Abstract
Objectives: Fractones are extracellular matrix structures that form a niche for neural stem cells and their immediate progeny in the subventricular zone of the lateral ventricle (SVZa), the primary neurogenic zone in the adult brain. We have previously shown that heparan sulphates (HS) associated with fractones bind fibroblast growth factor‐2 (FGF‐2), a powerful mitotic growth factor in the SVZa. Here, our objective was to determine whether the binding of FGF‐2 to fractone‐HS is implicated in the mechanism leading to cell proliferation in the SVZa.
Materials and methods: Heparitinase‐1 was intracerebroventricularly injected with FGF‐2 to N‐desulfate HS proteoglycans and determine whether the loss of HS and of FGF‐2 binding to fractones modifies FGF‐2 effect on cell proliferation. We also examined in vivo the binding of Alexa‐Fluor‐FGF‐2 in relationship with the location of HS immunoreactivity in the SVZa.
Results: Heparatinase‐1 drastically reduced the stimulatory effect of FGF‐2 on cell proliferation in the SVZa. Alexa‐Fluor‐FGF‐2 binding was strictly co‐localized with HS immunoreactivity in fractones and adjacent vascular basement membranes in the SVZa.
Conclusions: Our results demonstrate that FGF‐2 requires HS to stimulate cell proliferation in the SVZa and suggest that HS associated with fractones and vascular basement membranes are responsible for activating FGF‐2. Therefore, fractones and vascular basement membranes may function as a HS niche to drive cell proliferation in the adult neurogenic zone.
Introduction
Fibroblast growth factors (FGFs) compose a family of 24 growth factors that are key players in processes of cell proliferation and differentiation, in a wide variety of tissues and organs, during development and adulthood 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. In the adult and developing brain, FGF‐2 is a stimulator of neural stem and progenitor cell proliferation and differentiation 3, 5, 8, 9. Although not demonstrated in the adult brain, FGF‐2 is known as a heparin‐binding protein. This implies that FGF‐2 requires interactions with heparan sulphate proteoglycans (HSPG) in the extracellular matrix (ECM) near the cell surface of target cells to recognize and activate FGF receptors and ultimately exert its biological activity 1, 2, 3, 4, 5, 6, 7. In addition, binding of growth factors to HSPG is known to protect the growth factors from enzymatic degradation, and provides a mechanism for growth factor storage in the ECM 11. The question arises as to how FGF‐2 and other growth factors, with similar or antagonist effects, regulate neural stem and progenitor cell proliferation in the neurogenic niches. Does HSPG promote FGF‐2 and other growth factors in the adult neurogenic zones? We have recently demonstrated that N‐sulphated HSPGs are highly expressed along the neurogenic axis of the adult brain 12. We found that an N‐sulphated HSPG‐immunoreactive continuum forms a niche (favourable environment) for cell proliferation, through the germinal zones of the adult brain. These germinal zones comprise the subventricular zone (SVZ) of the lateral ventricle, primary site for adult neurogenesis 13, 14, the rostral migratory stream, the olfactory bulbs and the sub‐callosum zone, which all have also been recognized as specific sites for production of new neurons and glia in adulthood 15, 16. The N‐sulphated HSPG niche consists of ECM structure fractones, which we have previously characterized in the SVZ 17, 18, and of vascular basement membranes 12, 19. Fractones are small fragments of dense ECM material that are directly visible (without labelling) by transmission electron microscopy 17, 18. Along the SVZ of the lateral ventricle, multiple processes of neural stem cells and neural progenitor cells, converge toward each individual fractone 17. Fractones contain HSPG, collagen‐IV, nidogen and laminin 17, 20 and thus chemically resemble basement membranes 21. However, fractones have punctate morphology when observed by light microscopy and immunolabelling for its ECM components 12, 17, 18, 20, but exhibit fractal (branched) morphology when resolved using transmission electron microscopy 17, 18.
Based on our previous findings that biotinylated‐FGF‐2 in vitro binds N‐sulphated HSPG, present in fractones and blood vessels of the lateral ventricle walls 20, we hypothesize here that FGF‐2 functions as a heparin‐binding molecule for regulation of cell proliferation, in this major neural stem cell niche, and that structures responsible for heparin‐binding dependence are fractones and nearby vascular basement membranes. To determine whether stimulation of cell proliferation by FGF‐2 in the neural stem cell niche is extracellularly mediated by HSPG, here we compare effects of FGF‐2 alone with those of FGF‐2, after desulphation in vivo by heparitinase‐1 on cell proliferation in the SVZ of the lateral ventricle. As controls for effects of heparitinase‐1 in vivo, we examine outcome on cell proliferation, of injecting heparitinase‐1 only on disappearance of N‐sulphated HSPG immunoreactivity (N‐sulphated HSPG+) after heparitinase‐1 injection in vivo, and potential recovery of N‐sulphated HSPG+ after longer post‐injection periods. To further examine binding properties of FGF‐2, we also compare location of binding of biotinylate‐FGF‐2 and Alexa‐Fluor488‐FGF‐2, in vivo with N‐sulphated HSPG+.
Materials and methods
Animals and schedules of intracerebroventricular injection
Eight to 12‐week old male and female Balb/c mice (n = 42) were anesthetized with Ketamine/Xylazine mix (ratio 80/20) at 50 mg/kg of body weight. Two successive intracerebroventricular (ICV) injections (lasting 15 min each) were performed unilaterally at bregma +0.5 mm, +0.6 mm lateral to the midline, 2 mm depth with 1 μl of heparitinase‐1 (32 mU/injection, from Flavobacterium heparinum, Amsbio, Abingdon, UK), and/or 1 μl of FGF‐2 (0.5 μg per injection, human recombinant lo‐FGF‐2 17.3 kD produced by E. coli, InVitrogen, Carlsbad, CA, USA) diluted in artificial cerebrospinal fluid (CSF; Harvard Apparatus, Holliston, MA, USA) using micro‐4 microsyringe controller (WPI, Saratosa, FL, USA) connected to stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). ICV injections were performed sequentially on days 1 and 3 with either CSF + CSF (termed CSF condition) or CSF + FGF‐2 (FGF‐2 condition) or heparatinase‐1 + FGF‐2 (Hep + FGF‐2 condition) or heparitinase‐1 + CSF (Hep condition). A single intraperitoneal injection of bromodeoxyuridine (BrdU, 50 mg/kg of body weight; Sigma, St. Louis, MO, USA) was performed 6 h prior to animal termination, on day 5, to assess cell proliferation (pre‐mitotic cells). Brains were dissected and frozen in isopentane at −80 °C and stored at −20 °C without fixation. Animal experimental protocols followed NIH guidelines and was approved by the IACUC at the University of Hawaii.
In vivo binding of FGF‐2
FGF‐2 was either biotinylated using EZ‐link Micro‐sulfo‐NHS Biotinylation kit (21425; Pierce, Rockford, IL, USA) or directly linked to AlexaFluor488 (A30006; InVitrogen). After ICV injection, the binding of biotinylated‐FGF‐2 was revealed on frozen sections of brains, with streptavidin‐Texas red. When ICV injections of biotin/AlexaFluor488 only, or artificial CSF only, were carried out, no streptavidin‐Texas red or AlexaFluor488 signal was detected in the brains (data not shown).
Immunohistochemistry
Immunohistochemistry (IHC) was performed as previously described, on serial frozen sections generated using a Leica CM1900 cryostat (Leica Microsystems, Buffalo Grove, IL, USA), and was fixed in acetone at −20 °C just prior to IHC procedure 22. Fractones, blood vessels and choroid plexi were immunolabelled with anti‐laminin antibodies (1/1000, L9393; Sigma) 13, 15 or anti‐N‐sulphated glycosamine (10E4 antibody, 1/500; Seikagaku) 20, 23, then visualized with AlexaFluor‐647 goat anti‐rabbit antibody (1/400), AlexaFluor‐488 goat anti‐mouse IgM (1/400, A21042; InVitrogen) or Cy‐3 donkey anti‐mouse IgM (1/400, 715‐165‐140; Jackson Laboratories, Bar Harbor, ME, USA). For isotype control of 10E4, we immunolabelled frozen sections containing SVZa with IgM anti‐chondroitin sulphate CS56 (Abcam, Cambridge, MA, USA) (IgM). Visualization of chondroitin sulphates with CS56 yielded labelling very distinct from 10E4 labelling in the SVZa (Fig. 3e). Pre‐mitotic cells (S‐phase) were labelled with anti‐BrdU antibodies (1/500, OBT0030; Oxford Biotechnology, UK) as previously described 16, 20 and visualized with AlexaFluor‐488 goat anti‐rat antibody (1/400, A11006; InVitrogen) or AlexaFluor‐546 goat anti‐rat (1/400, A11081; InVitrogen). Results were recorded using a Zeiss confocal laser scanning microscope and images were processed using Adobe Photoshop CS3 (Adobe Systems, Mountain view, CA, USA). Adjustments for brightness and contrast were minimal. Control experiments for immunolabelling specificity were performed as previously described 17, 20, 22.
Figure 3.
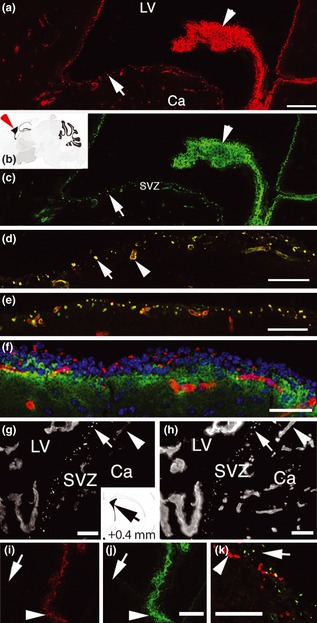
FGF‐2 binding in vivo matched location of fractone‐HSPG+. (a) Binding of biotinylated‐FGF‐2 visualized by streptavidin‐Texas red 22 h after ICV injection. Binding of biotinylated‐FGF‐2 was concentrated in the SVZa (arrow) and choroid plexus (CP) (arrowhead). Ca: caudate nucleus. (b) Location of images a‐f. (c) Immunolabelling for N‐sulphated‐HSPG (10E4, green) in the same section showing distribution pattern identical to binding of biotinylated‐FGF‐2. Numerous fractones, visualized as 10E4+ puncta (arrow in b) have concentrated biotinylated‐FGF‐2 (arrow in a). (d) High power field of the SVZa (indicated by an arrow in a) displaying biotinylated‐FGF‐2 binding and 10E4+ simultaneously. Yellow colouration indicates that the vast majority of 10E4+ fractones (arrow) and 10E4+ blood vessels, visualized as truncated cylinders (arrowhead), have bound FGF‐2. (e) Double immunolabelling for laminin (red) and N‐sulphated HSPG (green). (f) Isotype control for 10E4, showing IgM antibody (CS56) directed against chondroitin sulphates (green). The same secondary antibody (Alexa‐Fuor 488 goat anti‐mouse IgM) was used to reveal chondroitin sulphates (in e) and 10E4 (in b–d). IgM directed against chondroitin sulphates does not recognize fractones and blood vessels, which here are visualized by laminin+ (red). (g) Binding of AlexaFluor‐488‐FGF‐2 visualized 24 h after ICV injection. LV: lateral ventricle. (h) Laminin+ in the same section showing that Alexa‐Fluor488‐FGF‐2 binding matches location of laminin+ in fractones (arrow), CP (double arrowheads) and blood vessels (arrowheads) in the SVZa. Blood vessels located beyond the SVZa do not bind FGF‐2 (double arrows). (i) Loss of biotinylated‐FGF‐2 binding (red) in the SVZa 22 h after injection of heparitinase‐1 (arrow). (j) N‐sulphated‐HS+ (green). Biotinylated FGF‐2 binding and N‐sulphated HS+ remained in the CP stroma, suggesting lack of access of heparitinase‐1 to the CP, or rapid recovery of N‐sulphated‐HSPG+ and FGF‐2 binding in the CP (arrowhead). (k) Recovery of N‐sulphated‐HSPG+ (green) in the SVZa 48 h after heparitinase‐1 injection. Presence of 10E4+ puncta indicates that fractones have recovered heparan sulphates. Red colouration shows typical BrdU+ cell proliferation in the SVZa. Scale bars: 100 μm.
Cell counting and statistical methods
BrdU+ cells were counted in the anterior SVZ (SVZa) of the lateral ventricle, a major proliferation zone in the adult brain, in series of whole‐brain 25‐μm thick coronal sections, using a 20× Plan Apo objective and a DMIL Leica immunofluorescence microscope. BrdU+ cell counts were reported per lateral ventricle wall by increments of 0.1 mm, from 1.2 to 0.5 mm anterior to bregma. To compensate for over‐counting, observed numbers of BrdU+ cells were subjected to corrections given by Abercrombie's formula 24. Corrected numbers of BrdU+ cells were submitted to statistical analysis with XLStat. Means and respective standard deviations were reported per SVZa for each condition. One‐way ANOVA (a = 0.01), Tukey's and two‐sided Dunnett's tests were employed to compare each condition to the control (CSF condition) and REQWQ's procedure for multiple comparison tests were performed to evaluate significance of differences observed. Confidence interval of 99% was applied and a P‐value inferior to 0.001 was obtained for all comparisons except when comparing CSF and Hep conditions (P > 0.001). All statistical models applied had significant difference between 2 conditions.
Results
Upregulation of cell proliferation by FGF‐2 along the rostro‐caudal axis of the anterior SVZ
We first examined effects of FGF‐2 on cell proliferation along the rostro‐caudal axis of the anterior SVZ (SVZa). Figure 1a shows typical cell proliferation at 1.1 mm anterior to bregma (bregma +1.1 mm) after CSF injection (control). At this location, the lateral ventricle is collapsed (arrows). At bregma +1.1, mitogenesis was higher by a factor 5 in the SVZa, after injection of FGF‐2 (Fig. 1b arrowheads, 1c) in comparison to controls, that is injection with artificial CSF. Along the rostro‐caudal axis, the elevation was between 2 and 5‐fold (Fig. 1c) with average factor of 3.3 when merging results from bregma +1.2 to +0.5 mm (Fig. 2b).
Figure 1.
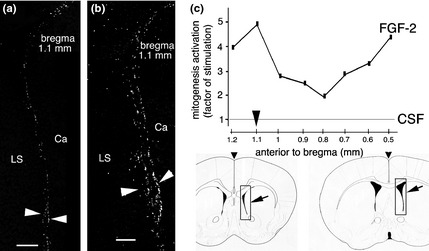
Upregulation of cell proliferation by FGF‐2 along the rostro‐caudal axis of the SVZa of the lateral ventricle (LV). (a) Cell proliferation was assessed by BrdU immunoreactivity in the SVZa of the LV after ICV injection of CSF (control condition). Proliferating cells (BrdU+ puncta) are located along collapsed walls of the LV (arrowhead). Ca: caudate nucleus; LS: lateral septal nucleus. (b) Increased cell proliferation after ICV injection of FGF‐2. Numerous BrdU+ puncta are found along the walls of the LV (arrowhead). (c) Graph showing upregulation of cell proliferation along the rostro‐caudal axis of the SVZa. Numbers in vertical axis indicate factors by which cell proliferation is increased after FGF‐2 injection (compared to cell proliferation after CSF injection, control). Data are reported at different locations along the SVZa, from +1.2 to +0.5 mm anterior to the bregma line. Schematic representations of the brain indicate locations where BrdU+ cells were counted (frames shown by arrows). Images (a) and (b) correspond to frames at +1.1 mm anterior to bregma. Scale bars: 100 μm.
Figure 2.
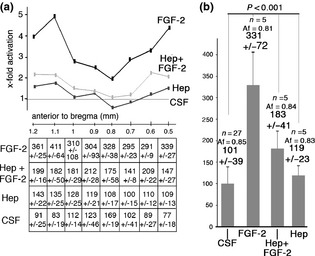
Upregulation of cell proliferation by FGF‐2 requires heparan sulphates. (a) Co‐injection of heparitinase‐1 and FGF‐2 (Hep + FGF‐2) strongly inhibited stimulatory effects of FGF‐2 on cell proliferation in the SVZa. Graph indicates the factor by which cell proliferation was increased along the rostro‐caudal axis of the SVZa. Results obtained after injection of heparitinase‐1 only, are also reported in the graph. All factors were normalized to values (number of BrdU+ cells) obtained after injection of CSF (control). Bottom panel shows mean values and standard deviations for all sections from 1.2 to 0.5 mm anterior to the bregma. (b) Graph showing BrdU+ cell average values for the entire zone from +1.2 to +0.5 mm anterior to the bregma. FGF‐2 upregulated cell proliferation by a factor 3.1. Co‐injection of heparitinase‐1 reduced the effect of FGF‐2 to a factor 1.8.
Upregulation of cell proliferation by FGF‐2 requires N‐sulphated HSPG
To determine whether the mitogenic effect of FGF‐2 was heparan sulphate‐dependant, we compared effects of FGF‐2 on cell proliferation in the SVZa after successive injections of CSF and FGF‐2, and of heparitinase‐1 and FGF‐2 [heparitinase‐1 cuts N‐sulphated chains of HSPG 25, 26]. In control experiments, we examined effects of injection of heparitinase‐1 only on cell proliferation in the SVZa. Heparitinase‐1 alone did not markedly increase the level of cell proliferation in the SVZa, compared to injection of CSF alone (Fig. 2b). When heparitinase‐1 was injected prior to FGF‐2, the mitogenic effect of FGF‐2 was drastically lower throughout the SVZa (Fig. 2a). Merging data from +1.2 to +0.5 mm to bregma, the level of cell proliferation in the SVZa increased by a factor 1.7 after heparitinase‐1+ FGF‐2, compared to a factor of 3.3 when FGF‐2 only was injected (Fig. 2b). These results demonstrate that heparan sulphates promote the mitogenic effect of FGF‐2 in the SVZa.
Binding of FGF‐2 in vivo co‐localizes with N‐sulphated HSPG immunoreactivity
Previously, we have demonstrated that biotinylated‐FGF‐2 binding in vitro in frozen brain sections co‐localizes with N‐sulphated HSPG, and that binding of biotinylated‐FGF‐2 in vivo coincides with laminin+ puncta or blood vessels, in the SVZa 20. However here, we did not demonstrate any correlation between FGF‐2 binding in vivo and N‐sulphated HSPG immunoreactivity. FGF‐2 was biotinylated or directly coupled to AlexaFluor‐488, then ICV injected in adult mice. Twenty‐two hours after injection, FGF‐2 binding sites were observed after incubation of sections with streptavidin‐Texas red (Fig. 3a), or were directly observed on the 488 nm channel by fluorescence microscopy (Fig. 3g). To determine whether binding loci of FGF‐2 in vivo would coincide with those of N‐sulphated‐HSPG, brain sections originating from animals that were injected with biotinylated FGF‐2 were immunolabelled with 10E4 antibody, raised against N‐sulphated HSPG. Binding sites of biotinylated‐FGF‐2 and of AlexaFluor‐488‐FGF‐2 almost perfectly matched patterns of immunoreactivity for N‐sulphated HSPG (Fig. 3a–d, 3g and 3h) as indicated by yellow colouration, overlap of 10E4 labelling (green) and FGF‐2 binding (red) (Fig. 3d). In the SVZa, most FGF‐2/N‐sulphated‐HSPG+ co‐labelling was found in fractones, which typically appeared as small puncta <5 μm in diameter 13, 16, although blood vessel walls (which are larger), and as truncated cylindrical structures, were also clearly visualized (Fig. 3d, arrowhead). Structural and ultrastructural differences between fractones and blood vessels has demonstrated in our previous studies 17, 18.
FGF‐2 binding was not found in the SVZa after heparitinase ‐1 treatment (dose equal to that used for investigating effects of heparitinase‐1 on cell proliferation) (Fig. 3i, arrow). N‐sulphated HSPG+ also disappeared from the SVZa after heparitinase‐1 injection (Fig. 3j), although N‐sulphated HSPG+ remained in the choroid plexus (Fig. 3j, arrowhead). This demonstrates that FGF‐2 binding in the SVZa required N‐sulphated‐HSPG. This also indicates that FGF‐2 was unable to bind in the SVZa with experimental conditions used to investigate heparitinase‐1 effects on cell proliferation. Thus, our results support the view that when FGF‐2 cannot bind fractones and SVZa blood vessels, it cannot stimulate cell proliferation in the SVZa.
To further validate specificity of FGF‐2 binding in the SVZa, we injected biotinylated‐FGF‐2 into the dorsal cortex, instead of into the lateral ventricle. Biotinylated‐FGF‐2 was captured by N‐sulphated‐HSPG+ fractones and blood vessels in the SVZa (Fig. 4f), demonstrating that FGF‐2 binding sites were independent of injection site in the brain. We also show that biotinylated FGF‐2 was captured by N‐sulphated‐HSPG+ fractones and blood vessels in the SVZ of the third ventricle after injection into the lateral ventricle (Fig. 4c). Together, these experiments demonstrate that capture of biotinylated‐FGF‐2 by fractones and vascular basement membranes of the SVZ was independent of injection site and was not restricted to the SVZa.
Figure 4.
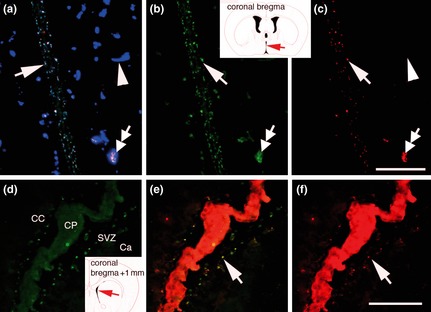
FGF‐2 capture by fractones and basement membranes was independent of injection site. (a–c) Binding FGF‐2 visualized by streptavidin Texas red in SVZ of the third ventricle, 22 h after ICV injection. FGF‐2 binding (red) and immunolabelling for laminin (blue), 10E4 (green) and are all displayed in image (a). Image (b) shows 10E4 only and image (c) shows FGF‐2 binding only. Due to construction of the third ventricle, fractones appear as two parallel lines (arrows). Numerous fractones (arrow in b) captured and concentrated biotinylated FGF‐2 (arrow in c). Specific blood vessels of the SVZ captured FGF‐2 (double arrow), most blood vessels did not (arrowheads). Inset indicates location of images. (d–f) 10E4 immunolabel (green, d) and binding of biotinylated FGF‐2 (red, f) displayed together in E, 22 h after injection in the dorsal cortex. Fractones of the lateral ventricle SVZ (arrow in E) wall concentrated FGF‐2 (arrow in f). Note that the choroid plexus also concentrated FGF‐2. Inset in (d) indicates the location of images. Scale bars: 100 μm.
To investigate toxicity induced by heparitinase‐1 treatment, we examined dynamic of recovery of N‐sulphated HSPG+ after heparitinase‐1 injection. Figure 3f shows that N‐sulphated HSPG+ had fully recovered 48 h after heparitinase‐1 injection. Thus, our experimental conditions were appropriate for examining effects of heparitinase‐1 on cell proliferation in the SVZa.
Discussion
FGF‐2 required heparan sulphates to stimulate cell proliferation in the SVZa
In this study, we demonstrated in vivo that stimulation of cell proliferation induced by FGF‐2 in the SVZa required heparin sulphates. This is the first demonstration that heparan sulphates mediate FGF‐2‐induced mitosis in the neural stem‐cell niche of the adult brain. We have recently shown that inhibition of cell proliferation by BMP‐7 in the SVZa neurogenic zone also requires heparan sulphates 27. This supports the view that heparan sulphates play a significant role in regulation of cell proliferation in the adult brain.
Loss of FGF‐2 effect on cell proliferation was associated with loss of heparan sulphates in fractones and SVZ vascular basement membranes
We have previously demonstrated that FGF‐2 primarily binds to SVZa fractones and blood vessels, and that a mix of heparitinase‐/heparitinase‐3 prevents this binding 20. Here, we used heparitinase‐1 to cut N‐sulphated HSPG specifically. We found that both biotinylated‐FGF‐2 and AlexaFluor‐488‐FGF‐2 bound in vivo to sites that match N‐sulphated HSPG+ fractones and blood vessel basement membranes, of the SVZa. Interestingly, most vascular basement membranes (besides those of the SVZa) were neither NH‐HS+ nor bound FGF‐2. Therefore, location of N‐sulphated HSPG may reflect a specific niche for FGF‐2 binding and activation. After in vivo heparitinase‐1 desulphation, both FGF‐2 binding to fractones/vascular basement membranes and FGF‐2‐mediated stimulation of cell proliferation were lost. Together, these results strongly suggest that FGF‐2 must bind N‐sulphated HSPG located into fractones and the SVZa vasculature, to stimulate cell proliferation.
Heparan sulphates act as captors and activators of growth factors, cytokines and chemokines
The importance of heparan sulphates as ECM receptors that bind and potentiate growth factors has previously been emphasized. HSPG have been implicated as crucial co‐receptors for interaction with a large variety of ligands including growth factors FGF‐1, FGF‐2 and FGF‐4 1, 2, 3, 4, 28, 29, 30, as well as other members of the FGF family, VEGF (vascular endothelial growth factor) 31, HB‐GAM (heparin‐binding growth‐associated molecule) 32, BMP (bone morphogenic protein) 27, 33, SCF (stem cell factor) 34, HGF (hepatocyte growth factor) 35, amphiregulin 36, transforming growth beta‐1 (TGFβ‐1) 37, cytokines such as granulocyte‐macrophage colony stimulating growth factor (GM‐CSF), 38, 39 (G‐CSF), IL (interleukins) 40 and IFN (interferons) 41, CC chemokines 42 and CXC chemokines 43, and diverse morphogens such as Shh (sonic hedgehog) 44 and Noggin 45. All these heparan sulphate‐binding signalling molecules influence neurogenesis in adulthood 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57. However, with the exception of BMP‐7 (our own previous study, 27) heparin‐binding dependence of these growth factors has not been reported in the adult brain.
Experimental validation, binding specificity, diffusion and toxicity of FGF‐2 and heparitinase‐1
In this study, we have demonstrated that binding of biotinylated‐FGF‐2 to fractones and vascular basement membranes in the SVZa is independent of site of injection (Fig. 4). FGF‐2 is known to slowly diffuse through brain tissue, and has slow clearance and low rate of metabolic degradation 58. Thus, biotinylated‐FGF‐2 can diffuse from the cortex to the sub‐cortical structures (on top of penetrating from the ventricles) prior to binding fractones and blood vessels in the SVZa. These characteristics explain why biotinylated‐FGF‐2 bound to fractones and vascular basement membranes of the SVZa 22 h after injection into the cortex (Fig. 4f).
Twenty‐two hours after injection into the cortex or lateral ventricle, biotinylated‐FGF‐2 was also visualized in the stroma (but not significantly in the epithelium) of the choroid plexus (Figs 3a arrowhead, 4f). This was predictable as FGF‐2 is capable of retrograde transport from the ventricle to the choroid plexus stroma, despite the CSF barrier formed by the choroid plexus epithelium 59, 60. Knowing that fibroblasts are ordinary targets for FGF‐2 61, we anticipated that biotinylated‐FGF‐2 would bind to fibroblasts in the stroma of the choroid plexus. In contrast to biotinylated‐FGF‐2, heparitinase‐1 was unable to benefit from retrograde transport through the CSF barrier of the choroids plexus and did not prevent binding of biotinylated‐FGF‐2 to the N‐sulphated‐HSPG in the choroid plexus stroma (Fig. 3i).
It is important to note that low molecular weight (17.3 kD) FGF‐2, also named lo‐FGF‐2, was used in this study. Lo‐FGF‐2 is incapable of causing chromatin compacting and cell death 62, 63. On the contrary, high molecular weight 23kD FGF‐2 (hi‐FGF‐2) has been shown to cause substantial cell death 63. Finally, we presume that heparitinase‐1 had no effect on cell death in our experimental procedures. According to results obtained by literature searching, 10 mU of heparitinase had no effect or reduced cell death (depending on cell layers) on retinal explants in culture 64.
In conclusion, our experiments demonstrate for the first time that N‐sulphated HSPGs are essential for stimulation of cell proliferation by FGF‐2 in the SVZa. Moreover, location of FGF‐2 binding in SVZa fractones and blood vessels, and necessity of this binding to mediate FGF‐2 stimulation of cell proliferation, strongly suggest that fractones and SVZa blood vessels specifically capture FGF‐2 to control the level of cell proliferation in the adult SVZa. High concentration of N‐sulphated HSPG in fractones and vascular basement membranes makes the SVZa unique and particularly suitable for recruiting and dispatching growth factors to neural stem and progenitor cells. We hypothesize a mechanism of FGF‐2 activation by fractone‐heparan sulphates in the context of the anatomy of the SVZa (Fig. 5). As FGF‐2 is produced highly by the choroid plexus epithelium 65, FGF‐2 may enter between ependymocytes via interstitial clefts 66 to finally reach fractones and neural stem cells in the SVZa. Knowing that numerous heparin‐binding growth factors and cytokines are produced by the choroid plexus and released in the ventricles 65, 67, we propose that fractones collect growth factors/cytokines from the ventricles and activate these signalling molecules in the ventricle walls (SVZ) via heparin‐binding controlled mechanisms 68.
Figure 5.
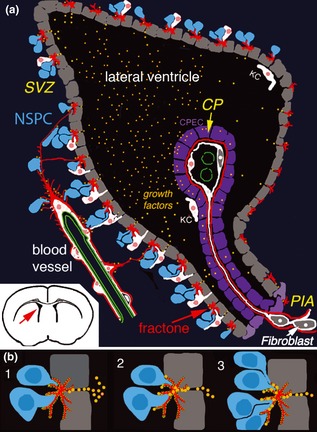
Hypothetical model of growth factor capture and activation in the SVZa neurogenic zone of the adult brain. (a) Overview of organization of the neurogenic zone (SVZ lateral ventricle). Neural stem cell and progenitor cells (NSPC) directly contact fractones just beneath the ependyma (cell layer facing the ventricle, represented in grey). Blood vessel, which also contacts NSPC, completes the neurogenic niche. Growth factors, such as FGF‐2, are secreted by the choroid plexus (CP), enter the interstitial clefts between ependymocytes, are captured by fractones and eventually by blood vessels of the niche. Kolmer cells (KC) and macrophages associated with fractones are represented in white. (b) Mechanism of FGF‐2 capture and activation in SVZ neurogenic zone. 1. FGF‐2 enters interstitial clefts. 2. FGF‐2 is captured by heparan sulphates (small yellow) located at the fractone surface, then is presented to its cognate receptors at the NSPC surface. 3. NSPC that are activated by FGF‐2, proliferate.
Acknowledgements
This work was supported by NIH R21 NS057675 and JSPS S09109. We thank the Histology and Imaging Core supported by RCMI G12‐RR003061 and COBRE P20‐RR016453. We thank Chloe Banel for proofreading this article.
References
- 1. Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DP (1991) Cell surface, heparin‐like molecules are required for binding of fibroblast growth factor to its high affinity receptor. Cell 64, 841–848. [DOI] [PubMed] [Google Scholar]
- 2. Guimond S, Maccarana M, Olwin BB, Lindhal U, Rapraeger AC (1993) Activating and inhibitory heparin sequences for FGF‐2 (basic FGF). Distinct requirements for FGF‐1, FGF‐2, and FGF‐4. J. Biol. Chem. 268, 23906–23914. [PubMed] [Google Scholar]
- 3. Nurcombe V, Ford MD, Wildshut JA, Barlett PF (1993) Developmental regulation of neural response to FGF‐1 and FGF‐2 by heparan sulfate proteoglycan. Science 260, 103–106. [DOI] [PubMed] [Google Scholar]
- 4. Aviezer D, Levy E, Safran M, Svahn C, Buddecke E, Schmidt A et al (1994) Differential structural requirements of heparin and heparan sulfate proteoglycans that promote binding of basic fibroblast growth factor to its receptor. J. Biol. Chem. 269, 114–121. [PubMed] [Google Scholar]
- 5. Brickman YG, Ford MD, Small DH, Bartlett PF, Nurcombe V (1995) Heparan sulfates mediate the binding of basic fibroblast growth factor to a specific receptor on neural precursor cells. J. Biol. Chem. 270, 24941–24948. [DOI] [PubMed] [Google Scholar]
- 6. Gomez‐Pinilla F, Vu L, Cotman CW (1995) Regulation of astrocyte proliferation by FGF‐2 and heparan sulfate in vivo. J. Neurosci. 5, 2021–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krufka A, Guimond S, Rapraeger AC (1996) Two hierarchies of FGF‐2 signaling in heparin: mitogenic stimulation and high‐affinity binding/receptor transphosphorylation. Biochemistry 35, 11131–11141. [DOI] [PubMed] [Google Scholar]
- 8. Kuhn HG, Winkler J, Kemperman G, Thal L, Gage FH (1997) Epidermal growth factor and fibroblast growth factor‐2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 17, 5820–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH (1999) Fibroblast growth factor‐2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 19, 8487–8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez AM, Taylor WM, Johanson CE, King JC, Leadbeater WE, Stopa EG et al (2010) Co‐localization and regulation of basic fibroblast growth factor and arginine vasopressin in neuroendocrine cells of the rat and human brain. Cerebrospinal Fluid Res. 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vlodavsky I, Friedmann Y (2001) Molecular properties and involvement of heparanases in cancer metastasis and angiogenesis. J. Clin. Invest. 108, 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mercier F, Arikawa‐Hirasawa E (2012) Heparan sulfate niche for cell proliferation in the adult brain. Neurosci. Lett. 510, 67–72. [DOI] [PubMed] [Google Scholar]
- 13. Das GD, Altman J (1970) Postnatal neurogenesis in the caudate nucleus an nucleus accumbens septi in the rat. Brain Res. 21, 122–127. [DOI] [PubMed] [Google Scholar]
- 14. Doetsch F, Garcia‐Verdugo JM, Alvarez‐Buylla A (1997) Cellular composition and three‐dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lois C, Alvarez‐Buylla A (1994) Long distance neuronal migration in the adult mammalian brain. Science 265, 1145–1148. [DOI] [PubMed] [Google Scholar]
- 16. Seri B, Herrera DG, Gritti A, Ferron S, Collado L, Vescovi A, Garcia‐Verdugo JM, Alvarez‐Buylla A (2006) Composition and organization of the SCZ: a large germinal layer containing neural stem cells in the adult mammalian brain. Cereb. Cortex 16, 103–111. [DOI] [PubMed] [Google Scholar]
- 17. Mercier F, Kitasako JT, Hatton GI (2002) Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J. Comp. Neurol. 451, 170–188. [DOI] [PubMed] [Google Scholar]
- 18. Mercier F, Kitasako JT, Hatton GI (2003) Fractones and other basal laminae in the hypothalamus. J. Comp. Neurol. 455, 324–340. [DOI] [PubMed] [Google Scholar]
- 19. Mercier F, Hatton GI (2004) Meninges and perivasculature as mediators of CNS plasticity In: Bittar EE, Herz I, eds. Non Neuronal Cells in the Nervous System: Function and Dysfunction. Amsterdam: Elsevier Bioscience; Adv. Mol. Cell. Biol. 31, 215–253. [Google Scholar]
- 20. Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa‐Hirasawa E et al (2007) Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor FGF‐2 from the extracellular milieu. Stem Cells. 25, 2146–2157. [DOI] [PubMed] [Google Scholar]
- 21. Timpl R (1989) Structure and biological activity of basement membrane proteins. Eur. J. Biochem. 180, 487–502. [DOI] [PubMed] [Google Scholar]
- 22. Mercier F, Hatton GI (2001) Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: role sin stem cell proliferation and morphological plasticity? J. Comp. Neurol. 431, 88–104. [DOI] [PubMed] [Google Scholar]
- 23. David G, Mei Bai X, Van der Schueren B, Cassiman JJ, Van der Berghe H (1992) Developmental changes in heparan sulfate expression: in situ detection with mAbs. J. Cell Biol. 119, 961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abercrombie M, Johnson ML (1946) Quantitative histology of Wallerian degeneration. I. Nuclear population in rabbit sciatic nerve. J. Anat. Lond. 80, 37–50. [PubMed] [Google Scholar]
- 25. Nader HB, Porcionatto MA, Tersariol ILS, Pinhal MAS, Oliveira FW, Moraes CT et al (1990) Purification and substrate specificity of heparitinase‐I and heparitinase‐II from Flavobacterium heparinum . J. Biol. Chem. 265, 16807–16813. [PubMed] [Google Scholar]
- 26. Raman K, Kuberan B (2010) Differential effects of heparitinase‐I and heparitinase‐III on endothelial tube formation in vitro. Biochem. Biophys. Res. Commun. 398, 191–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Douet V, Arikawa‐Hirasawa E, Mercier F (2012) Fractone‐heparan sulfates mediate BMP‐7‐inhibition of cell proliferation in the adult subventricular zone. Neurosci Lett. 528, 120–125. [DOI] [PubMed] [Google Scholar]
- 28. Bellosta P (2001) Identification of receptor and binding sites in fibroblast growth factor 4 by structure‐based mutagenesis. Mol. Cell. Biol. 21, 5946–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J, David G (1992) Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J. Biol. Chem. 267, 20435–20443. [PubMed] [Google Scholar]
- 30. Vlodavsky I, Folkman J, Sullivan R, Fridman R, Ishai‐Michaeli R, Sasse J et al (1987) Endothelial cell‐derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc. Nat. Acad. Sci. U.S.A. 84, 2292–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keck RG, Berleau L, Harris R, Keyt BA (1997) Disulfide structure of the heparan binding domain in vascular endothelial growth factor: characterization of post‐translational modifications in VEGF. Arch. Biochem. Biophys. 344, 103–113. [DOI] [PubMed] [Google Scholar]
- 32. Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H (1994) Isolation of a neuronal cell surface receptor of heparin binding growth‐associated molecule (HB‐GAM). Identification as N‐syndecan (syndecan‐3). J. Biol. Chem. 269, 12999–13004. [PubMed] [Google Scholar]
- 33. Khan SA, Nelson MS, Pan C, Gaffney PM, Gupta P (2008) Endogenous heparan sulfate and heparin modulate bone morphogenetic protein‐4 signaling and activity. Am. J. Physiol. Cell Physiol. 294, 1387–1397. [DOI] [PubMed] [Google Scholar]
- 34. Kishimoto S, Nakamura S, Oonuma F, Kanatani Y, Tanaka Y, Mori Y et al (2009) Human stem cell factor (SCF) is a heparin‐binding cytokine. J. Biochem. 145, 275–278. [DOI] [PubMed] [Google Scholar]
- 35. Zhou H, Casas‐Finet JR, Heath Coats R, Kaufman JD, Stahl SJ, Winfield PT et al (1999) Identification and dynamics of a heparin‐binding site in hepatocyte growth factor. Biochemistry 38, 14792–14802. [DOI] [PubMed] [Google Scholar]
- 36. Thorne BA, Plowman GD (1994) The heparin‐binding domain of amphiregulin necessitates the precursor pro‐region for growth factor secretion. Mol. Cell. Biol. 14, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lyon M, Rushton G, Gallagher JT (1997) The interaction of the transforming growth factor‐betas with heparin/heparan sulfate is isoform specific. J. Biol. Chem. 18, 18000–18006. [DOI] [PubMed] [Google Scholar]
- 38. Gordon MY, Riley GP, Watt SM, Greaves MF (1987) Compartmentalization of an hemopoietic growth factor (GM‐CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature 326, 403–405. [DOI] [PubMed] [Google Scholar]
- 39. Roberts R, Ghallager J, Spooncer E, Allen TD, Bloomfield F, Dexter TM (1998) Heparan‐sulfate bound growth factors: a mechanism for stromal cell mediated hematopoesis. Nature 332, 376–378. [DOI] [PubMed] [Google Scholar]
- 40. Webb LMC, Ehrengruber MU, Clark‐Lewis I, Baggiolini M, Rot A (1993) Binding to heparin enhances neutrophil responses to interleukin 8. Proc. Nat. Acad. Sci. U.S.A. 90, 7158–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernandez‐Botran R, Romanovskis O, Sun X, Spatola AF (2004) Linear basic peptides for targeting interferon‐g‐glycosaminoglycan interactions: synthesis and inhibitory properties. Chem. Biol. Drug Design. 63, 56–62. [DOI] [PubMed] [Google Scholar]
- 42. Oravecz T, Pall M, Wang J, Roderiquez G, Ditto M, Norcross MA (1997) Regulation of anti‐HIV‐1activity of Rantes by heparan sulfate proteoglycans. J. Immunol. 159, 4587–4592. [PubMed] [Google Scholar]
- 43. Campanella GSV, Lee EMJ, Sun J, Luster AD (2003) CXCR3 and heparin binding sites of the chemokine IP‐10 (CXCL10). J. Biol. Chem. 278, 17066–17074. [DOI] [PubMed] [Google Scholar]
- 44. Dierker T, Dreier R, Petersen A, Bordych C, Grobe K (2009) Heparan sulfate‐modulated, metalloprotease‐mediated sonic hedgehog release from producing cells. J. Biol. Chem. 284, 8013–8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paine‐Saunders S, Viviano BL, Economides AN, Saunders S (2002) Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J. Biol. Chem. 277, 2089–2096. [DOI] [PubMed] [Google Scholar]
- 46. Kosaka N, Kodama M, Sasaki H, Yamamoto Y, Takeshita F, Takahama Y et al (2006) FGF‐4 regulates neural progenitor cell proliferation and neuronal differentiation. FASEB J. 20, E623–E629. [DOI] [PubMed] [Google Scholar]
- 47. Jin K, Mao XO, Sun Y, Xie L, Greenberg DA (2002) Stem Cell factor stimulates neurogenesis in vitro and in vivo. J. Clin. Invest. 110, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lim DA, Tramontin AD, Travejo JM, Herrera DG, Garcia‐Verdugo JM, Alvarez‐Buylla A (2000) Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713–726. [DOI] [PubMed] [Google Scholar]
- 49. Schneider A, Kruger C, Steiglede T, Weber D, Pitzer C, Laage R et al (2005) The hematopoeitic factor G‐CSF is a neuronal ligand that counteracts programmed cell death and drive neurogenesis. J. Clin. Invest. 115, 2083–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang TW, Zhang H, Gyetko MR, Parent JM (2011) Hepatocyte growth factor acts as a mitogen and chemoattractant for postnatal subventricular zone‐olfactory bulb neurogenesis. Mol. Cell. Neurosci. 48, 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lai K, Kaspar BK, Gage FH, Schaffer DV (2003) Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 6, 21–27. [DOI] [PubMed] [Google Scholar]
- 52. Wachs FP, Winner B, Couillard‐Despres S, Schiller T, Aigner R, Winkler J et al (2006) Transforming growth factor‐beta1 is a negative modulator of adult neurogenesis. J. Neuropathol. Exp. Neurol. 65, 358–370. [DOI] [PubMed] [Google Scholar]
- 53. Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S et al (2006) Microglia activated by IL‐4 and IFN‐ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 31, 149–160. [DOI] [PubMed] [Google Scholar]
- 54. Falk A, Frisen J (2002) Amphiregulin is a mitogen for adult neural stem cells. J. Neurosci. Res. 69, 757–762. [DOI] [PubMed] [Google Scholar]
- 55. Hienola A, Pekkanen M, Raulo E, Ventolla P, Rauvala H (2004) HB‐GAM inhibits proliferation and enhances differentiation of neural stem cells. Mol. Cell. Neurosci. 26, 75–88. [DOI] [PubMed] [Google Scholar]
- 56. Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Nat. Acad. Sci. U.S.A. 99, 11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tran PB, Ren D, Veldhouse TJ, Miller RJ (2004) Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J. Neurosci. Res. 76, 20–34. [DOI] [PubMed] [Google Scholar]
- 58. Gonzalez AM, Carman LS, Ong M, Ray J, Gage FH, Shults CW et al (1994) Storage, metabolism, and processing of 125I‐fibroblast growth factor‐2 after intracerebral injection. Brain Res. 665, 285–292. [DOI] [PubMed] [Google Scholar]
- 59. Mufson EJ, Kroin JS, Sendera TJ, Sobreviela T (1999) Distribution and retrograde transport of trophic factors in the central nervous system: functional implications for the treatment of neurodegenerative diseases. Prog. Neurobiol. 57, 451–484. [DOI] [PubMed] [Google Scholar]
- 60. Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger‐Chodobska J (2005) The choroid plexus‐cerebrospinal fluid system: from development to aging, Cur. Topics Dev. Biol. 71, 52. [DOI] [PubMed] [Google Scholar]
- 61. Delehedde M, Lyon M, Gallaghet JT, Rudland PS, Fernig DG (2002) Fibroblast growth factor‐2 binds to small heparin‐derived oligosaccharodes and stimulates a sustained phosphorylation of p42/44 mitogen‐activated protein kinase and proliferation of rat mammalian fibroblasts. Biochem. J. 366, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ma X, Dang X, Claus P, Hirst C, Fandrich RR, Jin Y et al (2007) Chromatin compaction and cell death by high molecular weight FGF2 depend on its nuclear localization, intracrine ERK activation and engagement of mitochondria. J. Cell. Physiol. 213, 690–698. [DOI] [PubMed] [Google Scholar]
- 63. Pasumarthi KB, Doble BW, Kardami E, Cattini PA (1994) Over‐expression of CUG or AUG‐initiated forms of basic fibroblast growth factor in cardiac myocytes results in similar effects on mitosis and protein synthesis but distinct nuclear morphologies. J. Mol. Cell. Cardiol. 26, 1045–1060. [DOI] [PubMed] [Google Scholar]
- 64. Erlich RB, Werneck CC, Mourao PAS, Linden R (2003) Major glycosaminoglycans species in the developing retina: synthesis, tissue distribution and effects upon cell death. Exp. Eye Res. 77, 157–165. [DOI] [PubMed] [Google Scholar]
- 65. Johanson C, McMillan P, Tavares R, Spangenberger A, Duncan J, Silverberg G et al (2004) Homeostatic capabilities of the choroid plexus epithelium in Alzheimer's disease. Cerebrospinal Fluid Res. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brightman MW (2002) The brain's interstitial clefts and their glial walls. J. Neurocytol. 31, 569–603. [DOI] [PubMed] [Google Scholar]
- 67. Stopa EG, Berzin TM, Kim S, Song P, Kuo‐LeBlanc V, Rodriguez‐Wolf M et al (2001) Human choroid plexus growth factors: what are the implications for CSF dynamics in Alzheimer's disease? Exp. Neurol. 167, 40–47. [DOI] [PubMed] [Google Scholar]
- 68. Mercier F, Schnack J, Saint Georges Chaumet M (2011) Fractones: home and conductors of the neural stem cell niche In: Alvarez‐Buylla A, Parent JM, Sawamoto K, Seki T, eds. Neurogenesis in the Adult Brain, pp. 109–136. New York/Heidelberg: Springer Verlag Pub. [Google Scholar]


