Abstract
Objective: Fibroblasts appear to modulate osteoclastogenesis, but their precise role in this process remains unclear. In this work, paracrine‐mediated osteoclastogenic potential of different human fibroblasts was assessed.
Materials and methods: Fibroblast‐conditioned media (CM) from foetal skin (CM1), adult skin (CM2) and adult gingiva (CM3) were used to promote osteoclastogenesis of osteoclast precursor cells. Cultures supplemented with macrophage‐colony stimulating factor (M‐CSF) and receptor activator of nuclear factor‐κB ligand (RANKL) were used as controls.
Results: All fibroblast cultures expressed FSP‐1, M‐CSF and RANKL and produced osteoprotegerin (OPG); gingival fibroblasts presented lowest expression of osteoclastogenic genes and higher production of OPG. All fibroblast CM were able to induce osteoclastogenesis. CM1 showed behaviour similar to positive controls, and slightly higher osteoclastogenic potential than CM, from adult ones. Gingival fibroblasts revealed lowest osteoclastogenic ability. Presence of anti‐MCSF or anti‐RANKL partially inhibited osteoclastogenesis promoted by CM, although the former antibody revealed higher inhibitory response. Differences among the osteoclastogenic effect of CM were noted, mainly in expression of genes involved in differentiation and activation of osteoclast precursor cells, c‐myc and c‐src, and less regarding functional related parameters.
Conclusions: Fibroblasts are able to induce osteoclastogenesis by paracrine mechanisms, and age and anatomical location affect this ability. Other factors produced by fibroblasts, in addition to M‐CSF and RANKL, appear to contribute to observed osteoclastogenic potential.
Introduction
Osteoclastogenesis is a complex process that occurs in discrete areas of bone tissue, the bone multicellular units (BMUs). In healthy bone, osteoclast formation and activation are strictly regulated by numerous types of cell physical, autocrine and paracrine cross‐talk involving among others, osteoclasts, osteoblasts and fibroblasts (1, 2, 3). Bone metabolic disorders can occur from imbalances in cell communication among these cell types (4).
Relevance of osteoblasts in the osteoclastogenic process has been extensively documented (4), however, fibroblasts also appear to be an important player in this process (1, 4). Fibroblasts are the most abundant cells of connective tissue; they are morphologically and functionally heterogeneous, depending on their location and activity (5, 6, 7). Although a relevant function of fibroblasts is to produce extracellular matrix, to maintain structural integrity of tissues, they also secrete numerous molecules indispensable for development and function of other cell types (8). Regarding bone tissue, fibroblasts express a variety of molecules known to be implicated in the osteoclastogenic process, including macrophage‐colony stimulating factor (M‐CSF) and receptor activator of nuclear factor‐κB ligand (RANKL), two growth factors sufficient to promote osteoclastogenesis in vitro (1). Also, some pro‐inflammatory cytokines produced by fibroblasts, such as IL‐1, TNF‐α, and IL‐6 (8, 9), appear to stimulate osteoclast formation and activation (3, 10, 11, 12, 13). Another important cytokine also produced by fibroblasts, IL‐17, has a positive effect on osteoclastogenesis (9). It has also been reported that fibroblasts express vascular endothelial growth factor (VEGF) (14), an important angiogenic factor that also induces osteoclastogenesis (15, 16).
In spite of this, only few studies address osteoclastogenic potential of fibroblasts (10, 17, 18, 19, 20, 21, 22) and thorough knowledge of their role in osteoclast differentiation and activation has not been established. This appears to be a very important subject as, in addition to that mentioned above, fibroblasts are assuming growing relevance in many inflammatory joint disorders associated with high rates of osteolysis, such as rheumatoid arthritis, where it has been proposed that fibroblasts are key players in osteoclast formation and activation (9, 14). Moreover, although osteoclasts are usually located in bone tissue, they can also be found in cutaneous nodules in rare systemic disorder multicentric reticulohistiocytosis, and in further soft tissue lesions (23, 24, 25, 26, 27). Thus, as it happens in bone, where mesenchymal cells modulate osteoclastogenesis, in those conditions, stromal cells, such as fibroblasts, can have a central role in regulation of osteoclast development.
In this context, and considering heterogeneity of fibroblasts, the aim of this work has been to evaluate osteoclastogenic potential of human fibroblasts from different origins, mediated by paracrine mechanisms, in the absence of direct physical cell interactions. To accomplish this, conditioned media collected from cell cultures of three different human fibroblast lineages (foetal skin, adult skin and adult gingiva) were used to promote osteoclastogenesis, of human peripheral blood mononuclear cells (PBMC). Cultures performed in absence or presence of recombinant M‐CSF and RANKL, classic inducers of in vitro osteoclastogenesis (1), were used as negative and positive control, respectively. Cell cultures were assessed for osteoclast‐related markers, including bone resorbing activity.
Materials and methods
Conditioned media from fibroblast cultures
Explants were collected from healthy donors aged 30–35 years. Three independent primary cultures from three distinct donors were performed for each fibroblastic origin.
Primary cultures were established by culturing explants of human foetal skin, adult skin and adult gingiva in α‐minimal essential medium (α‐MEM) containing 10% foetal bovine serum, 100 IU/ml penicillin, 2.5 μg/ml streptomycin, 2.5 μg/ml amphotericin B and 50 μg/ml ascorbic acid. Culture medium was replaced twice a week. Cultures were maintained in a 5% CO2 humidified atmosphere at 37 °C. Cell passage was performed by treatment of 70–80% confluent cultures with 0.05% trypsin in 0.5 mm EDTA. Cells from the second passage, cultured at 5 × 104 cell/cm2, were used to prepare conditioned media.
After reaching in the region of 50% confluence (4–5 days), second passage cell cultures were maintained for a further period of 7 days without medium change. Subsequently, culture medium was collected and centrifuged at 550 g for 10 min, aliquoted and stored at −20 °C. After recovering conditioned media, cell layers were assessed for total protein content, to normalize conditioned media used to supplement PBMC cultures. In addition, fibroblast cell layers were characterized for expression of fibroblast and osteoclastogenic genes, by RT‐PCR.
Conditioned media from fibroblast cultures of foetal skin (conditioned media 1, CM1), adult skin (CM2) and adult gingiva (CM3) were used as potential osteoclastogenic inducers.
Gene expression of fibroblast cultures by RT‐PCR analysis
RNA was extracted from fibroblast cultures after recovering the conditioned media. RNA was isolated using RNeasy® Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. RNA was quantified by evaluating absorbance of samples, at 260 nm. Expression of GAPDH (glyceraldehyde‐3‐phosphate dehydrogenase), FSP‐1 (fibroblast‐specific protein 1 gene), M‐CSF and RANKL genes was performed by RT‐PCR. For that, 0.5 μg of cell RNA was reverse transcribed and amplified (25 cycles) using the Titan One Tube RT‐PCR System (Roche, Basel, Switzerland), with annealing temperature of 55 °C; primers used are listed in Table 1. RT‐PCR products were analysed on a 1% (w/V) agarose gel. Densitometric analysis of bands obtained by RT‐PCR was performed with imagej (National Institutes of Health, USA) 1.41 software and values obtained were normalized for the corresponding GAPDH value of each experimental condition.
Table 1.
Primers used on RT‐PCR analysis of cell cultures used as a source of conditioned media
| Gene | 5′ Primer | 3′ Primer |
|---|---|---|
| GADPH | 5′‐CAGGACCAGGTTCACCAACAAGT‐3′ | 5′‐GTGGCAGTGATGGCATGGACTGT‐3′ |
| FSP‐1 | 5′‐CTCTGGAGAAGGCCCTGGAT‐3′ | 5′‐TTCTTCCTGGGCTGCTTATC‐3′ |
| M‐CSF | 5′‐CCTGCTGTTGTTGGTCTGTC‐3′ | 5′‐GGTACAGGCAGTTGCAATCA‐3′ |
| RANKL | 5′‐GAGCGCAGATGGATCCTAAT‐3′ | 5′‐TCCTCTCCAGACCGTAACTT‐3′ |
Osteoprotegerin quantification of conditioned media
OPG quantification was performed using the Osteoprotegerin Human ELISA Kit (Abcam, Cambridge, UK) following the manufacturer’s instructions. After detection, absorbance of samples was measured at 450 nm using an ELISA plate reader (Wellscan WS050; Denley, Franklin, USA). Results were expressed as pg/ml.
Human peripheral blood mononuclear cell cultures
PBMC were isolated from blood of healthy donors aged 25–35 years of age, as described previously (28). Briefly, blood was diluted with phosphate‐buffered saline (PBS; 1:1), and applied on to Ficoll‐Paque™ PREMIUM (GE Healthcare Bio‐Sciences, Piscataway, USA). Samples were centrifuged at 400 g for 30 min and PBMC were recovered and washed twice in PBS. Typically, around 70 × 106 PBMC were obtained for each 100 ml of processed blood.
PBMC were seeded at density of 3 × 106 cells/cm2. Cells were cultured in α‐MEM supplemented with 30% (V/V) human serum (from the same donor from which cells were obtained), 2 mm l‐glutamine, 100 IU/ml penicillin, 2.5 μg/ml streptomycin, 2.5 μg/ml amphotericin B, in the following experimental conditions: absence of recombinant growth factors and conditioned media (base medium); presence of both recombinant M‐CSF (R&D Systems, Minneapolis, USA) and RANKL (Insight Biotechnology, Wembley, UK) (positive control); presence of CM1‐CM3.
M‐CSF and RANKL were used at concentration of 25 and 40 ng/ml, respectively, based on previously published work (28). CM1–CM3 were used at 10% (V/V), based on preliminary experiments in which osteoclast precursor cell cultures were supplemented with 5–20% conditioned media and assessed for TRAP activity; concentrations that elicited maximum TRAP activity were chosen. Foetal bovine serum (present in conditioned media) did not affect TRAP activity of PBMC cell cultures (data not shown). When indicated, antibodies anti‐MCSF and anti‐RANKL (Abcam) were included in culture media, at 0.5 μg/ml. Cultures were maintained in a 5% CO2 humidified atmosphere at 37 °C for 21 days. Culture medium was replaced once a week. At the end of the culture period, cell cultures were characterized for protein content and several osteoclastic features, as follows:
Protein quantification. Total protein of cell cultures was quantified by Bradford’s method (29), using bovine serum albumin as standard. Shortly afterwards, post‐washing PBMC cultures in PBS, cells were solubilized in 0.1 m NaOH and treated using Coomassie® Protein Assay Reagent (Fluka, Milwaukee, USA). Samples were incubated for 2 min at room temperature and absorbance was quantified at 600 nm using an ELISA plate reader (Wellscan WS050; Denley). Results were expressed as mg/ml.
Expression of osteoclastic genes by RT‐PCR analysis. PBMC cultures were assessed by RT‐PCR for expression of osteoclastic genes, namely, osteoclastic differentiation gene c‐myc, osteoclastic activation gene c‐src (30), and osteoclastic markers TRAP, cathepsin K (CATK) and carbonic anhydrase 2 (CA2). RT‐PCR analysis was performed as described for the fibroblast cultures. Primers used are listed in Table 2.
Table 2.
Primers used on RT‐PCR analysis of PBMC cultures
| Gene | 5′ Primer | 3′ Primer |
|---|---|---|
| GAPDH | 5′‐CAGGACCAGGTTCACCAACAAGT‐3′ | 5′‐GTGGCAGTGATGGCATGGACTGT‐3′ |
| c‐myc | 5′‐TACCCTCTCAACGACAGCAG‐3′ | 5′‐TCTTGACATTCTCCTCGGTG‐3′ |
| c‐src | 5′‐AAGCTGTTCGGAGGCTTCAA‐3′ | 5′‐TTGGAGTAGTAGGCCACCAG‐3′ |
| TRAP | 5′‐ACCATGACCACCTTGGCAATGTCTC‐3′ | 5′‐ATAGTGGAAGCGCAGATAGCCGTT‐3′ |
| CATK | 5′‐AGGTTCTGCTGCTACCTGTGGTGAG‐3′ | 5′‐CTTGCATCAATGGCCACAGAGACAG‐3′ |
| CA2 | 5′‐GGACCTGAGCACTGGCATAAGGACT‐3′ | 5′‐AAGGAGGCCACGAGGATCGAAGTT‐3′ |
Presence of actin rings and vitronectin and calcitonin receptors. PBMC cultures were washed twice in PBS and treated with 3.7% (V/V) para‐formaldehyde for 15 min. After fixation, cells were permeabilized for 5 min with 0.1% (V/V) Triton X‐100 and stained for actin with 5 U/ml Alexa Fluor® 647‐phalloidin (Invitrogen, California, USA), and for vitronectin receptor (VNR) and calcitonin receptor (CTR) with 50 μg/ml mouse IgGs anti‐VNR and IgGs anti‐CTR (R&D Systems), respectively. Detection of IgGs anti‐VNR and IgGs anti‐CTR was performed with 2 μg/ml Alexa Fluor1 488‐goat anti‐mouse IgG. Stained cultures were observed using confocal laser scanning microscopy (CLSM).
TRAP activity; number of multinucleate TRAP‐positive cells. TRAP activity was assayed using the para‐nitrophenilphosphate (pNPP) hydrolysis assay. Briefly, PBMC were washed twice in PBS and solubilized in 0.1% (V/V) Triton X‐100. After solubilization, cell extracts were incubated in 12.5 mm pNPP in 0.04 m tartaric acid and 0.09 m citrate (pH 4.8), for 1 h at 37 °C. Samples were treated with 5 m NaOH, and absorbance was measured at 405 nm in an ELISA plate reader (Wellscan WS050; Denley). Results are expressed as nmol/min.mgprotein −1.
PBMC cultures were fixed in 3.7% formaldehyde for 10 min and then washed in distilled water. Cells were stained for TRAP with acid phosphatase, leucocyte (TRAP) kit (Sigma, Sigma‐Aldrich, Missouri, USA), according to the manufacturer’s instructions. Shortly afterwards, cells were incubated in the dark at 37 °C for 1 h, in presence of naphtol AS‐BI 0.12 mg/ml, 6.76 mm tartrate and 0.14 mg/ml fast garnet GBC. After incubation, cells were washed and stained with haematoxylin. Multinucleate (4–8 nuclei) and TRAP‐positive (purple/dark red) cells were quantified.
Calcium phosphate resorption assay. PBMC were cultured on BD BioCoat™ Osteologic™ Bone Cell Culture Plates (BD Biosciences, New Jersey, USA). After 21 days of culture, cells were removed with 6% NaOCl and 5.2% NaCl, following manufacturer’s instructions. Calcium phosphate layers were visualized by phase contrast light microscopy. Image analysis of resorbed areas was performed using imagej 1.41 software.
Statistical analysis
Results presented in this work were gathered from three separate experiments using cell cultures from three different patients. There were three replicates for each experimental situation. Groups of data were evaluated using two‐way analysis of variance and no significant differences in patterns of cell behaviour were found. Statistical differences found between control and experimental conditions were determined by Bonferroni’s method. Values of P ≤ 0.05 were considered to be significant. This study was reviewed and approved by the local Research Ethics Committee.
Results
Gene expression of fibroblast cultures
Figure 1a,b present expression of housekeeping gene, GAPDH, fibroblast marker gene FSP‐1 (31) and osteoclastogenic genes, M‐CSF and RANKL, assessed in fibroblast cultures used as source of conditioned media. GAPDH was highly expressed in all cell cultures. FSP‐1 expression was observed in all fibroblast cultures, but adult fibroblasts (from skin and gingiva) expressed significantly lower levels of it. Expression of osteoclastogenic factors M‐CSF and RANKL were significantly higher in skin (adult and foetal) fibroblasts compared to gingival fibroblasts.
Figure 1.
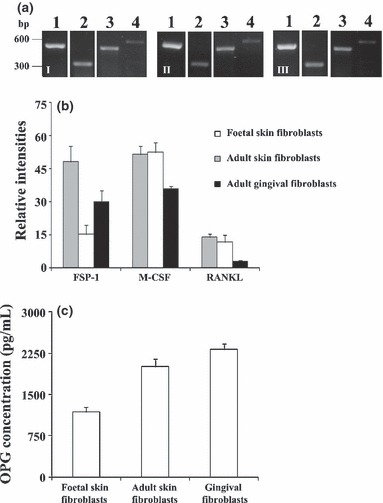
RT‐PCR analysis of fibroblast cultures used as source of conditioned media (CM). (a) Gene expression of GAPDH (lane 1), FSP‐1 (lane 2), M‐CSF (lane 3) and RANKL (lane 4). (i) Foetal skin fibroblasts; II – Adult skin fibroblasts; III – Gingival fibroblasts. (b) Densitometric analysis of the RT‐PCR bands normalized to GAPDH. (c) OPG quantification of fibroblast conditioned media. Results are an average of three independent sets of experiments.
Quantification of OPG present in conditioned media
As observed in Fig. 1c, all fibroblast conditioned media contained high concentrations of OPG. CM1 revealed lowest levels of the protein, followed by CM2 and, finally, CM3, which had concentration of OPG that was in the region of double that present on CM1.
Osteoclast differentiation and activation of PBMC
Total protein content. PBMC cultures performed in absence of any recombinant growth factor or conditioned medium (base medium) displayed low protein content, but supplementation with both recombinant M‐CSF and RANKL (positive control) sharply increased (about six times) total protein content (Fig. 2, group I). With regard to cultures supplemented with fibroblast conditioned media (Fig. 2, group II), all displayed higher values of cell protein compared to cultures performed in base medium. CM1 or CM2 elicited a response similar to that observed for the positive control, whereas cultures performed in presence of CM3 presented lower values (about 70%).
Figure 2.
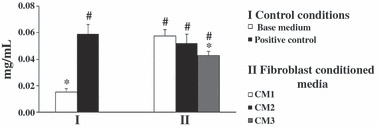
Protein content of PBMC cultures. Cell cultures were performed for 21 days in the absence (base medium) or presence (positive control) of recombinant M‐CSF and RANKL, or supplemented with conditioned media from foetal skin fibroblasts (CM1), adult skin fibroblasts (CM2) and adult gingival fibroblasts (CM3). *Significantly different from positive control. #Significantly different from cultures performed in base medium. Data were collected from three independent experiments.
Expression of osteoclast related genes by RT‐PCR analysis. Gene expression by PBMC cultures of housekeeping gene GAPDH and osteoclast‐related markers is presented in Fig. 3. GAPDH was highly expressed by each cell culture (Fig. 3a). Cultures supplemented with M‐CSF and RANKL (positive control) expressed genes associated with differentiation (c‐myc), activation (c‐src) and function (TRAP, CATK and CA2) of osteoclastic cells. CM1 supplementation elicited a response similar to control. Cultures treated with CM2 displayed lower expression of c‐myc (∼50%), c‐src (∼30%) and CA2 (∼20%), but similar expression of TRAP and CATK. CM3 supplementation resulted in lower expression of all markers, particularly c‐myc (∼80%), c‐src (∼60%) and CA2 (∼40%), and small decreases was observed of TRAP and CATK (∼20%).
Figure 3.
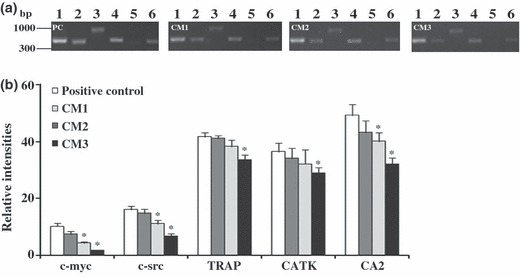
RT‐PCR analysis of PBMC cultures. Cell cultures were maintained for 21 days in the presence of both M‐CSF and RANKL (positive control, PC), or supplemented with conditioned media from foetal skin fibroblasts (CM1), adult skin fibroblasts (CM2) and adult gingival fibroblasts (CM3). (a) Gene expression of GAPDH (lane 1), c‐myc (lane 2), c‐src (lane 3), TRAP (lane 4), CATK (lane 5) and CA2 (lane 6). (b) Densitometric analysis of RT‐PCR bands normalized to GAPDH. *Significantly different from positive control. Three independent experiments were performed.
Presence of actin rings and vitronectin and calcitonin receptors. PBMC cultures supplemented with recombinant M‐CSF and RANKL or fibroblast conditioned media showed presence of cells displaying actin rings and positive for VNR and CTR, as assessed by CLSM. In addition, amounts of cells with these features seemed to be somehow correlated with results obtained for TRAP activity and staining. Representative result of this is shown in Fig. 4.
Figure 4.
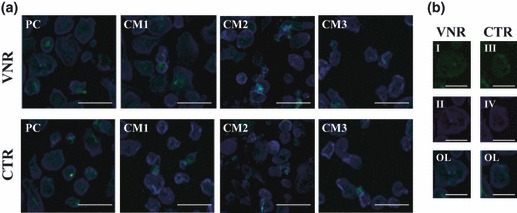
CLSM representative images of PBMC cultures, after 21 days. (a) Positive control (PC) and cultures supplemented with conditioned media from foetal skin fibroblasts (CM1), adult skin fibroblasts (CM2) and adult gingival fibroblasts (CM3). (b) Detail of osteoclasts stained green, VNR (I) or CTR (III) and blue for actin (II and IV); OL – overlay. Bars: 60 μm (a) and 100 μm (b).
TRAP activity; multinucleate TRAP‐positive cells. PBMC cultures maintained in base medium displayed low TRAP activity (Fig. 5a, group I). Supplementation with recombinant M‐CSF and RANKL increased TRAP activity about three times. Presence of fibroblast‐conditioned media (Fig. 5a, group II) also caused significant increase in TRAP activity, by around twice for CM2 and CM3, and three times for CM1. Compared with positive control, CM1 yielded similar TRAP activity, but supplementation with CM2 or CM3 resulted in lower vales (around 30%).
Figure 5.
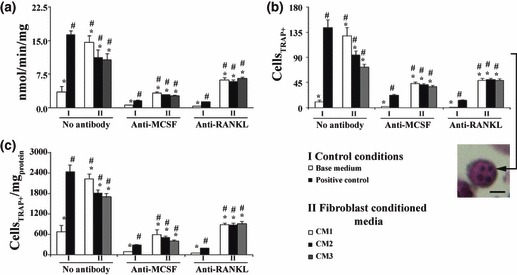
TRAP activity (a) and histochemical staining (b and c) of PBMC cultures. Cultures performed in the absence (base medium) or presence (positive control) of recombinant M‐CSF and RANKL, or supplemented with conditioned media from foetal skin fibroblasts (CM1), adult skin fibroblasts (CM2) and adult gingival fibroblasts (CM3). In parallel, cell cultures were treated with antibodies anti‐M‐CSF and anti‐RANKL. Multinucleate cells positive for TRAP were counted (b) and value obtained was normalized to total protein content (c). *Significantly different from positive control. #significantly different from cultures performed in base medium. Black bar in the representative image of a multinucleate cell positive for TRAP represents 50 μm. Results are a mean of three independent experiments.
In the presence of anti‐MCSF antibody, TRAP activity decreased significantly in all tested conditions, being in the region of 6–8 times lower in control conditions and 3–4 times lower in CM supplemented cell cultures. Treatment with antibody anti‐RANKL also yielded a decrease in enzyme activity of about 10 and 1.6–2 times in control conditions and PBMC cultures performed in the presence of CM1–CM3, respectively.
Quantification of multinucleate cells positive for TRAP is shown as absolute cell number and after normalization by total protein content Fig. 5b,c. The pattern was similar to that observed for TRAP activity in all tested conditions.
Calcium phosphate resorption activity. Resorption activity of PBMC on calcium phosphate coated culture plates, as observed by light phase contrast microscopy, is visualized in Fig. 6a. Resorbed areas were measured and expressed as percentage of total area (Fig. 6b). Cell cultures performed in base medium displayed low ability to reabsorb calcium phosphate, with only few isolated lacunae being observed (Fig. 6a). Presence of recombinant M‐CSF and RANKL, resulted in significant increase in resorption activity (about six times). Supplementation with CM1 elicited similar response. However, resorption activity was slightly lower in presence of CM2 (although without statistical significance) and significantly lower for CM3 treatment, with decrease of around 25%.
Figure 6.
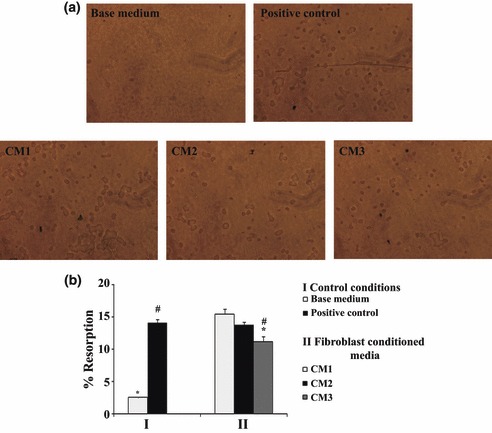
Calcium phosphate resorption activity of PBMC cultures. Cell cultures were performed in absence (base medium) or presence of recombinant M‐CSF and RANKL (positive control), or supplemented with conditioned media from foetal skin fibroblasts (CM1), adult skin fibroblasts (CM2) and adult gingival fibroblasts (CM3). (a) Representative images of calcium phosphate layers after cell removal. (b) Quantification of resorbed areas expressed as percentage of total area. *Significantly different from positive control. #Significantly different from cultures performed in base medium. Three independent experiments were performed for each condition.
Discussion
Although the exact role of fibroblasts on bone metabolism is not well established, it is known that they might be important players in the process (1, 4). In many inflammatory joint disorders, as well as in conditions where osteoclastic cells are found in extraskeletal tissues (9, 23, 24, 25, 26, 27), fibroblasts appear to be the main candidates for a key role in modulation of osteoclastogenesis. A relevant matter in this issue that deserves further investigation is the significance of heterogeneity found among different types of fibroblasts (5, 6, 7). In this work, conditioned media from skin (foetal and adult) and gingiva (adult) fibroblast cultures (from donors with no pathological conditions) were tested as potential promoters of PBMC osteoclast differentiation, activation and function.
Gene expression profile of the fibroblast cultures used as source of conditioned media exhibited differences between foetal and adult skin fibroblasts, and also between adult skin and gingival fibroblasts. Expression of the fibroblast marker FSP‐1 (31) was significantly higher in foetal fibroblasts compared to adult fibroblasts (skin and gingival), and gingival fibroblasts displayed lowest expressions. However, regarding osteoclastogenic inducers M‐CSF and RANKL, significant differences were noted only between skin and gingival fibroblasts with again, the latter expressing significantly lower levels, especially RANKL. These observations are consistent with previous studies reporting ability of fibroblasts to express these factors (10, 17, 18) and those showing that RANKL expression was very low in gingival fibroblasts (20). In addition, although all fibroblast cultures produced high levels of OPG, an inhibitory molecule known to have a key role in the osteoclastogenic process (1, 2, 4), highest levels were achieved in adult fibroblasts, particularly by gingival fibroblasts. Taken together, present results also reflect the heterogeneity among fibroblasts, as observed previously (5, 6, 7).
Conditioned media from skin and gingival fibroblasts supported survival of PBMC at a degree similar to or lower than positive controls, that is, PBMC supplemented with recombinant RANKL and M‐CSF. Total protein content, which provides information regarding adherent cell layer at the end of the culture period, was similar in cultures supplemented with CM from foetal skin and from adult fibroblasts, but lower in the presence of CM from gingival fibroblasts. This observation might be related, at least partially, to the lower expression of M‐CSF by these cells, which can lead to low osteoclastic progenitor survival, according to the proposed role of this growth factor on osteoclastogenesis (32).
PBMC supplemented with fibroblast conditioned media expressed genes associated with differentiation (c‐myc), activation (c‐src) and functional proteins (TRAP, CATK and CA2) of osteoclasts. Moreover, cells showed high TRAP activity associated with multinuclear cells, presence of actin rings, vitronectin and calcitonin receptors and calcium phosphate resorption activity. In addition, resorption activity was consistent with the number of TRAP‐positive multinucleate cells, suggesting that the counted cells were active osteoclasts. However, differences were found between foetal and adult skin fibroblasts and also between adult skin and gingival fibroblasts. CM1 was shown to be slightly more osteoclastogenic than CM2, and CM3 elicited lower response. It is worthwhile noting that differences observed were particularly significant for expression of c‐myc and c‐src genes. Regarding this, it has been reported that c‐myc is a downstream target of RANKL and that its expression is required for RANKL‐induced osteoclastogenesis (33). Moreover, expression of c‐src appears to be pivotal for formation and activity of human osteoclasts (34). On the other hand, differences among CM supplementation were lower regarding expression of genes associated with functional activity, TRAP, CATK and CA2. For this, differences observed in expression of TRAP and CATK were similar, which is in agreement with previous studies reporting that TRAP mRNA shows strong correlation with CATK mRNA, supporting its role in bone matrix degradation in collaboration with CATK (35).
Here, differences found in osteoclastogenic effects of fibroblast conditioned media might be related, at least partially, to differences of M‐CSF and/or RANKL expression, and of OPG production. Gingival fibroblasts showed lower expression of M‐CSF and RANKL, and high concentration of OPG was determined after CM3, probably contributing to low osteoclastogenic induction of PBMC by CM3. It is known that RANKL is a growth factor mainly engaged in late osteoclast differentiation events (36) and gingival fibroblasts showed only residual expression of this factor. In addition, OPG acts as a soluble decoy receptor for RANKL, sequestering it, and, thus, inhibiting osteoclastogenesis (31). Interestingly, foetal and adult skin fibroblasts expressed similar levels of both M‐CSF and RANKL, but OPG concentration after CM2 was higher than that after CM1. This can help explain why osteoclastogenic potential of CM1 seems to be slightly higher than that of CM2. To assess influence of M‐CSF and RANKL present in conditioned media on observed osteoclastogenesis, PBMC cultures were treated with antibodies raised against those growth factors. Both antibodies elicited decrease in osteoclast differentiation, which reveals that the corresponding growth factors are involved in osteoclastogenesis induced by paracrine mechanisms, by fibroblasts. However, relative decrease induced by anti‐MCSF was higher than that achieved by anti‐RANKL, which suggests that M‐CSF production by fibroblasts has a more important role on osteoclast differentiation than RANKL production. Nevertheless, inhibition observed in PBMC cultures treated with the antibodies was lower than that observed in positive controls, suggesting that fibroblast molecules other than M‐CSF and RANKL present in that conditioned medium play an important role in modulation of the osteoclastogenic process. As observed previously (9, 14, 27, 37, 38, 39, 40, 41, 42), a variety of molecules produced by fibroblasts can affect responsiveness of other cell types (monocytes, osteoclasts, macrophages) in the bone environment (43, 44, 45), in addition to M‐CSF and RANKL. To better understand osteoclastogenic features of fibroblast conditioned media, presence of relevant molecules, and mechanisms underlying their action on osteoclasts are being evaluated.
With regard to previous similar studies, it was observed that conditioned media derived from human periodontal ligament fibroblasts, either treated or not with cytokines, stimulated release of calcium from bone organ cultures (19, 21, 22). A similar result was also observed for gingival fibroblasts (19). Interestingly, differences in osteoclastogenic profile of fibroblasts of different origins has also been proposed in a study showing that oral fibroblasts may stimulate resorption in a way different from that of established fibroblast cell lines and an epidermal cell line (21). More recently, de Vries et al. (20) have shown that conditioned medium from gingival fibroblasts has the ability to inhibit osteoclast formation and activation in the presence of recombinant M‐CSF and RANKL. Moreover, in a previous report, employing co‐cultures of mouse osteoclast precursors and fibroblasts, including skin fibroblasts, it was observed that fibroblasts have the ability to promote osteoclastogenesis (27). The present work here related is consistent with reported studies showing that fibroblasts have the ability to induce varying degrees of osteoclastogenesis, although scarcity of studies and great heterogeneity of fibroblasts and the experimental protocols, render establishment of patterns difficult. Biological significance of the role of fibroblast cells on osteoclastogenesis has not yet been clearly established. Although skin fibroblasts have the potential to induce a high degree of osteoclast differentiation, this process does not occur in normal skin, highlighting complexity of the osteoclastogenic process, which involves several cell types, and the resulting network of cross‐talk may dictate fate of osteoclast precursor cells. However, there is growing evidence of involvement of fibroblasts in pathological conditions associated with a high rate of osteolysis (9, 24, 27, 42).
In conclusion, the present work shows that human fibroblasts were able to promote osteoclastogenesis in the absence of physical cell–cell interactions, and this ability seems to involve other molecules in addition to M‐CSF and RANKL. This potential appears to be present in foetal tissues and is maintained through adulthood. Significant differences were noted between fibroblasts from different origins, namely adult skin and gingival fibroblasts. Also, differences appear to exist between foetal and adult fibroblasts of the same origin, as suggested by the results from the skin fibroblasts. In addition, differences were noted in expression of genes, c‐myc and c‐src, involved in differentiation and activation of osteoclast precursors, and with lower significance regarding functional related parameters. Elucidation of the role of role fibroblasts in osteoclast biology would contribute to better understanding of bone metabolism, with the possibility of new therapeutic strategies in diseases associated with osteolysis.
Acknowledgements
The authors thank Faculty of Dental Medicine from University of Porto, Portugal, for financial support.
References
- 1. Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423, 337–342. [DOI] [PubMed] [Google Scholar]
- 2. Bruzzaniti A, Baron R (2006) Molecular regulation of osteoclast activity. Rev. Endocr. Metab. Disord. 7, 123–139. [DOI] [PubMed] [Google Scholar]
- 3. Vaananen HK, Laitala‐Leinonen T (2008) Osteoclast lineage and function. Arch. Biochem. Biophys. 473, 132–138. [DOI] [PubMed] [Google Scholar]
- 4. Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS (2008) The cell biology of bone metabolism. J. Clin. Pathol. 61, 577–587. [DOI] [PubMed] [Google Scholar]
- 5. Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA et al. (1994) Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin. Immunol. 72, 283–292. [DOI] [PubMed] [Google Scholar]
- 6. Phipps RP, Borrello MA, Blieden TM (1997) Fibroblast heterogeneity in the periodontium and other tissues. J. Periodontal Res. 32, 159–165. [DOI] [PubMed] [Google Scholar]
- 7. Sorrell JM, Caplan AI (2004) Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 117, 667–675. [DOI] [PubMed] [Google Scholar]
- 8. Kurosu H, Kuro OM (2009) Endocrine fibroblast growth factors as regulators of metabolic homeostasis. Biofactors 35, 52–60. [DOI] [PubMed] [Google Scholar]
- 9. Walsh NC, Crotti TN, Goldring SR, Gravallese EM (2005) Rheumatic diseases: the effects of inflammation on bone. Immunol. Rev. 208, 228–251. [DOI] [PubMed] [Google Scholar]
- 10. Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 473, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mundy GR (1996) Bone‐resorbing cells In Research TASfBaM , ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, pp. 16–24. Lippincott‐Raven Publishers, New York. [Google Scholar]
- 12. Osdoby P, Martini MC, Caplan AI (1982) Isolated osteoclasts and their presumed progenitor cells, the monocyte, in culture. J. Exp. Zool. 224, 331–344. [DOI] [PubMed] [Google Scholar]
- 13. Suda T, Takahashi N (1996) Cells of bone: osteoclast generation In: Bilezikian JP, Rodan GA, eds. Principles of Bone Biology, pp. 87–102. Academic Press, San Diego. [Google Scholar]
- 14. Koreny T, Tunyogi‐Csapo M, Gal I, Vermes C, Jacobs JJ, Glant TT (2006) The role of fibroblasts and fibroblast‐derived factors in periprosthetic osteolysis. Arthritis Rheum. 54, 3221–3232. [DOI] [PubMed] [Google Scholar]
- 15. Kohno S, Kaku M, Tsutsui K, Motokawa M, Ohtani J, Tenjo K et al. (2003) Expression of vascular endothelial growth factor and the effects on bone remodeling during experimental tooth movement. J. Dent. Res. 82, 177–182. [DOI] [PubMed] [Google Scholar]
- 16. Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S et al. (1999) Vascular endothelial growth factor can substitute for macrophage colony‐stimulating factor in the support of osteoclastic bone resorption. J. Exp. Med. 190, 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashizume M, Hayakawa N, Mihara M (2008) IL‐6 trans‐signalling directly induces RANKL on fibroblast‐like synovial cells and is involved in RANKL induction by TNF‐alpha and IL‐17. Rheumatology (Oxford) 47, 1635–1640. [DOI] [PubMed] [Google Scholar]
- 18. Kaku M, Motokawa M, Tohma Y, Tsuka N, Koseki H, Sunagawa H et al. (2008) VEGF and M‐CSF levels in periodontal tissue during tooth movement. Biomed. Res. 29, 181–187. [DOI] [PubMed] [Google Scholar]
- 19. Cochran DL, Rouse CA (1993) The effect of conditioned medium from connective tissue fibroblasts and epithelium on calcium release from mouse calvarial organ culture. Arch. Oral Biol. 38, 61–65. [DOI] [PubMed] [Google Scholar]
- 20. de Vries TJ, Schoenmaker T, Wattanaroonwong N, van den Hoonaard M, Nieuwenhuijse A, Beertsen W et al. (2006) Gingival fibroblasts are better at inhibiting osteoclast formation than periodontal ligament fibroblasts. J. Cell. Biochem. 98, 370–382. [DOI] [PubMed] [Google Scholar]
- 21. Saito S, Rosol TJ, Saito M, Ngan PW, Shanfeld J, Davidovitch Z (1990) Bone‐resorbing activity and prostaglandin E produced by human periodontal ligament cells in vitro. J. Bone Miner. Res. 5, 1013–1018. [DOI] [PubMed] [Google Scholar]
- 22. Saito S, Saito M, Ngan P, Lanese R, Shanfeld J, Davidovitch Z (1990) Effects of parathyroid hormone and cytokines on prostaglandin E synthesis and bone resorption by human periodontal ligament fibroblasts. Arch. Oral Biol. 35, 845–855. [DOI] [PubMed] [Google Scholar]
- 23. Athanasou NA, Quinn J, Ferguson DJP, McGee JOD (1991) Bone resorption by macrophage polykaryons of giant cell tumour of tendon sheath. Br. J. Cancer 63, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Codriansky KA, Runger TM, Bhawan J, Kantarci A, Kissin EY (2008) Multicentric reticulohistiocytosis: a systemic osteoclastic disease? Arthritis Rheum. 59, 444–448. [DOI] [PubMed] [Google Scholar]
- 25. Khan ZM, Cockerell CJ (1997) Atypical fibroxanthoma with osteoclast‐like multinucleated giant cells. Am. J. Dermatopathol. 19, 174–179. [DOI] [PubMed] [Google Scholar]
- 26. Neale S, Kristelly R, Gundle R, Quinn JMW, Athanasou NA (1997) Giant cells in pigmented villo nodular synovitis express an osteoclast phenotype. J. Clin. Pathol. 50, 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quinn JM, Horwood NJ, Elliott J, Gillespie MT (2000) Fibroblastic stromal cells express receptor activator of NF‐kB ligand and support osteoclast differentiation. J. Bone Miner. Res. 15, 1459–1466. [DOI] [PubMed] [Google Scholar]
- 28. Nicholson GC, Malakellis M, Collier FM, Cameron PU, Holloway WR, Gough TJ et al. (2000) Induction of osteoclasts from CD14‐positive human peripheral blood mononuclear cells by receptor activator of nuclear factor kappaB ligand (RANKL). Clin. Sci. (Lond.) 99, 133–140. [PubMed] [Google Scholar]
- 29. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 30. Zhao Q, Shao J, Chen W, Li YP (2007) Osteoclast differentiation and gene regulation. Front. Biosci. 12, 2519–2529. [DOI] [PubMed] [Google Scholar]
- 31. Okada H, Danoff TM, Fischer A, Lopez‐Guisa JM, Strutz F, Neilson EG (1998) Identification of a novel cis‐acting element for fibroblast‐specific transcription of the FSP1 gene. Am. J. Physiol. 275, 306–314. [DOI] [PubMed] [Google Scholar]
- 32. Pixley FJ, Stanley ER (2004) CSF‐1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 14, 628–638. [DOI] [PubMed] [Google Scholar]
- 33. Battaglino R, Kim D, Fu J, Vaage B, Fu XY, Stashenko P (2002) c‐myc is required for osteoclast differentiation. J. Bone Miner. Res. 17, 763–773. [DOI] [PubMed] [Google Scholar]
- 34. de Vries TJ, Mullender MG, van Duin MA, Semeins CM, James N, Green TP et al. (2009) The Src inhibitor AZD0530 reversibly inhibits the formation and activity of human osteoclasts. Mol. Cancer Res. 7, 476–488. [DOI] [PubMed] [Google Scholar]
- 35. Logar DB, Komadina R, Preželj J, Ostanek B, Trošt Z, Marc J (2007) Expression of bone resorption genes in osteoarthritis and in osteoporosis. J. Bone Miner. Metab. 25, 219–225. [DOI] [PubMed] [Google Scholar]
- 36. Wada T, Nakashima T, Hiroshi N, Penninger JM (2006) RANKL‐RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 12, 17–25. [DOI] [PubMed] [Google Scholar]
- 37. Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ (2000) A role for TGFbeta(1) in osteoclast differentiation and survival. J. Cell Sci. 113, 2445–2453. [DOI] [PubMed] [Google Scholar]
- 38. Kaneda T, Nojima T, Nakagawa M, Ogasawara A, Kaneko H, Sato T et al. (2000) Endogenous production of TGF‐beta is essential for osteoclastogenesis induced by a combination of receptor activator of NF‐kappa B ligand and macrophage‐colony‐stimulating factor. J. Immunol. 165, 4254–4263. [DOI] [PubMed] [Google Scholar]
- 39. Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S et al. (2000) Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL‐RANK interaction. J. Exp. Med. 191, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sato K, Takayanagi H (2006) Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr. Opin. Rheumatol. 18, 419–426. [DOI] [PubMed] [Google Scholar]
- 41. Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T et al. (2000) Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 43, 259–269. [DOI] [PubMed] [Google Scholar]
- 42. Udagawa N, Kotake S, Kamatani N, Takahashi N, Suda T (2002) The molecular mechanism of osteoclastogenesis in rheumatoid arthritis. Arthritis Res. 4, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mandelin J, Li TF, Hukkanen M, Liljestrom M, Salo J, Santavirta S et al. (2005) Interface tissue fibroblasts from loose total hip replacement prosthesis produce receptor activator of nuclear factor‐kappaB ligand, osteoprotegerin, and cathepsin K. J. Rheumatol. 32, 713–720. [PubMed] [Google Scholar]
- 44. Sabokbar A, Itonaga I, Sun SG, Kudo O, Athanasou NA (2005) Arthroplasty membrane‐derived fibroblasts directly induce osteoclast formation and osteolysis in aseptic loosening. J. Orthop. Res. 23, 511–519. [DOI] [PubMed] [Google Scholar]
- 45. Wei X, Zhang X, Zuscik MJ, Drissi MH, Schwarz EM, O’Keefe RJ (2005) Fibroblasts express RANKL and support osteoclastogenesis in a COX‐2‐dependent manner after stimulation with titanium particles. J. Bone Miner. Res. 20, 1136–1148. [DOI] [PubMed] [Google Scholar]


