Abstract
Objectives
Mangifera indica L. (mango) stem bark aqueous extract (MSBE) that has antioxidant, anti‐inflammatory and immunomodulatory properties, can be obtained in Cuba. It is rich in polyphenols, where mangiferin is the main component. In this study, we have tested DNA damage and protection effects of MSBE and mangiferin on primary human lymphocytes and lymphoblastoid cells.
Material and methods
Cell suspensions were incubated with the products (50–1000 μg/ml) for experiments on damage induction, and evaluation of any potential protective effects (5–100 μg/ml) for 60 min at 37 °C. Irradiation was performed using a γ‐ray source, absorbed dose 5 Gy. At the end of exposure, DNA damage, protection and repair processes were evaluated using the comet assay.
Results
MSBE (100–1000 μg/ml) induced DNA damage in a concentration dependent manner in both cell types tested, primary cells being more sensitive. Mangiferin (200 μg/ml) only induced light DNA damage at higher concentrations. DNA repair capacity was not affected after MSBE or mangiferin exposure. On the other hand, MSBE (25 and 50 μg/ml) and mangiferin (5–25 ug/ml) protected against gamma radiation‐induced DNA damage.
Conclusions
These results show MSBE has protector or harmful effects on DNA in vitro depending on the experimental conditions, which suggest that the extract could be acting as an antioxidant or pro‐oxidant product. Mangiferin was involved in protective effects of the extract.
Introduction
Recently, there has been considerable interest in antioxidant and free radical scavenging properties of many plants and their components 1. Specifically, polyphenols compose a broad family of naturally occurring physiologically active compounds, and different biological activities have been attributed to these molecules. For example, antioxidant activity is important in prevention of cardiovascular diseases. These molecules are also often used as antiviral, anti‐inflammatory, anti‐tumoural and immunomodulator agents 2, 3.
Mangifera indica (common name, mango) is a plant widely used in traditional medicine in different regions of the world. In Cuba, aqueous extract can be obtained from the stem bark of this plant (MSBE). It is the active pharmaceutical ingredient of Vimang formulations, and has been used for treatment of a variety of diseases and pathological states for a number of years 4, 5. Phytochemical investigation of MSBE has led to the isolation of seven phenolic constituents: mangiferin, gallic acid, 3,4‐dihydroxy benzoic acid, gallic acid methyl ester, gallic acid propyl ester (+)‐catechin (−)‐epicatechin, benzoic acid and benzoic acid propyl ester 6, 7. The extract also contains triterpenes, phytosterols, fatty acids and microelements. Ethnomedical reports show welfare improvement in cancer patients after MSBE exposure, including for those undergoing chemotherapy and radiotherapy 4, 5. Also, MSBE has potent antioxidant effects as shown in both in vitro and in vivo models 3, 8, 9, 10, 11, 12; it is also analgesic and anti‐inflammatory and has immunomodulator activities 13, 14, 15, 16, 17, 18.
Mangiferin, the main component of the extract 6, 7, is a glucosylxanthone found in large quantities not only in the stem bark but also in leaves and fruit of M. indica. It exhibits several beneficial pharmacological properties such as being anti‐viral, anti‐inflammatory, hypoglycaemic, anti‐diabetic, analgesic, anti‐tumour and having antioxidant activities 19, 20, 21, 22, 23, 24.
Toxicological screening of MSBE has shown low potential for general toxicity. The extract has only been reported to be toxic to animals when injected intraperitoneally, and after acute exposure 25. Trials have been conducted to study whether it has any mutagenic and/or genotoxic potential: in vitro (Ames and micronucleus assays) and in vivo (micronucleus and comet assays, in mice), from which results were negative 26, 27; such tests are considered sufficient for evaluating genotoxic effects of a new product under investigation. Nevertheless, MSBE is rich in polyphenols, molecules with proven genotoxic effects under certain conditions. It has been previously observed, that many antioxidants can exhibit pro‐oxidant effects through generation of reactive oxygen species (ROS) such as the hydroxyl radical, which causes DNA damage 28, 29, 30, 31, 32. Taking high sensitivity of comet assay into account, specially to detect oxidative DNA damage, we decided to use this assay to test effects of MSBE and mangiferin on DNA strand breaks in human primary lymphocytes and lymphoblastoid cells. On the other hand, antioxidant properties of M. indica L. and reports from traditional medicine suggest that it could have radioprotective activity. Thus, in this study, we have evaluated in vitro effects of MSBE and mangiferin isolated from it, on DNA damage, and protection against, gamma radiation‐induced using the comet assay, in human primary lymphocytes and lymphoblastoid cells.
Materials and methods
Chemicals
Low‐melting‐point agarose, phosphate‐buffered saline, dimethyl sulphoxide (DMSO) and histopaque 1077 were purchased from Sigma. All other reagents and solvents used were of analytical reagent grade.
Plant material
Mangifera indica Linneo (Anacardiaceae) plants were collected from cultivated fields located in the region of Pinar del Rio, Cuba. Voucher specimens of the plant (Code: 41722) were deposited at the Herbarium of Academy of Sciences, of the Institute of Ecology and Systematics of the Ministry of Science, Technology and Environmental, La Habana, Cuba and authenticated by Ramona Prieto MSc, curator, and Isora Baró MSc, Director of the Herbarium. Stem bark extract of M. indica was prepared by decoction for 1 h. The extract was concentrated by evaporation and spray dried using Niro Atomizer Standard Spray Drying (Soeborg, Denmark), to obtain a fine homogeneous brown powder with 30–60 μm particle size of (MSBE); this melts at 210–215 °C, with decomposition 33. MSBE is used as the standardized pharmaceutical active ingredient of Vimang formulations; its chemical composition has been characterized by chromatographic (planar, liquid and gas) methods, mass spectrometry and UV/VIS spectrophotometry 6, 7, 34.
Mangiferin (1,3,6,7‐tetrahydroxy xanthone‐C2‐β‐d‐glucoside) was isolated from the MSBE by extraction with methanol, according to the described standard method 6, 7. This was supplied by the Laboratory of Analytical Chemistry, Center of Pharmaceutical Chemistry (Havana, Cuba) with 90% purity assessed with a validated HPLC‐based method 6, 7. MSBE and mangiferin were dissolved in 1% DMSO for following tests. DMSO has been shown to have no genotoxic effect 35.
Cell preparations
Human lymphocytes were isolated from fresh whole blood as described Schmezer et al. 36. Cells were obtained from healthy volunteers (Blood Bank, University of Heidelberg) and were purified using the Lymphoprep kit (Nycomed Pharma, Oslo, Norway). Immediately thereafter, cells were cryopreserved in 10% DMSO, 90% foetal calf serum (FCS) by cooling in a freezer (Neolab, Heidelberg, Germany). Frozen cells were kept at −80 °C until the day of assay. Frozen samples were then rapidly thawed, washed in RPMI‐1640 medium and adjusted to a concentration of 1 × 106 cells per ml, in the medium supplemented with 10% FCS and 2 mm l‐glutamine. After being cultured for 20 h at 37 °C with 5% CO2, cells were employed in the experiments.
Human lymphoblastoid cell line (AT516F) was obtained from the German Center for Cancer Research Cell Bank, Heidelberg, Germany. Monolayer cultures were grown in RPMI medium supplemented with 10% foetal calf serum, 2 mm glutamine and antibiotics. Cells were maintained at 37 °C in an atmosphere with 5% CO2 and 95% air, with higher than 95% humidity. For obtaining cell suspensions, cells were detached by treatment with 0.25% trypsin/0.02% EDTA at 37 °C.
Cell viability
Cell viability was measured using the trypan blue exclusion assay, before and after exposure to different concentrations of the products or gamma irradiation. Each aliquot of cells was suspended in 0.4% trypan blue and cells were scored for inclusion or no inclusion, of the dye. Values obtained were expressed as percentage cell viability, calculated in relation to non‐treated cells (100% value).
Effect of MSBE and mangiferin on DNA damage in primary lymphocytes and lymphoblastoid cells
Cell suspensions were incubated with different concentration ranges of both products as appropriate for the experiments (50–1000 μg/ml to assess damage, and 5–100 μg/ml to evaluate protective effects) for 60 min at 37 °C in an incubator, in the dark, together with untreated control and samples treated with 5 Gy used as positive control. At the end of exposure, DNA damage was evaluated on both cell type suspensions.
Effect of MSBE and mangiferin on DNA repair in primary lymphocytes and lymphoblastoid cells
Samples on slides were incubated with MSBE and mangiferin, in RPMI medium for 15, 30 and 60 min at 37 °C. They were then lysed and electrophoresis was performed.
Effect of MSBE and mangiferin on DNA damage induced by γ‐radiation, in primary lymphocytes
After incubation periods with the product concentrations, cell suspensions were mixed with low melting agarose, but just before lysis, each sample was exposed to 5 Gy using a 137Cs‐source at 12 Gy/min; they were then incubated overnight at 4 °C. Electrophoresis was then performed.
Alkaline single‐cell gel electrophoresis (comet assay)
The comet assay was performed under alkaline conditions according to the procedure of Sing et al. 37 with slight modifications. Fifty microlitre cell suspensions were mixed with 350 μl of 0.7% low melting agarose kept at 42 °C. Fifty microlitre aliquots of this cell suspension were pipetted on two areas of Comet Slides™ (Trevigen Inc., Gaithersburg, MD, USA). These samples were then placed on ice for 10 min to accelerate gelling of the agarose layer, then transferred to pre‐chilled lysis solution (100 mm Na2EDTA, 10 mm Tris, 2.5 mm NaCl, 1% sodium sarcosinate, 1% Triton X‐100, 10% DMSO, pH 10) and incubated overnight at 4 °C.
After lysis, slides were placed in a horizontal gel electrophoresis tank with a high‐pH electrophoresis buffer (1 mm EDTA, 300 mm NaOH, pH 13). Samples remained in electrophoresis buffer for 20 min to allow unwinding of DNA. Electrophoresis was then performed for 20 min at 25 V and 300 mA. Following electrophoresis, samples were fixed for 5 min in ice‐cold absolute ethanol and air‐dried. They were then stained with 50 μl SYBR Green solution (Molecular Probes, Inc., Leiden, The Netherlands) diluted 1:10 000 in TE buffer (10 mm Tris–HCl/1 mm EDTA, pH 7.5). Slides were examined using a fluorescence microscope with camera attached, connected to a personal computer‐based image analysis system (Komet 5.0, Kinetic Imaging Ltd., Liverpool, UK). For each analysis, 50 individual cells were calculated by gel and two slides were prepared by treatment in each experiment. DNA damage was expressed as tail moment. DNA migration was directly expressed as mean tail intensity from each slide from the experiments, according to guidelines as described 38.
Statistical analysis
Values were expressed as mean ± SEM. All data were checked using the Kolomogorov–Smirnov test and homogeneity of variances was assessed using the Bartlet test. Statistical significance between control and treated groups was evaluated using the Mann–Whitney test. P < 0.05 was accepted to be statistically significant.
Results
Cell viability
Neither MSBE nor mangiferin induced significant reduction in cell viability, this being >85% over all series (Fig. 1a,b). These results reveal that both extract and mangiferin did not reduce viability of human primary lymphocytes nor cells of the lymphoblastoid line, after 1 h exposure of up to 1000 μg/ml.
Figure 1.
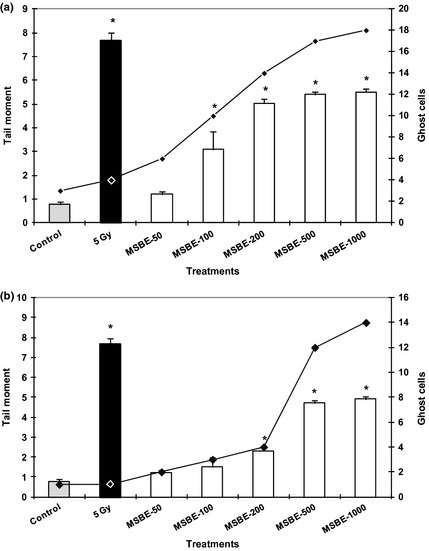
Effect of Mangifera indica L. extract ( MSBE 50, 100, 200, 500, 1000 µg/ml) on strand breaks in human peripheral lymphocytes (a) and lymphoblastoid cell line (b) after 60 min of incubation expressed as tail moment in Comet assay ( y ‐axis on the left). As a positive control, cells were exposed to γ‐irradiation (5 Gy). Untreated cells served as negative controls. Ghost cells (y‐axis on the right) represent number of observed cells with total DNA fragmentation. Data from three independent determinations are expressed as mean ± SEM, *P < 0.05 with respect to control group (Mann–Whitney U‐test).
Effect of MSBE and mangiferin on DNA damage and on DNA repair
DNA strand breaks and the repair process in the lymphocytes and lymphoblastoid cells were measured using the comet assay. Figure 1a and 1b shows DNA damage induced by MSBE at 37 °C for 60 min; degree of DNA damage was expressed as tail moment. MSBE induced DNA damage in both types of cell (primary lymphocytes and lymphoblastoid line) after 60 min exposure at higher concentrations (100–1000 μg/ml). Damage increased dependent upon concentration increase, compared to control cells. This effect was also observed time exposure dependently (0–60 min) (data not shown). Mangiferin though induced only light DNA damage at higher concentration tested (200 μg/ml); this was not statistically different from control cells (data not shown). On the other hand, the results show that primary human lymphocytes were more sensitive than lymphoblastoid cells, to DNA damage.
We also checked the ability of DNA to repair changes caused by the extract (Fig. 2a,b) and mangiferin on cells. Data suggest that DNA strand breaks started to be repaired 15 min after removal MSBE to be completely repaired 60 min after stopping exposure of cells to the extract. Repair did not change after mangiferin treatment (data not shown). In this sense, it is possible to affirm that DNA repair capacity was not affected after MSBE or mangiferin exposure.
Figure 2.
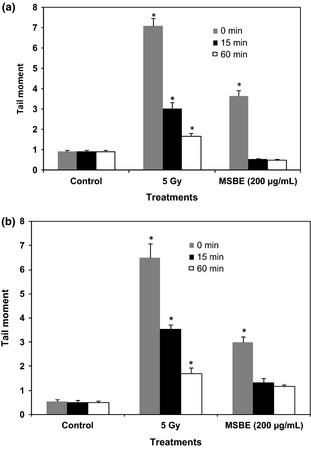
Effect of Mangifera indica L. extract ( MSBE ) on DNA repair in human peripheral lymphocytes (a) and lymphoblastoid cell line (b) after 60 min of incubation expressed as tail moment in Comet assay. As a positive control, cells were exposed to γ‐irradiation (5 Gy). Untreated cells served as negative controls. The times (0, 15 and 60 min) represent the incubation period for detecting the capacity of cells to repair DNA damage induced by the extract or γ‐irradiation. Data from three independent determinations are expressed as mean ± SEM, *P < 0.05 with respect to control group (Mann–Whitney U‐test).
Effect of MSBE and mangiferin on DNA damage induced by gamma radiation
Figure 3a and 3b shows effects of MSBE and mangiferin pre‐treatment on human lymphocyte damage by induced by gamma radiation. As can be seen, low concentrations of MSBE (25–50 μg/ml) and mangiferin (5–25 μg/ml) protected against radiation‐induced DNA damage in these cells.
Figure 3.
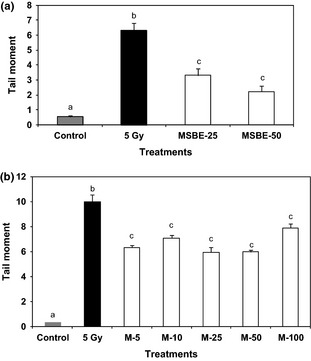
(a) Effects of Mangifera indica L. extract ( MSBE 0, 25, 50 µg/ml) and (b) mangiferin (M 0, 5, 10 25, 50, 100 µg/ml) on the radiation‐induced DNA damage in primary human lymphocytes after 60 min of incubation expressed as tail moment in Comet assay. As a positive control, cells were exposed to γ‐irradiation (5 Gy). Untreated cells served as negative controls. Data from three independent determinations are expressed as mean ± SEM. Treatments not sharing the same letters are significantly different by the Mann–Whitney U‐test (P < 0.05).
Discussion
Comet assay is a sensitive method for detecting DNA strand breaks at the level of individual cells. The alkaline version is a method that enables detection of the broadest spectrum of DNA damage, as induction of double‐and single‐strand DNA breaks. These lesions can be, as well as by others causes, a consequence of oxidative damage produced by increase of ROS in cells 28, 39, 40, 41.
Yet, pro‐oxidative activity is related to induction of free radical damage to such non‐lipids as DNA, protein and carbohydrates, and to be the cause of many genotoxic effects observed 28, 29, 42, 43, 44. There are lines of evidence showing that most plant‐derived polyphenolic antioxidants, including flavonoids and tannins, act as pro‐oxidants either alone or in the presence of transition metals. For example, it is known that flavonoids can either enhance or inhibit formation of hydroxyl radicals by Fenton‐type reactions 44, 45. In turn, there are reports concerning genotoxic effects associated with pro‐oxidant activity, for gallic acid, its derivates and tannic acid by 1 h exposure to concentrations higher than 60 μm, in human lymphocytes. Tannic acid, for example, in the presence of Cu (II), causes DNA degradation through generation of ROS, structural features being one important factor for both antioxidant action and generation of hydroxyl radicals.
Mangifera indica L. (mango) stem bark aqueous extract is a complex mixture of compounds, where polyphenols (40–60%) are the chemical entity most represented 7, 34, 46. These have powerful antioxidant properties both at in vitro and in vivo assays 3, 8, 9, 11, 12 without pro‐oxidant activity. The present study shows that higher concentrations of MSBE induce DNA damage in both cell types tested, which could be associated with ROS generation by polyphenols present in the extract under these experimental conditions. This suggests that MSBE could be acting as an antioxidant and as a pro‐oxidant product, depending on the conditions.
On the other hand, catechol functional groups present in numerous flavonoids and other polyphenols are oxidized during cell protection against free radical production, generating semiquinone radicals and quinines 47, 48. These compounds have been described to be potentially toxic, due to (among other reasons) their ability to induce DNA damage; this type of groups are present in mangiferin. In this sense, exhibition of genotoxic activity by this xanthone could be expected. Our results suggest that mangiferin (up to 200 μg/ml) was not genotoxic in lymphocytes or lymphoblastoid cells. Thus, at least under these experimental conditions, mangiferin was not involved in genotoxic effects induced by the extract.
Mangiferin and tannins (tannic and gallic acids) have exhibited pro‐apoptotic activity in mammalian cells and this property seems very close to their pro‐oxidant activities 49. Many authors report that increase of comet type 4 damage can be associated with induction of apoptosis 50. This was observed after MSBE exposure; the genotoxic damage could be associated with induction of apoptosis. However, lately, this hypothesis has been refuted. The sub‐cellular variant of comet assay using different pH conditions during electrophoresis (12.1 and 13) as described by Kasamatsu et al. 51 was used for studying the nature of DNA damage. Results of this assay demonstrated that MSBE induced damage to naked DNA of isolated lymphocytes under both pH conditions for unwinding and electrophoresis, which are statistically significant for 50 and 100 μg/ml MSBE in pH 13 and pH 12.1, respectively. These results also indicate that the genotoxic effect was higher at pH 13 electrophoresis conditions than at pH 12.1. This can be related to unwinding and electrophoresis at pH 13, which permits detection of different types of DNA damage, such as single‐strand breaks, double‐strand breaks and alkali‐labile sites (ALS). The same step at pH 12.1 does not detect ALS. Thus, it could be suggested that, in these experimental conditions, MSBE extract principally induces formation of ALS. ALS is produced by action of specific radical oxygen species and action of alkaline medium 2. Thus, these data suggest that MSBE induces strand breaks to DNA by different mechanisms from the apoptotic process, revealed by sub‐cellular comet assay performed under experimental conditions where cellular components necessary to develop apoptosis are not present.
Previous results have shown that the extract did not induce single‐strand breaks or alkali‐labile sites in blood peripheral lymphocytes of treated animals compared to controls, revealed by comet assay. MSBE induced cytotoxic activity, determined as cell viability or polychromatic erythrocytes/normochromatic erythrocytes (PCE/NCE) ratio, but neither increased frequency of micronucleate binucleate cells in culture of human blood nor in treated mouse bone 26. DNA damage observed by comet assay can be repaired and for this reason no clastogenic effects are observed in micronucleus assays. Our results are also in agreement with the close relationship observed between influence of redox environment in proximity to the DNA molecule, and genotoxic activity observed for this kind of molecule 41. Other factors such as absorption, distribution and metabolism of the extract could contribute to differences observed between in vitro and in vivo assays. Similar results have been observed for other polyphenols; for example, quercetin induces DNA damage in vitro, but it is not a genotoxic compound in vivo. Results of the present study must be analysed as part of the genotoxic evaluation of MSBE in which there have been different levels of DNA damage.
Humans are exposed to ionizing radiation in diagnostic and therapeutic purposes and in occupational settings, amongst others. Ionizing radiation damages DNA by affecting the fidelity of DNA replication, causing genomic instability that may result in mutagenesis and carcinogenesis 52. In many cases, lesions observed are caused by generation of free radicals. There are reports that indicate how products isolated from plants have received special attention for their radioprotective properties, as in many cases, they are better tolerated and have minor adverse effects compared to synthetic radioprotective agents 53.
Taking into account the antioxidant effects reported for MSBE and the ethnomedical reports observed, in this study we evaluated consequences of low concentrations of MSBE and mangiferin on human lymphocytes and cells of a lymphoblastoid line, on gamma radiation‐induced damage (Fig. 3a,b). Results show that MSBE (25 and 50 μg/ml) protected against radiation‐induced DNA damage in this cells, which agree with its antioxidant profile. In this sense, probably, the mechanism involved in these radioprotective effects could be related to scavenging of radiation‐induced free radicals. Recent studies support that MSBE and mangiferin have scavenger effects on free radicals 12 and reduce lipid peroxidation in both in vitro and in vivo models 3, 9, 10, 11. They may also contribute to reducing DNA damage resulting from exposure to gamma radiation, as lipid peroxidation has been reported as an inductor of damage to DNA 54.
In this investigation, we also confirmed that mangiferin (5–25 μg/ml) protected DNA from gamma radiation‐induced damage, which suggests that this polyphenol is at least in part responsible of radioprotective effects observed for the extract. This result is consistent with other preliminary reports 53 have observed that mangiferin reduced the frequency of radiation‐induced micronucleate binucleate cells in cultures of human peripheral blood lymphocytes. These authors also associated the radioprotective effects observed with antioxidant properties of mangiferin under those experimental conditions.
In conclusion, these findings affirm that mangiferin could be involved in protective effects of MSBE against radiation‐induced damage to DNA in human lymphocytes. However, M. indica L. aqueous stem bark extract and mangiferin, its main component, exhibit DNA damage as well as protective in vitro effects under certain conditions, which suggest that this extract might act as either an antioxidant or a pro‐oxidant agent.
Acknowledgements
The authors thank Prof. Helmut Bartsch and Prof. Peter Schmezer for hosting Idania Rodeiro (I.R.), and Mr. Reinhard Gliniorz for technical support in the Division Epigenomics and Cancer Risk Factors, German Cancer Research Center (DKFZ), Im Neuenheimer Feld 280, 69120 Heidelberg, Germany. I.R. is also grateful to the Alexander von Humboldt Foundation for her research fellowship.
References
- 1. Rice‐Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant‐derived polyphenolic flavonoids. Free Rad. Res. 22, 375–383. [DOI] [PubMed] [Google Scholar]
- 2. Duarte Silva I, Gaspar J, Gomes da Costa G, Rodrigues AS, Laires A, Rueff J (2000) Chemical features of flavonols affecting their genotoxicity. Potential implications in their use as therapeutical agents. Chem. Biol. Interact. 124, 29–51. [DOI] [PubMed] [Google Scholar]
- 3. Martínez SG, Delgado HR, Pérez D, Garrido GG, Núñez Sellés AJ, León FOS (2000) Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang). Phytother. Res. 14, 424–427. [DOI] [PubMed] [Google Scholar]
- 4. Guevara M, Montalvo A, Álvarez A, Garrido G, Páez E, Delgado R (2002) Mangifera indica L. uso etnomédico en Cuba. Rev. Cubana Farm. 36, 166–168. [Google Scholar]
- 5. Tamayo D, Mari E, González S, Guevara M, Garrido G, Delgado R et al (2001) Vimang as natural antioxidant supplementation in patients with malignant tumours. Minerva Med. 92, 95–97. [Google Scholar]
- 6. García Rivera D, Hernández I, Merino N, Luque Y, Álvarez A, Martín Y et al (2011) Mangifera indica L. extract (Vimang) and mangiferin reduce the airway inflammation and Th2 cytokines in murine model of allergic asthma. J. Pharm. Pharmacol. 63, 1336–1345. [DOI] [PubMed] [Google Scholar]
- 7. Nuñez‐Selles A, Castro H, Aguero‐Aguero J, Gonzalez J, Naddeo F, De Simone F et al (2002) Isolation and quantitative analysis of phenolic antioxidants, free sugars and polyphenols from Mango (Mangifera indica L.) stem bark aqueous decoction used in Cuba as a nutritional supplement. J. Agric. Food Chem. 50, 762–766. [DOI] [PubMed] [Google Scholar]
- 8. Martínez SG, Jalil EC, Giuliani A, León FOS, Ram S, Delgado HR et al (2000) Mangifera indica L. Extract (Vimang) reduces ischaemia‐induced neuronal loss and oxidative damage in Gerbil brain. Free Radic. Res. 32, 1–3. [DOI] [PubMed] [Google Scholar]
- 9. Martínez G, Giuliani A, Leon OS, Perez G, Nuñez Selles AJ (2001) Effect of Mangifera indica L. extract (QF808) on protein and hepatic microsome peroxidation. Phytother. Res. 15, 581–585. [DOI] [PubMed] [Google Scholar]
- 10. Pardo GL, Delgado R, Velho JA, Curti C, Vercesi AE (2005) Mangiferin, a natural occurring glucosyl xanthone, increases susceptibility of rat liver mitochondria to calcium‐induced permeability transition. Arch. Biochem. Biophys. 439, 184–193. [DOI] [PubMed] [Google Scholar]
- 11. Remirez D, Shahrzard T, Delgado R, Sarandi A, O'Brien P (2005) Preventing hepatocyte oxidative stress cytotoxicity with Mangifera indica L. extract (Vimang). Drug Metabol. Drug Interact. 21, 19–29. [DOI] [PubMed] [Google Scholar]
- 12. Garrido G, Gonzalez D, Romay C, Nuñez‐Selles AJ, Delgado R (2008) Scavenger effect of a mango (Mangifera indica L.) food supplement's active ingredient on free radicals produced by human polymorphonuclear cells and hypoxanthine–xanthine oxidase chemiluminescence systems. Food Chem. 107, 1008–1014. [Google Scholar]
- 13. Garrido G, Gonzalez D, Delporte C, Backhouse N, Quintero G, Nuñez‐Selles AJ et al (2001) Analgesic and anti‐inflammatory effects of Mangifera indica L. extract (Vimang). Phytother. Res. 15, 18–21. [DOI] [PubMed] [Google Scholar]
- 14. Garrido G, Delgado R, Lemus Y, Rodríguez J, García D, Núñez‐Sellés AJ (2004) Protection against septic shock and suppression of tumor and nitric oxide production on macrophages and microglia by a standard aqueous extract of Mangifera indica L (Vimang) Role of mangiferin isolated from extract. Pharmacol. Res. 50, 165–172. [DOI] [PubMed] [Google Scholar]
- 15. Garrido G, Gonzalez D, Lemus Y, Garcia D, Lodeiro L, Quintero G et al (2004) In vivo and in vitro anti‐inflammatory activity of Mangifera indica L. extract (Vimang®). Pharmacol. Res. 50, 143–149. [DOI] [PubMed] [Google Scholar]
- 16. Garrido G, Blanco‐Molina M, Sancho R, Macho A, Delgado R, Muñoz E (2005) An aqueous stem bark extract of Mangifera indica (Vimang®) inhibits T cell proliferation and TNF‐induced activation of nuclear transcription factor NF‐κB. Phytother. Res. 19, 211–215. [DOI] [PubMed] [Google Scholar]
- 17. Garcia D, Delgado R, Ubeira F, Leiro J (2002) Modulation of rat macrophage function by the Mangifera indica L. extracts Vimang and mangiferin. Intern. Immunopharmacol. 2, 797–806. [DOI] [PubMed] [Google Scholar]
- 18. Leiro J, Garcia D, Arranz J, Delgado R, Sanmartin M, Orallo F (2004) An Anacardiaceace preparation reduces the expression of inflammation‐related genes in murine macrophages. Intern. Inmunopharmacol. 4, 991–1003. [DOI] [PubMed] [Google Scholar]
- 19. Guha S, Ghosal S, Chattopadhyay U (1996) Antitumor, immunomodulatory and anti‐HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy 42, 443–451. [DOI] [PubMed] [Google Scholar]
- 20. Garrido G, Valdes M (2012) Advances in pharmacological and toxicological investigations with the stem bark aqueous extract of the mango tree (Mangifera indica L). Rev. Farmacol. Chile 5, 63–93. [Google Scholar]
- 21. Garrido G, Martínez G, Pardo G, García D, Hernández P, Rodeiro I et al (2007) Recent advances in the research & development of an aqueous stem bark extract obtained from Mangifera indica L In. Capasso A, ed. Recent Developments in Plants Research, pp. 169–192. Kerala, India: Research Signpost. [Google Scholar]
- 22. Zheng MS, Lu ZY (1990) Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chin. Med. J. (Engl.) 103, 160–165. [PubMed] [Google Scholar]
- 23. Rodeiro I, Donato T, Hernandez I, Martinez I, Castell JV, Gómez‐Lechon MJ (2008) Potencial hepatoprotective effects of new Cuban natural products in rat hepatocytes culture. Tox. In Vitro 22, 1242–1249. [DOI] [PubMed] [Google Scholar]
- 24. Rajendran P, Ekambaram G, Sakthisekaran D (2008) Protective role of mangiferin against benzo(a)pyrene induced lung carcinogenesis in experimental animals. Biol. Pharm. Bull. 31, 1053–1058. [DOI] [PubMed] [Google Scholar]
- 25. Garrido G, Rodeiro I, Hernandez I, Garcia G, Perez G, Merino N et al (2009) In vivo acute toxicological studies of an antioxidant extract from Mangifera indica L (Vimang) Drug Chem . Toxicol. 32, 53–58. [DOI] [PubMed] [Google Scholar]
- 26. Rodeiro I, Cancino L, González JE, Morffi J, Garrido G, González RM et al (2006) Evaluation of the genotoxic potential of Mangifera indica l. extract (Vimang), a new natural product with antioxidant activity. Food Chem. Toxicol. 44, 1707–1713. [DOI] [PubMed] [Google Scholar]
- 27. Rodeiro I, Hernandez S, Morffi J, Herrera JA, Gómez‐Lechón MJ, Delgado R et al (2012) Evaluation of genotoxicity and DNA protective effects of mangiferin, a glucoxylxanthone isolated from Mangifera indica L. stem bark extract. Food Chem. Toxicol. 50, 3360–3366. [DOI] [PubMed] [Google Scholar]
- 28. Labieniec M, Gabryelak T, Falconi G (2003) Antioxidant and pro‐oxidant effects of tannins in digestive cells of the freshwater mussel Unio tumidus . Mutat. Res. 539, 19–28. [DOI] [PubMed] [Google Scholar]
- 29. Oikawa S, Furukawaa A, Asada H, Hirakawa K, Kawanishi S (2003) Catechins induce oxidative damage to cellular and isolated DNA through the generation of reactive oxygen species. Free Radic. Res. 37, 881–890. [DOI] [PubMed] [Google Scholar]
- 30. Yen GC, Duh PD, Tsai HL, Huang SL (2003) Pro‐oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 67, 1215–1222. [DOI] [PubMed] [Google Scholar]
- 31. Synder D, Gillies PJ (2002) Evaluation of the clastogenic DNA intercalative and topoisomerase II interactive properties of bioflavonoids in Chinese hamster V79 cells. Environ. Mol. Mutagen. 4, 266–276. [DOI] [PubMed] [Google Scholar]
- 32. Nelofer S, Ahmand A, Hadi S (2000) Anti‐oxidant, pro‐oxidant properties of tannic acid and its binding to DNA. Chem. Biol. Interact. 125, 177–189. [DOI] [PubMed] [Google Scholar]
- 33. Acosta‐Esquijarosa J, Jáuregui‐Haza U, Amaro‐González D, Sordo‐Martínez L (2009) Spray drying of aqueous extract of Mangifera indica L (Vimang): Scale up for the process. World Appl. Sci. J. 6, 408–412. [Google Scholar]
- 34. Nuñez‐Selles A (2005) Antioxidant therapy: myth or reality? J. Braz. Chem. Soc. 16, 699–710. [Google Scholar]
- 35. Aye M, Di Giorgio C, De Mo M, Botta A, Perrin J, Courbiere B (2010) Assessment of the genotoxicity of three cryoprotectants used for human oocyte vitrification: dimethyl sulfoxide, ethylene glycol and propylene glycol. Food Chem. Toxicol. 48, 1905–1912. [DOI] [PubMed] [Google Scholar]
- 36. Schmezer P, Rajaee‐Behbahani N, Risch A, Thiel S, Rittgen W, Drings P et al (2001) Rapid screening assay for mutagen sensitivity and DNA repair capacity in human peripheral blood lymphocytes. Mutagenesis 16, 25–30. [DOI] [PubMed] [Google Scholar]
- 37. Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp. Cell Res. 175, 184–191. [DOI] [PubMed] [Google Scholar]
- 38. Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H et al (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35, 206–221. [DOI] [PubMed] [Google Scholar]
- 39. Wilms LC, Hollman PC, Boots AW, Kleinjans JCS (2005) Protection by quercetin and quercetin‐rich fruit juice against induction of oxidative DNA damage and formation of BPDE‐DNA adducts in human lymphocytes. Mutat. Res. 585, 155–162. [DOI] [PubMed] [Google Scholar]
- 40. Collins AR, Duthie SJ, Dobson VL (1993) Direct enzymatic detection of endogenousbase damage in human lymphocyte DNA. Carcinogenesis 14, 1733–1735. [DOI] [PubMed] [Google Scholar]
- 41. Collins AR (2005) The Comet assay for DNA damage and repair. Principles, applications, and limitations. Mol. Biothecnol. 26, 249–259. [DOI] [PubMed] [Google Scholar]
- 42. Mersch‐Sundermann V, Kassie F, Bohmer S, Lu WQ, Wohlfarth R, Sobel R et al (2004) Extract of Toxicodendron quercifolim caused genotoxicity and antigenotoxicity in bone marrow cells of CD1 mice. Food Chem. Toxicol. 42, 1611–1617. [DOI] [PubMed] [Google Scholar]
- 43. Miura T, Muraoka S, Fujimoto Y (2003) Inactivation of creatine kinase induced by quercetin with horseradish peroxidase and hydrogen peroxide: pro‐oxidative and anti‐oxidative actions of quercetin. Food Chem. Toxicol. 41, 759–765. [DOI] [PubMed] [Google Scholar]
- 44. Khan NS, Ahmad A, Hadi SM (2000) Anti‐oxidant, pro‐oxidant properties of tannic acid and its binding to DNA. Chem. Biol. Interact. 125, 177–189. [DOI] [PubMed] [Google Scholar]
- 45. Puppo A (1992) Effects of flavonoids on hydroxil radical formation by Fenton type reactions; influence of the iron chelator. Phytochemistry 31, 85–88. [Google Scholar]
- 46. Nuñez‐Selles AJ, Delgado R, Garrido G, García D, Guevara M, Pardo G (2007) The paradox of natural products as pharmaceuticals. Experimental evidences of a mango stem bark extract. Pharmacol. Res. 55, 351–358. [DOI] [PubMed] [Google Scholar]
- 47. Awad HM, Boersma MG, Boeren S, Van Bladeren PH, Vervoort J, Rietjens IMCM (2001) Structure‐activity study on the quinone/quinone methide chemistry of flavonoids. Chem. Res. Toxicol. 14, 398–408. [DOI] [PubMed] [Google Scholar]
- 48. Awad HM, Boersma MG, Vervoort J, Rietjens IM (2000) Peroxidase‐catalyzed formation of quercitin quinone methide‐glutathione adducts. Arch. Biochem. Biophys. 378, 224–233. [DOI] [PubMed] [Google Scholar]
- 49. Tang SY, Whiteman M, Jenner A, Yong EL, Halliwell B (2004) Characterization of antioxidant and antiglycation properties and isolation of active ingredients from Chinese medicines. Free Radic. Biol. Med. 15, 1575–1587. [DOI] [PubMed] [Google Scholar]
- 50. Choucroun P, Gillet D, Dorange G, Sawicki B, Dewitte JD (2001) Comet assay and early apoptosis. Mutat. Res. 478, 89–96. [DOI] [PubMed] [Google Scholar]
- 51. Kasamatsu T, Kohda K, Kawazoe Y (2004) Comparison of chemically induced DNA breakage in cellular and subcellular systems using the Comet assay. Mut. Res. 369, 1–6. [DOI] [PubMed] [Google Scholar]
- 52. Turner ND, Brady LA, Ford J, Lupton JR (2002) Opportunities for nutritional amelioration of radiation‐induced cellular damage. Nutrition 18, 904–912. [DOI] [PubMed] [Google Scholar]
- 53. Ganesh CJ, Venkatasubbaiah AV (2005) Effect of mangiferin on radiation‐induced micronucleus formation in culture human peripheral blood lymphocytes. Environ. Mol. Mutagen. 46, 12–21. [DOI] [PubMed] [Google Scholar]
- 54. Marnett LJ (2002) Oxy radicals, lipid peroxidation and DNA damage. Toxicology 27, 219–222. [DOI] [PubMed] [Google Scholar]


