperfusion (n = 20) | P value (test value) | |
|---|---|---|---|
| Mean age, y (SD) | 71.9 (10.3) | 70.3 (12.2) | 0.813 (Z = ‐0.239) |
| Female, % | 8 (42.1) | 6 (30.0) | 0.431 (χ 2 = 0.620) |
| Hypertension, % | 15 (78.9) | 13 (65) | 0.333 (χ 2 = 0.936) |
| Diabetes, % | 4 (21.1) | 5 (25.0) | 1.000 (χ 2 = 0.086) |
| Smoking, % | 5 (26.3) | 5 (25.0) | 0.925 (χ 2 = 0.009) |
| Hyperlipidemia, % | 6 (31.6) | 9 (45) | 0.389 (χ 2 = 0.742) |
| Atrial fibrillation, % | 10 (52.6) | 12 (60) | 0.643 (χ 2 = 0.215) |
| History of stroke/transient ischemic attack, % | 2 (10.5) | 6 (30.0) | 0.235 (χ 2 = 2.27) |
| Median baseline NIHSS (SD) | 11.5 (4.7) | 10.9 (7.6) | 0.461 (Z = −0.761) |
| ONT, min (SD) | 256 (86.2) | 217 (64.7) | 0.813 (Z = −1.55) |
| OIT, min (SD) | 206 (77.2) | 182 (66.7) | 0.380 (Z = −0.90) |
| Average volume on acute DWI, mL (SD) | 29.9 (36.9) | 8.6 (8.4) | 0.084 (Z = −1.73) |
| Average acute hypoperfusion (Tmax > 6 seconds) volume, mL (SD) | 94.2 (67.1) | 76.0 (45.7) | 0.667 (Z = −0.450) |
SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; ONT, onset to needle time; OIT, onset to acute MR perfusion imaging time.
The Optimal Threshold of Ischemic Penumbra
Infarct in patients with no reperfusion would grow to encroach the entire penumbra. The value that best predict their final infarct would be the optimal threshold of ischemic penumbra. A significantly infarct growth was seen between baseline and 24‐h DWI imaging (29.9 ± 36.9 mL vs. 79.5 ± 74.1 mL, Z = −3.743, P < 0.001).
Volume‐based Analysis
Wilcoxon paired signed‐rank test showed that volume defined by the following perfusion parameters was of no significant difference with 24‐h DWI lesion volume: Tmax > 6 seconds (Z = −0.121, P = 0.904), rMTT>150% (Z = −1.248, P = 0.212),rMTT > 175% (Z = −0.684, P = 0.494), rCBF < 40% (Z =& thinsp;−0.241, P = 0.809), rCBV < 40% (Z = −0.114, P = 0.910), and rCBV < 50% (Z = −1.112, P = 0.266). Volumetric agreement of these parameters with the 24‐h diffusion lesion was then performed. Tmax > 6 seconds defined volume had the best Bland–Altman 95% limits of agreement with the 24‐h diffusion lesion (see Table 3. Bland–Altman plots could be found in the online‐only supplemental figures).
Table 3.
Bias, 95% LoA between thresholds‐based lesion volume and final infarct
| Groups | Thresholds | Ratioa | 95%LoA | Differenceb | 95%LoA | Percent differencec | 95%LoA | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | Lower limit | Upper limit | Lower limit | Upper limit | |||||
| No reperfusion (n = 19) | Tmax > 6 seconds | 1.05 | −0.23 | 2.33 | −0.9 | −98.1 | 96.3 | −13.7 | −145 | 118 |
| rCBF < 40% | 0.90 | −0.27 | 2.06 | 2.4 | −114 | 118 | −29.8 | −161 | 101 | |
| rMTT > 150% | 0.90 | −0.7 | 2.6 | −12.5 | −164 | 139 | −39.7 | −196 | 116 | |
| rCBV < 40% | 0.90 | −0.27 | 2.06 | 13.5 | −130 | 157 | −25.6 | −176 | 125 | |
| rMTT > 175% | 1.4 | −1.2 | 3.9 | 25.2 | −130 | 180 | −5.3 | −163 | 153 | |
| rCBV < 50% | 0.73 | −0.38 | 1.83 | −21.7 | −168 | 125 | −58.2 | −191 | 74.8 | |
| Reperfusion (n = 20) | Tmax > 10 seconds | 4.2 | −11.4 | 19.7 | 10.5 | −36.1 | 57.0 | 39.2 | −159 | 238 |
| rMTT > 200% | 3.8 | −11.0 | 18.6 | 5.3 | −28.1 | 38.7 | 37.5 | −120 | 195 | |
| rMTT > 225% | 2.4 | −6.5 | 11.4 | −2.5 | −33.4 | 28.4 | −1.9 | −180 | 176 | |
| rMTT > 250% | 2.6 | −7.9 | 13.0 | 1.1 | −89.0 | 91.3 | −36.3 | −245 | 173 | |
| rCBF < 20% | 3.1 | −9.1 | 15.3 | 1.5 | −37.5 | 40.5 | 16.7 | −173 | 206 | |
| rCBV < 20% | 4.5 | −17.8 | 26.8 | 4.1 | −30.8 | 38.9 | 34.7 | −130 | 199 | |
LoA, limits of agreement.
aRatio = [thresholds‐based lesion volume / 24‐hour DWI lesion volume] versus average.
bDifference = [thresholds‐based lesion volume −24‐hour DWI lesion volume] versus average.
cPercent difference = [100*(threshold‐based lesion volume −24‐hour lesion volume)/average] versus average.
Voxel‐based Analysis
ROC curve analysis demonstrated that the best MR perfusion threshold to match the 24‐h diffusion lesion was Tmax > 6.1 second. Voxels in patients being reperfused less than 10% were also tested; their results performed quite similar to that among patients reperfused less than 30% (see Table 4). As Tmax 6.1 seconds was quite close to Tmax 6 seconds, and Tmax is generally calculated in 1‐ to 2‐second increments because of the timing between each imaging acquisition, Tmax > 6 seconds was defined as the optimal threshold in ROC curve analysis.
Table 4.
Best thresholds in each MR perfusion map by ROC curve analysis for defining the final infarct in no reperfusion group
| Parameters | AUC | Thresholds | Sensitivity | Specificity | Youden index | |
|---|---|---|---|---|---|---|
| Reperfused less than 30% (n = 19) | Tmax | 0.783 | 6.1 seconds | 72.3% | 74.3% | 0.466 |
| rMTT | 0.711 | 143% | 68.2% | 65.2% | 0.334 | |
| rCBF | 0.667 | 44% | 57.1% | 68.7% | 0.258 | |
| rCBV | 0.604 | 50% | 54.2% | 62.1% | 0.163 | |
| Reperfused less than 10%(n = 5) | Tmax | 0.804 | 6.6 seconds | 74.9% | 73.5% | 0.490 |
| rMTT | 0.700 | 144% | 66.3% | 66.1% | 0.323 | |
| rCBF | 0.682 | 42% | 55.3% | 73.7% | 0.290 | |
| rCBV | 0.623 | 51% | 51.7% | 69.4% | 0.211 |
AUC, area under curve.
The Optimal Threshold of Infarct Core
The infarct in patients reperfused would be stabilized at its border without transgressing into penumbral territories. The value that best predicts the final infarct should be the optimal threshold of infarct core.
Volume‐based Analysis
Wilcoxon paired signed‐rank test showed that volume defined by the following perfusion parameters was of no significant difference with 24 h‐DWI lesion volume: Tmax > 10 seconds (Z = −1.755, P = 0.079), rMTT > 200% (Z = −1.755, P = 0.079),rMTT>225% (Z = −0.336, P = 0.737), rMTT>250% (Z = −1.792, P = 0.073), rCBF < 20% (Z = −0.859, P = 0.391), and rCBV < 20% (Z = −1.008, P = 0.313). Volumetric agreement of these parameters with the 24‐h diffusion lesion was performed. rMTT > 225% defined volume was proved of best agreement with the 24‐h diffusion lesion (see Table 3. Bland–Altman plots could be found in the online‐only supplemental figures.).
Voxel‐based Analysis
ROC curve analysis demonstrated that the best MR perfusion threshold to match the 24‐h DWI lesion was Tmax > 5.6 seconds. Considering that the optimal thresholds determined by two measurements were distinct, we also tested voxels in patients being reperfused ≥ 90%. Similarly, Tmax>6 seconds still performed as the optimal threshold (see Table 5). Moreover, significant infarct growth, not DWI reversal, was found in 24‐h DWI images in comparison of baseline infarct (8.6 ± 8.4 mL vs. 17.0 ± 14.3 mL, Z = −3.435, P = 0.001). It was even observed in patients being reperfused ≥ 90% (9.4 ± 8.7 mL vs. 18.7 ± 15.3 mL, Z = −2.999, P = 0.003) (see Figure 1).
Table 5.
Best thresholds in each MR perfusion map by ROC curve analysis for defining the final infarct in reperfusion group
| Parameters | AUC | Thresholds | Sensitivity | Specificity | Youden index | |
|---|---|---|---|---|---|---|
| Reperfused over 70% (n = 20) | Tmax | 0.793 | 5.6 seconds | 76.8% | 70.3% | 0.471 |
| rMTT | 0.618 | 127% | 66.1% | 51.7% | 0.177 | |
| rCBF | 0.604 | 43% | 40.4% | 74.6% | 0.150 | |
| rCBV | 0.587 | 49% | 48.9% | 65.2% | 0.141 | |
| Reperfused over 90% (n = 17) | Tmax | 0.799 | 6.0 seconds | 78.6% | 79.7% | 0.483 |
| rMTT | 0.604 | 121% | 73.5% | 41.5% | 0.151 | |
| rCBF | 0.589 | 42% | 41.5% | 72.4% | 0.139 | |
| rCBV | 0.576 | 49% | 52.8% | 61.4% | 0.132 |
Figure 1.
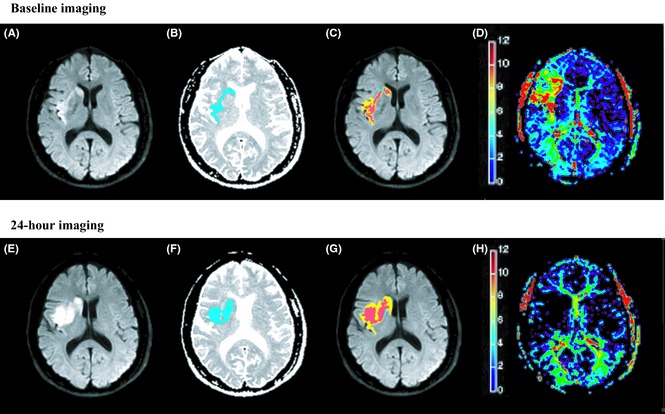
A 56‐year‐old male obtained intravenous thrombolysis at 218 min after stroke onset. The DWI lesion (A and E. areas of visual hyperintensity) was coregistered with the hypointensity area on ADC map (B and F. blue areas). The baseline infarct (C. red areas indicate A overlap B) volume was 2 mL. Comparing with the baseline Tmax map (D. based on the color bar, signals covered from yellow to red indicated Tmax > 6 seconds tissues), 24‐h Tmax map (H.) confirmed that he achieved total reperfusion after thrombolysis. The final infarct (G. red areas) volume on 24‐h DWI was 9.2 mL, which proved the infarct progression.
Discussion
This is the first Chinese population‐based study on MR perfusion parameters to determine the optimal thresholds of ischemic penumbra and infarct core. Our study confirmed the premise about evolution of ischemic penumbra among Chinese acute ischemic stroke patients, and both volume and voxel‐based analysis proved Tmax > 6 seconds as the optimal threshold in predicting the distribution of ischemic penumbra. However, the premise about infarct core was invalid given that thresholds for infarct core were distinct between two methods.
Our second premise was borne out in analysis of optimal threshold to define ischemic penumbra. In previous studies, Tmax map was almost valid in identifying perfusion lesion. Thresholds varied from 2 to 6 seconds, depending on the populations and applied methods. Taking the volumetric method, substudies from both Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution study (DEFUSE) and EPITHET have illustrated that Tmax > 4 seconds or >6 seconds more accurately predicted penumbral salvage and infarct growth than Tmax>2 seconds 2. In voxel‐by‐voxel analysis, Tmax > 5.5 seconds (sensitivity 88%, specificity 89%, AUC = 0.95) was the best threshold to match positron emission tomography (PET) CBF < 20 mL/100 g/min 12. However, each method has its own limitation. Using volumetric analysis, for being lack of position information, the final infarct volume was considered of underestimating the true distribution of ischemic penumbra at admission 4, 5. Comparing with volumetric method, the coregistered voxel approach give the advantage of more precisely identifying the location and extent of perfusion lesion, but the analysis is relatively complicated and the coregistration may introduce potential error which would be magnified in the process. In this study, our strength lie in the mutual validation, which performed volume and voxel‐based analyses separately among the same cohort patients, and further confirmed Tmax > 6 seconds as the mutual optimal threshold to identify ischemic penumbra. This result indicates that, to some extent, the volume of Tmax > 6 seconds will be applicable to form the favorable penumbral pattern in predicting the clinical outcome. A recent result from DEFUSE 2, using the definition of <10% reperfusion, also confirmed a high correlation between volume of Tmax > 6 seconds and final infarct volume (r = 0.86) 13. Thus, we are looking forward to the performance of this threshold in future trials from China.
Prior studies selected patients achieving reperfusion to identify the optimal threshold of infarct core based on the assumption that the infarct would be stable if reperfusion achieved 14, 15, 16. Our study did not support this premise. The obvious infarct growth still existed, even was seen in patients with over 90% reperfusion. Three possible reasons may explain this “reperfusion phenomenon”. (1) Perfusion imaging‐based reperfusion may not fully represent the real reperfusion in cerebral microcirculation 17. (2) Tissue viability in different patients is varied. Even slight perfusion deficit may lead to irreversible injury in ischemic tissue, while some of others may endure more severe hypoperfusion. That means, specific ischemic tissue may be prone to die even achieved early reperfusion which is called invalid reperfusion 18. (3)Theoretically, infarct progression is stopped as soon as successful reperfusion occurs. However, as the exact time of reperfusion was unknown, the infarct would be allowed to grow over the penumbra before reperfusion occurred.
Of note, acute DWI volume seems to provide a reasonable surrogate of irreversibly injured lesion. In our study, final infarct volumes were virtually not smaller than the baseline DWI, supporting that sustained DWI lesion reversal is infrequent in patients accept reperfusion therapy within 6 h after stroke onset. A recent study just demonstrated that DWI reversal is rarely seen but more likely to occur in those with smaller baseline lesions, which should not significantly affect the accuracy of penumbra definition 19.
Our study has limitations attributed to its retrospective nature and small number of included patients. Also, our reperfusion imaging was obtained relatively late. The use of 24‐h DWI to define infarct core in reperfused patients is not ideal unless we know the exact time of reperfusion. Therefore, it is possible that if reperfusion was measured earlier, then some of the patients would be excluded from the reperfusion group. As the discovery of the reperfusion phenomenon, results of studies using 24‐h DWI to assess acute perfusion status in reperfused patients should be interpreted with cautious. Additionally, patients enrolled were received intravenous thrombolysis, the final infarct in no reperfusion group might be consequently smaller than that in studies in which thrombolysis was not performed.
An additional limitation is that although ROC curve analysis is accurate enough to identify tissue died or not, applying it among voxels of all patients which may violate the assumption that each unit in the sample is independent. Up until now, several clinical trials, such as perfusion and angiography imaging substudy of the third international stroke trial (IST‐3), have taken this into account, and comparisons between existing thresholds are under testing 20. Nevertheless, it is currently unknown whether future trials can avoid this limitation. For the present, it appears that current threshold which was mutually tested in our study is most rational and reliable.
In summary, this is the first to demonstrate MR perfusion thresholds among Chinese thrombolytic candidates. Tmax > 6 seconds, within 6 h after stroke onset, provides the most accurate estimate of ischemic penumbra. However, none of PWI parameters is reliable to predict infarct core may associate with that reperfusion occurred within 24 h after thrombolysis is not valid to restrain infarct growth. Acute DWI may still be quite reliable to estimate the infarct core before more rational premise is approved in future studies.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. Bland‐Altman plots of volume differences between threshold‐based perfusion lesion and infarct core.
Table S1. Comparison of baseline clinical and imaging variables between patients accepted intravenous rtPA within 4.5 h or out of 4.5 h.
Acknowledgments
This work was supported by the Science Technology Department of Zhejiang Province (2013C13G2010032), Zhejiang Provincial Natural Science Foundation (LR12H09001), and the National Natural Science Foundation of China (81171095 & 81471170). The perfusion analysis software (MIStar) used in this study was provided to the site as part of their involvement in the INternational Stroke Perfusion Imaging REgistry (INSPIRE, www.inspire.apollomit.com/), study funded by the National Health and Medical Research Council of Australia.
References
- 1. Wang Y, Liao X, Zhao X, et al. Using recombinant tissue plasminogen activator to treat acute ischemic stroke in China: analysis of the results from the Chinese National Stroke Registry (CNSR). Stroke 2011;42:1658–1664. [DOI] [PubMed] [Google Scholar]
- 2. Olivot JM, Mlynash M, Thijs VN, et al. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke 2009;40:1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma H, Zavala JA, Teoh H, et al. Penumbral mismatch is underestimated using standard volumetric methods and this is exacerbated with time. J Neurol Neurosurg Psychiatry 2009;80:991–996. [DOI] [PubMed] [Google Scholar]
- 4. Ogata T, Christensen S, Nagakane Y, et al. The effects of alteplase 3 to 6 hours after stroke in the EPITHET‐DEFUSE combined dataset: post hoc case‐control study. Stroke 2013;44:87–93. [DOI] [PubMed] [Google Scholar]
- 5. Nagakane Y, Christensen S, Brekenfeld C, et al. EPITHET: positive Result After Reanalysis Using Baseline Diffusion‐Weighted Imaging/Perfusion‐Weighted Imaging Co‐Registration. Stroke 2011;42:59–64. [DOI] [PubMed] [Google Scholar]
- 6. Shih LC, Saver JL, Alger JR, et al. Perfusion‐weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke 2003;34:1425–1430. [DOI] [PubMed] [Google Scholar]
- 7. Carrera E, Jones PS, Iglesias S, et al. The vascular mean transit time: a surrogate for the penumbra flow threshold? J Cereb Blood Flow Metab 2011;31:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mlynash M, Lansberg MG, De Silva DA, et al. Refining the definition of the malignant profile: insights from the DEFUSE‐EPITHET pooled data set. Stroke 2011;42:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 10. Lansberg MG, Lee J, Christensen S, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke 2011;42:1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell BC, Tu HT, Christensen S, et al. Assessing response to stroke thrombolysis: validation of 24‐hour multimodal magnetic resonance imaging. Arch Neurol 2012;69:46–50. [DOI] [PubMed] [Google Scholar]
- 12. Zaro‐Weber O, Moeller‐Hartmann W, Heiss WD, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke 2010;41:2817–2821. [DOI] [PubMed] [Google Scholar]
- 13. Wheeler HM, Mlynash M, Inoue M, et al. Early diffusion‐weighted imaging and perfusion‐weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke 2013;44:681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiao Y, Zhu G, Patrie J, et al. Optimal perfusion computed tomographic thresholds for ischemic core and penumbra are not time dependent in the clinically relevant time window. Stroke 2014;45:1355–1362. [DOI] [PubMed] [Google Scholar]
- 15. Kidwell CS, Wintermark M, De Silva DA, et al. Multiparametric MRI and CT models of infarct core and favorable penumbral imaging patterns in acute ischemic stroke. Stroke 2013;44:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soares BP, Chien JD, Wintermark M. MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: everything that recanalizes does not necessarily reperfuse!. Stroke 2009;40:S24–S27. [DOI] [PubMed] [Google Scholar]
- 17. Barber PA. Magnetic resonance imaging of ischemia viability thresholds and the neurovascular unit. Sensors (Basel) 2013;13:6981–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dani KA, Thomas RG, Chappell FM, et al. Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: definitions and thresholds. Ann Neurol 2011;70:384–401. [DOI] [PubMed] [Google Scholar]
- 19. Asdaghi N, Campbell BC, Butcher KS, et al. DWI Reversal Is Associated with Small Infarct Volume in Patients with TIA and Minor Stroke. AJNR Am J Neuroradiol 2014;35:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wardlaw JM, von Kummer R, Carpenter T, et al. Protocol for the perfusion and angiography imaging sub‐study of the Third International Stroke Trial (IST‐3) of alteplase treatment within six‐hours of acute ischemic stroke. Int J Stroke DOI: 10.1111/j.1747-4949.2012.00946.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Bland‐Altman plots of volume differences between threshold‐based perfusion lesion and infarct core.
Table S1. Comparison of baseline clinical and imaging variables between patients accepted intravenous rtPA within 4.5 h or out of 4.5 h.


