Abstract
Abstract. All too often media attention clouds the reality that there are many types of stem cell. The embryos, bone marrow and umbilical cord blood (UCB) are the three most used sources. However, despite what it would appear, embryonic stem cells have not been the first to yield life‐saving cures at present. Faster routes to clinical intervention have been using adult stem cells that can be sourced from bone marrow and from cord blood, and that are readily accessible and are more ethically acceptable to the general public. Both these non‐embryonic sources have been able to provide sufficient numbers of cells to allow development of clinical translational protocols. Bone marrow‐derived cells have been used successfully in myocardial infarct therapy where relining by endothelial tissue has allowed limited reperfusion to damaged cardiac tissue. UCB have also demonstrated significant success for around 20 years in haematotransplantation. With a global human population in excess of 6 billion, births thus UCB, remain the largest untouched source of stem cells available every year. UCB also provide a distinct advantage over other adult stem cells due to the length of the telomere and also due protected immunological status of the developing neonatal environment. The total mutation load in the UCB populations is clearly likely to be significant less than in adult tissues.
INTRODUCTION
The non‐scientific public could be forgiven for thinking that there is only one type of stem cell – the embryonic stem cell. Media attention to embryonic stem cells (ESCs) is responsible for this, but the attention is problematic, because it clouds the reality that major scientific hurdles have not been overcome to use ESCs in the treatment of disease. On the other hand, adult stem cells have already demonstrated potential for therapeutic intervention. While it could be argued that animal ESC use has been interesting, translation of discoveries to humans is challenging because they have three main limitations: first, the limited amount of tissue producible from one embryo either with or without use of feeder layers (Klimanskaya et al. 2005; Martin et al. 2005); second, the limitation of producing homogeneous tissue (embryonic stem lines grown for more than 80 passages have been shown to acquire mutations) (Krtolica 2005); and, third, the immunological reality that when tissue is produced, it will gain human leucocyte antigens and be recognizable to a host immune system as being foreign, and thus risk rejection (Tajima et al. 2003; Kofidis et al. 2005). Although television and newspapers report ‘breakthroughs’ in embryonic stem transplantation (usually infrequent and usually subprimate animal), their goals remain, as yet, unattained for human therapy.
Tissue engineering and stem cell biology are fast‐evolving fields with an exciting future, but we must be careful not to provide unfounded expectations, controversies and hype. So what are stem cells and how can they be defined? A true stem cell is best described as a cell capable of differentiating into one or more cell types as well as maintaining an immature stem cell pool, by self‐renewal and asymmetric division (Krtolica 2005). Throughout ontogeny, stem cells have historically been grouped into two distinct categories embryonic or adult stem cells. We would now add umbilical cord blood (UCB) as a source of stem cells, a third category, because they possess differences to both embryonic stem and traditional adult sourced bone marrow.
Embryonic stem cells are formed post‐fertilization and are termed totipotent by their ability to produce tissues for three germ layers plus trophoblast precursors. Strictly speaking, ESCs are those that form the entire being (human ESCs forming a whole human), and embryonic stem cell lines are those that are experimentally isolated from the inner cell mass of the pre‐implantation blastocyst (Stojkovic et al. 2004; Atala 2005). Despite what is inaccurately reported, embryonic stem lines can only be described as pluripotent, because no one at the time of writing this manuscript has been able to produce every single type of human cell from one embryonic stem line. The reason for this is simple – we do not yet know every individual type of cell in the human system – the technology to analyse that does not yet exist. The ethical debate and issues surrounding the use of embryos for research and/or clinical applications is still passionate and calls for objective education of the public, scientists, physicians and politicians (Landry & Zucker 2004).
Adult stem cells have historically been considered to be located in adult tissue and to be mulipotent, capable of differentiation into one or several lineages. Adult stem cells also organize tissue regeneration and repair after stress or trauma (Quesenberry et al. 2005). UCB is perhaps one of the most readily available sources of adult stem cells, and in some quarters it is felt that UCB should be given a new category of its own. That UCB has been in existence from an extremely early point post‐fertilization, proposes a question – is this a source for adult stem cells, or is there a possibility that a group of stem cells exists that transcends many of the traditional barriers laid down by the conventional embryonic vs. adult lobby.
Here, we focus on recent progress made in studies on UCB by our research group, in which a unique group of stem cells with embryonic properties has been derived from UCB and applied to a three‐dimensional model of hepatic tissue, bio‐engineering in microgravity‐simulating bioreactors. Limited numbers of adult stem cells have often hindered their clinical development, necessitating expansion of the cells. However, historically, ex vivo expansion of cells while restricting their differentiation has proven a difficult task. We have investigated some of the characteristics of cell populations available from UCB we have developed these intriguing cells for tissue engineering and regeneration protocols, including the use of microgravity‐simulated bioreactor culture to expand our stem cells, ex vivo. We have accessed rotating culture devices originally developed with NASA (National Aeronautics and Space Administration) to successfully expand stem cell populations of extreme immaturity prior to loading the cells on to three‐dimensional scaffolds, allowing defined hepatocyte generation. Perhaps one of the most exciting characteristics of such extremely immature cells is that they can show an in vitro growth pattern similar to that of embryoid bodies seen during embryonic stem cell culture. We believe the creation of clinical grade protocols for rapid harvest and processing of UCB can reveal cell populations ranging from mature cells right back to stem cell groups of extreme immaturity and useful for both diagnostic and therapeutic development. UCB adult stem cells are a thrilling therapeutic prospect and with the advent of international cord blood banking, provide an increasing resource of stem cells useful for global treatment of disease.
UMBILICAL CORD BLOOD AS A USEFUL SOURCE OF STEM CELLS FOR CLINICAL APPLICATION
Adult stem cells (ADS) are immature cell groups capable of tissue repair and maintaining tissue homeostasis in a niche‐specific controlled microenvironment (Heissig et al. 2005; Li & Xie 2005). ADS offer a faster route to clinical intervention than embryonic stem cells, providing a cell source that is both readily accessible and more ethically acceptable to the public. Although ADS have been characterized in many tissues (including neural, muscular, hepatic and cardiovascular), haematopoietic stem cells remain the most characterized ADS population with clinical application. Bone marrow (BM), mobilized peripheral blood and UCB have provided sufficient quantities of cells to develop clinical translation protocols, including the development of BM as a cellular therapy for myocardially infarcted cardiac tissues and the use of UCB in haematotherapy (Stamm et al. 2003; Cohen & Nagler 2004; Perin & Silva 2004).
Umbilical cord blood provides distinct advantages over other adult stem cell sources. In terms of ontogeny, UCB cells are at the intermediate point between embryonic/foetal and adult life, have longer telomeres per chromosome and high cell proliferation potential. UCB units also have reduced risk of viral contamination (cytomegalovirus or Epstein–Barr virus). Haematological transplantation using UCB has further demonstrated reduced incidence in graft‐versus‐host disease during allogeneic grafts (Rocha et al. 2000; Rubinstein et al. 1999) and UCB cells display better tolerance for human leucocyte antigen (HLA) mismatches, when compared to BM (probably due to the immaturity of the immune cells, control by UCB dendritic cells and/or natural killer cells) (Cohen & Nagler 2004; Liu et al. 2004). UCB is also a valuable source of stem cells in terms of access and supply. With a worldwide population in excess of 6 billion and a global birth rate around 100 million per year, it remains the largest untouched source of stem cells available. The non‐invasive nature of its collection confers UCB with a distinct advantage over any other sources of adult or embryonic stem cells, because its cells can be easily characterized and banked. Efficient UCB banking constitutes a powerful and readily accessible cell source for allogeneic transplants particularly for patients with rare HLA phenotypes (e.g. some ethnic minorities) but also siblings and unrelated recipients. These UCB units are used routinely for haematotherapy, mainly paediatric haematological transplantation.
In the UK, the public National Blood Service Cord Blood Bank currently stores over 7000 UCB units. All are HLA‐phenotyped, screened for microbiological infections and banked following current good manufacture and practice standards (Watt & Contreras 2005). In the USA, over 40 000 UCB units are banked and managed by the National Marrow Donor Programme. Further to this, the ‘Bone Marrow Donors Worldwide’ registry collected data from 38 cord blood registries from 21 countries and facilitates direct access to over 200 000 UCB units worldwide (from private and public banks; http://www.bmdw.org). Our own Newcastle Cord Blood Bank has transplanted cord blood into patients for both haematotherapy and immunotherapy successfully, including multiple cord blood units into single patients.
UMBILICAL CORD BLOOD STEM CELLS AND CHALLENGES
Umbilical cord blood's main drawback is the limited number of cells available from a single unit. For instance, the number stem/progenitor cells present in an average UCB unit was believed to represent only 5% of the optimal dose required for adult haematopoietic transplantation (2–4 × 106/kg) (Gilmore et al. 2000). This supposed limitation focused research and development towards safe and reliable technologies for ex vivo expansion of the cells, pooling and cryopreservation of them (Querol et al. 2000; Ende et al. 2001). Our research group has spent years investigating available primitive stem cell populations available in UCB. Historically, clinicians have quantified and predicted haematological graft success using CD34 (a cell surface sialomucin) expression as the gold standard marker for stem/progenitor cells. Using multiparametric phenotyping, we have demonstrated that UCB contains a complex succession of heterogeneous stem and progenitor cell groups up‐ and down‐regulating a range of surface antigens including CD133, CD34, CD38, CD7, CD90 and more, as they differentiated (Forraz et al. 2002a; McGuckin et al. 2003a). High definition laser‐scanning confocal microscopy imaging enabled us to understand cell adhesion molecules’ role in stem cell development, maintenance and differentiation, and three‐dimensional interactions with surrounding cells and extracellular matrix components (McGuckin et al. 2003b). By integrating these data together, we moved away from positive cell isolation methods targeting progenitor cell groups (CD34+ or CD133+) that, at least for a vast majority, are committed towards haematopoiesis. This led us to progressively refine our stem cell separation strategy by removing haematopoietic mature and progenitor cells in order to yield pre‐haematopoietic stem cells from UCB (Forraz et al. 2004). Interestingly, this extremely immature, perhaps lineage‐restricted stem cell group proved to bear differentiation multipotential into blood and also into non‐haematopoietic phenotypes, including neural and hepatic tissues (2002b, 2004, 2003; 2003c, 2004a, 2004b).
EMBRYONIC STEM CELLS FROM UMBILICAL CORD BLOOD?
Because we could reproducibly identify and select UCB stem cells with multitissue differentiation capability, and further because UCB cells are ontogenically young, we hypothesized that highly primitive and discrete circulating stem cell groups could remain from embryonic development in UCB. We then optimized a series of stringent and rapid cell separation methods whereby sequential immunomagnetic removal of mature cells in UCB combined to standardized subculture protocols, has revealed reproducibly immature stem cell groups with astonishing embryonic characteristics. We have called these cord blood‐derived embryonic‐like stem cells (CBEs). UCB units were collected post‐birth from caesarean section donors and were submitted to centrifugal density separation to remove most erythrocytes, granulocytes and platelets. The UCB mononucleate cell fraction was further depleted of nucleate red cells precursors, myeloid and lymphoid progenitors, and leucocytes at various stages of differentiation, by sequential immunomagnetic cell separation targeting glycophorin‐A, CD33, CD7 and CD45 surface antigens (Forraz et al. 2004; McGuckin et al. 2004b, 2005). Post‐selection, lineage‐restricted cells represented a highly rare and specific cell group from UCB, as little as 0.16 ± 0.02% of the initial mononucleate cell fraction (n = 21). The lineage‐restricted stem cells were then grown at high concentration and low volume (to promote cell–cell interaction and endogenous growth factor release) in microtissue flasks with Iscove's modified Dulbecco's medium supplemented with human recombinant thrombopoietin, flt3 ligand and c‐kit ligand (McGuckin et al. 2005). For every 10 UCB units submitted to this protocol six yielded CBEs with similar growth patterns, consisting of a majority of cells (70%) adhering to the microflasks’ matrix and forming tight organized embryoid body‐like colonies after only 1 week (Fig. 1).
Figure 1.
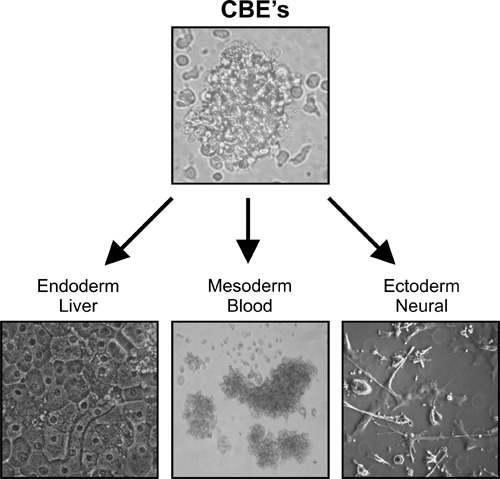
Cord blood‐derived embryonic‐like cells (CBEs) can be harvested and tissues produced, using cord blood, for all three germ layers. Highlighted here, are blood, hepatic and neural tissues.
We confirmed the embryonic similarity of the CBEs’ nature by testing immunoreactivity against a selection of human embryonic stem cell markers. Notably CBEs expressed stage‐specific embryonic antigens‐3 and ‐4 (SSEA‐3 and SSEA‐4) that are sialoproteins expressed by human ESCs, but did not express SSEA‐1, confirming their undifferentiated phenotype (Thomson et al. 1998). CBEs were further immunoreactive for tumour rejection antigens (TRA) 1‐60 and TRA 1‐81 and ESC‐specific extracellular matrix sialylated keratin sulfate proteoglycans, which most likely contributed to the embryoid body‐like nature of their clustered organization (Hoffman & Carpenter 2005). CBEs also expressed pluripotency transcription factor Oct‐4 involved in differentiation inhibition and ESC self‐renewal (Matin et al. 2004; Gerrard et al. 2005), and demonstrated high potential for cell population expansion in liquid culture. Following a short lag phase, CBE colonies could be propagated significantly for 14 weeks and over, with 389 maximal fold expansion at week 7 (2, 3), exponential CBE population growth occurring between week 2 and week 7. Following slowing down in proliferation, CBEs underwent a maintenance phase (week 9 to week 14) that coincided with near‐confluence on the substrata and colonies no longer releasing non‐adherent single cells into the supernatant. During this plateau phase, CBEs could be dissociated with trypsin and be re‐plated into second generation liquid cultures where they expanded exponentially for over 6 weeks (Fig. 3); they could also be frozen down and thawed without affecting their in vitro proliferation potential. CBEs might thus (i) have the ability to self‐limit their growth via cell–cell contact or release of soluble growth inhibitors, (ii) require sustained embryonic‐like stimuli for further propagation or (iii) be inhibited by the in vitro surface geography of the microflask, preventing further free enlargement of the colony. CBEs are currently being tested in novel feeder‐free systems specific for ESCs (Klimanskaya et al. 2005; Wang et al. 2005).
Figure 2.
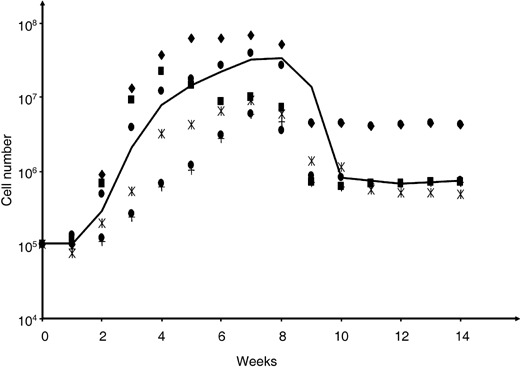
Phased growth characteristics of primary Cord blood‐derived embryonic‐like cell colonies growing in vitro.
Figure 3.
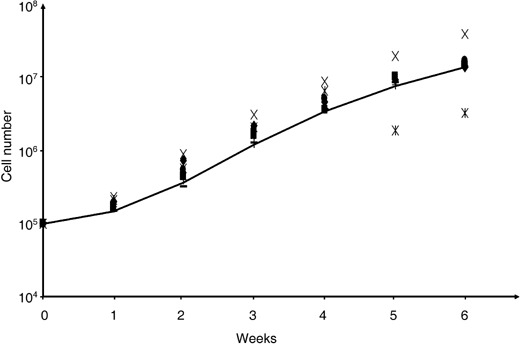
Cord blood‐derived embryonic‐like cells can be subpropagated and reseeded for continued controlled growth and expansion.
Criticism, from the ESC community that we could not produce such cells from a frozen cord blood unit, was made irrelevant in 2006 when we repeated the work on frozen units successfully, including the demonstration of expression of the stem cell marker Nanog (Fig. 4). Taken together, our protocols highlight the tremendous potential for the establishment of UCB‐derived stem cell lines with embryonic properties.
Figure 4.
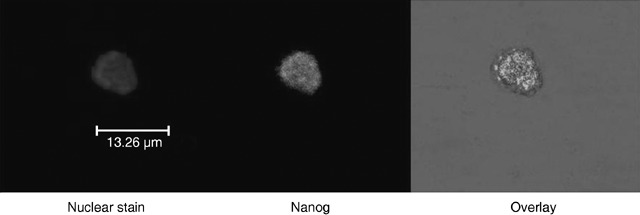
Example of CBE staining for embryonic markers from frozen banked umbilical cord blood. In this case, staining of the stem cell marker Nanog is highlighted.
HEPATIC FORMATION USING CORD BLOOD‐DERIVED EMBRYONIC‐LIKE STEM CELLS IN THREE‐DIMENSIONAL MICROGRAVITY TISSUE BIO‐ENGINEERING
Regenerative medicine for clinical translation of stem cell technology into cellular therapeutics requires development of three‐dimensional tissue bio‐engineering systems integrating adequate cell sources, bio‐compatible matrices, growth factors and physical/mechanical forces. A major limitation to robust tissue bio‐engineering development is access to sufficient numbers of homogeneous immature primary cell groups. CBEs were therefore tested for three‐dimensional tissue bio‐engineering potential towards human hepatic progenitors. The cells were dissociated from two‐dimensional microflasks and were seeded into microgravity‐simulating Rotating Cell Culture Systems (RCCS, Synthecon Inc., Houston, TX, USA –Fig. 5) in Iscove's modified Dulbecco's medium, supplemented with human hepatocyte growth factor, basic‐fibroblast growth factor, epidermal growth factor and c‐kit ligand to induce hepatic differentiation (1, 5). Synthecon RCCS microbioreactors are based on NASA‐derived high aspect ratio vessel technology and have unique properties to model microgravity for tissue bio‐engineering, by enabling (i) reduced shear stress with balanced gravitational, centrifugal and Coriolis forces; (ii) adjustable rotation speed that minimizes circular orbit deviations; and (iii) optimized transport of nutrients and oxygen (convective bulk flow/passive diffusion).
Figure 5.

Synthecon microbioreactor for sustained three‐dimensional generation of tissues, such as hepatic tissue illustrated in Fig. 1.
The CBEs were stimulated by hepatic differentiating cues in the RCCS for 4 weeks. RCCS maintained cell growth and caused cellular aggregation as reflected by a decrease in single cell number in the microbioreactor system. The resulting three‐dimensional constructs were positive for functional hepatic progenitor cell markers including cytokeratin 18, α‐foetoprotein and albumin (Fig. 1). The NASA‐derived microgravity simulating technology confirmed its suitability when applying stem cells to three‐dimensional tissue production. CBE‐derived hepatic progenitors (endodermal origin) are currently being further tested for functionality and clinical application (transplantation and/or drug discovery biosensor). Furthermore, although we could derive tissue from all three germ layers in a two‐dimensional culture environment, CBEs’ ability to differentiate into ectodermal and mesodermal three‐dimensional tissue constructs also continues to be investigated (Fig. 1) (Forraz et al. 2002b, 2004; McGuckin et al. 2004b, 2005).
REWRITING THE STEM CELL RULES
Our isolation of CBEs may provide an explanation for the presence of multipotent stem cells in adult tissues and go towards overcoming the debate on transdifferentiation and plasticity (Quensenberry et al. 2005). Our findings strongly support the existence of circulating embryonic stem cell‐like somatic ancestors during foetal development that are maintained at birth and relocate to adult tissues including to the BM (Jiang et al. 2002; Korbling & Estrov 2003; McGuckin et al. 2005). Postnatal embryonic stem‐like cells might subsequently, in adult life, be able to form the unique reservoirs of adult stem cells whose properties have been reported extensively in the literature (Nowlin 2005; Prindull 2005; Quesenberry et al. 2005). Our CBEs provide a viable alternative to the use of human ESC sources in terms of ethical controversy and convenient accessibility, not least for research and understanding of stem cells. Even when national laws permit the use of human ESC lines, they have a poor success rate technically. However, several thousands of UCB units have already been banked and immunophenotyped, showing the potential of CBE cell production for stem cell researchers to refine their techniques prior to the use of any stem cell population.
Our advancements in stem cell biology propose a new possibility, and by their very nature, UCB‐derived primitive stem cells with ESC characteristics, may provide the first real publicly acceptable stem cell source. Should we wait 50 years for ESCs to be ready for patients, when UCB and BM stem cell sources can provide help to our patients in the short term? The debate on religion and ethics must be held, but it must also refer to the science and must not allow hype and controversy – and more particularly the media – to hijack the debate. If it does, patients will suffer. Stem cell medicine must not be a race.
Both authors declare no conflicts of interest.
REFERENCES
- Atala A (2005) Tissue engineering, stem cells and cloning: current concepts and changing trends. Expert Opin. Biol. Ther. 5, 879–892. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Nagler A (2004) Umbilical cord blood transplantation – how, when and for whom? Blood Rev. 18, 167–179. [DOI] [PubMed] [Google Scholar]
- Ende N, Lu S, Alcid MG, Chen R, Mack R (2001) Pooled umbilical cord blood as a possible universal donor for marrow reconstitution and use in nuclear accidents. Life Sci. 69, 1531–1539. [DOI] [PubMed] [Google Scholar]
- Forraz N, Pettengell R, Deglesne PA, McGuckin CP (2002a) AC133+ umbilical cord blood progenitors demonstrate rapid self‐renewal and low apoptosis. Br. J. Haematol. 119, 516–524. [DOI] [PubMed] [Google Scholar]
- Forraz N, Pettengell R, McGuckin CP (2002b) Haemopoietic and neuroglial progenitors are promoted during cord blood ex vivo expansion. Br. J. Haematol. 119, 888. [DOI] [PubMed] [Google Scholar]
- Forraz N, Pettengell R, McGuckin CP (2004) Characterization of a lineage‐negative stem‐progenitor cell population optimized for ex vivo expansion and enriched for LTC‐IC. Stem Cells 22, 100–108. [DOI] [PubMed] [Google Scholar]
- Forraz N, San‐Choi B, Pettengell R, Morgan R, McGuckin CP (2003) Characterization of a lineage‐negative stem‐progenitor cell population optimized for ex vivo expansion and enriched for LTC‐IC. Am. Soc. Cell Biol. Annu. Meeting, San Francisco, USA 1, 333–333. [DOI] [PubMed] [Google Scholar]
- Gerrard L, Zhao D, Clark AJ, Cui W (2005) Stably transfected human embryonic stem cell clones express OCT4‐specific green fluorescent protein and maintain self‐renewal and pluripotency. Stem Cells 23, 124–133. [DOI] [PubMed] [Google Scholar]
- Gilmore GL, Depasquale DK, Lister J, Shadduck RK (2000) Ex vivo expansion of human umbilical cord blood and peripheral blood CD34(+) hematopoietic stem cells. Exp. Hematol. 28, 1297–1305. [DOI] [PubMed] [Google Scholar]
- Heissig B, Ohki Y, Sato Y, Rafii S, Werb Z, Hattori K (2005) A role for niches in hematopoietic cell development. Hematology 10, 247–253. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Carpenter MK (2005) Characterization and culture of human embryonic stem cells. Nat. Biotechnol. 23, 699–708. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM (2002) Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp. Hematol. 30, 896–904. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R (2005) Human embryonic stem cells derived without feeder cells. Lancet 365, 1636–1641. [DOI] [PubMed] [Google Scholar]
- Kofidis T, Debruin JL, Tanaka M, Zwierzchoniewska M, Weissman I, Fedoseyeva E, Haverich A, Robbins RC (2005) They are not stealthy in the heart: embryonic stem cells trigger cell infiltration, humoral and T‐lymphocyte‐based host immune response. Eur. J. Cardiothoracic Surg. 28, 461–466. [DOI] [PubMed] [Google Scholar]
- Korbling M, Estrov Z (2003) Adult stem cells for tissue repair – a new therapeutic concept? N. Engl. J. Med. 349, 570–582. [DOI] [PubMed] [Google Scholar]
- Krtolica A (2005) Stem cell: balancing aging and cancer. Int. J. Biochem. Cell Biol. 37, 935–941. [DOI] [PubMed] [Google Scholar]
- Landry DW, Zucker HA (2004) Embryonic death and the creation of human embryonic stem cells. J. Clin. Invest. 114, 1184–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie T (2005) Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21, 605–631. [DOI] [PubMed] [Google Scholar]
- Liu E, Law HK, Lau YL (2004) Tolerance associated with cord blood transplantation may depend on the state of host dendritic cells. Br. J. Haematol. 126, 517–526. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Muotri A, Gage F, Varki A (2005) Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 11, 228–232. [DOI] [PubMed] [Google Scholar]
- Matin MM, Walsh JR, Gokhale PJ, Draper JS, Bahrami AR, Morton I, Moore HD, Andrews PW (2004) Specific knockdown of Oct4 and beta2‐microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells 22, 659–668. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Pearce D, Forraz N, Tooze JA, Watt SM, Pettengell R (2003a) Multiparametric analysis of immature cell populations in umbilical cord blood and bone marrow. Eur. J. Haematol. 71, 341–350. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Baradez MO, Lojo‐Rial C, Wertheim D, Whiting K, Watt SM, Pettengell R (2003b) Colocalization analysis of sialomucins CD34 and CD164. Stem Cells 21, 162–170. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Liu WM (2003c) diaminofluorene stain detects erythroid differentiation in immature haemopoietic cells treated with EPO, IL‐3, SCF, TGFbeta1, MIP‐1alpha and IFNgamma. Eur. J. Haematol. 70, 106–114. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Allouard Q, Pettengell R (2004a) Umbilical cord blood stem cells can expand hematopoietic and neuroglial progenitors in vitro . Exp. Cell Res. 295, 350–359. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Pettengell R, Thompson A (2004b) Thrombopoietin, flt3‐Ligand and c‐Kit‐Ligand modulate HOX gene expression in expanding cord blood CD133 cells. Cell Prolif. 37, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, Tilton R, Denner L (2005) Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Proliferation 38, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlin A (2005) The promise of stem cells. RN 68, 48–52; quiz 53. [PubMed] [Google Scholar]
- Perin EC, Silva GV (2004) Stem cell therapy for cardiac diseases. Curr. Opin. Hematol. 11, 399–403. [DOI] [PubMed] [Google Scholar]
- Prindull G (2005) Hypothesis: cell plasticity, linking embryonal stem cells to adult stem cell reservoirs and metastatic cancer cells? Exp. Hematol. 33, 738–746. [DOI] [PubMed] [Google Scholar]
- Querol S, Capmany G, Azqueta C, Gabarro M, Fornas O, Martin‐Henao GA, Garcia J (2000) Direct immunomagnetic method for CD34+ cell selection from cryopreserved cord blood grafts for ex vivo expansion protocols. Transfusion 40, 625–631. [DOI] [PubMed] [Google Scholar]
- Quesenberry PJ, Dooner G, Colvin G, Abedi M (2005) Stem cell biology and the plasticity polemic. Exp. Hematol. 33, 389–394. [DOI] [PubMed] [Google Scholar]
- Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, Gluckman E (2000) Graft‐versus‐host disease in children who have received a cord‐blood or bone marrow transplant from an HLA‐identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N. Engl. J. Med. 342, 1846–1854. [DOI] [PubMed] [Google Scholar]
- Rubinstein P, Adamson JW, Stevens C (1999) The placental/umbilical cord blood program of the new york blood center. A progress report. Ann. NY Acad. Sci. 872, 328–334; discussion 334–335. [DOI] [PubMed] [Google Scholar]
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schumichen C, Nienaber CA, Freund M, Steinhoff G (2003) Autologous bone‐marrow stem‐cell transplantation for myocardial regeneration. Lancet 361, 45–46. [DOI] [PubMed] [Google Scholar]
- Stojkovic M. Lako M. Strachan T, Murdoch A (2004) Derivation, growth and applications of human embryonic stem cells. Reproduction (Cambridge, England) 128, 259–267. [DOI] [PubMed] [Google Scholar]
- Tajima A, Tanaka T, Ebata T, Takeda K, Kawasaki A, Kelly JM, Darcy PK, Vance RE, Raulet DH, Kinoshita K, Okumura K, Smyth MJ, Yagita H (2003) Blastocyst MHC, a putative murine homologue of HLA‐G, protects TAP‐deficient tumor cells from natural killer cell‐mediated rejection in vivo . J. Immunol. 171, 1715–1721. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Wang L, Li L, Menendez P, Cerdan C, Bhatia M (2005) Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood 105, 4598–4603. [DOI] [PubMed] [Google Scholar]
- Watt SM, Contreras M (2005) Stem cell medicine: umbilical cord blood and its stem cell potential. Semin. Fetal Neonatal Med. 10, 209–220. [DOI] [PubMed] [Google Scholar]


