Abstract
Objectives
Many studies have demonstrated that the clock gene, brain and muscle ARNT‐like 1 (Bmal1), is directly related to bone ageing by affecting age‐related changes to mesenchymal stem cells (MSCs). As a main developmental signal, Wnt may play an important role in this process. Here, we have aimed to elucidate whether Bmal1 positively regulates osteogenesi via Wnt pathways.
Materials and methods
Bone marrow stromal cells were cultured in basic and in osteo‐induction medium with Wnt signalling inhibitor Dkk1 and Bmal1 transfection. Proliferation and osteogenesis of MSCs, expression of Bmal1 and activation of Wnt signalling were investigated by flow cytometry, senescence‐associated β‐galactosidase (SA‐β‐gal) staining, real‐time quantitative PCR and western blot analysis.
Results
Expression of Bmal1 (specially after 7 days osteo‐induction), activation of Wnt signalling and osteo‐related factors fell significantly during osteo‐induction after Dkk1 addition. When cellular Bmal1 was increased by transfection, osteogenesis inhibition by Dkk1 was rescued to a certain extent with activation of Wnt signalling. However, Dkk1 did not significantly affect proliferation or senescence of MSCs during early periods of culture.
Conclusion
These findings demonstrated that Bmal1 and Wnt signalling may have a synergistic effect at a particular stage of osteogenesis. Inhibition of Wnt signalling did not greatly affect ageing of MSCs through early passages.
Introduction
Some studies have shown that reduction in capability of self‐healing and regeneration is the primary cause of ageing 1. Such changes may be due to reduction in stem‐cell proliferation and differentiation. Osteoporosis is a major symptom of mammalian ageing 2 and is an important part of the process, closely related to bone marrow stromal cells (BMSCs) 3. Proliferation and differentiation abilities of mesenchymal stem cells (MSCs) decline with ageing 4, specially osteogenetic ability 5. As osteoblast progenitors, changes in BMSCs inevitably result in osteoporosis, reduced bone mass and further complications.
However, numbers of experiments have demonstrated that changes in circadian gene expression are probably internal reasons for cell senescence, specially core circadian component brain and muscle ARNT‐like 1 (Bmal1 gene). Researchers have found that mice lacking Bmal1 (Bmal1−/−) show early ageing symptoms compared to wild‐type littermates 6. These signs include shorter lifespan, arthropathy, osteoporosis and other ageing precursors. Bmal1−/− mice 40 weeks of age have obvious weight loss, as occurs in osteoporosis. After rescuing Bmal1 expression in Bmal1−/− mice, long‐term survival has been restored, and early ageing mitigated 7.
Study of gene function should be traced to changing processes of metabolic transduction. Wnt signalling, specially the canonical Wnt pathway (Wnt/β‐catenin signalling), plays a significant role in cell proliferation and differentiation. Although the exact role of Wnt signalling in senescence of cells and organs remains controversial, most studies support the idea that its activity can delay the ageing process 8, 9, 10, however, recent studies have provided contradictory results 11, 12. Such controversial findings may be attributed to differences in Wnt signal intensity, cell culture conditions in vitro, and cell manipulation 13. Nevertheless, importance of Wnt signalling in cell ageing been confirmed 11, 14. The Wnt/β‐catenin pathway involves the ligand (Wnt), transmembrane receptor (Frizzled), cytoplasmic protein (β‐catenin), nuclear transcription factor (TCF) and further components 15, 16. When Wnt protein level is low, β‐catenin is hydrolysed by phosphorylation‐dependent proteolytic enzymes induced by degradation of the glycogen synthase kinase 3β complex, Axin, and adenomatous polyposis coli. Thus, β‐catenin level is kept low, which inhibits transcription mediated by T‐cell factor/lymphoid enhancer factor. When the Wnt ligand interacts with its transmembrane receptor, degradation of the complex is inhibited, β‐cateninfirst accumulates in the cytoplasm then enters the nucleus to activate transcription of target genes mediated by TCF 17.
Dkk1, a protein inhibitor of Wnt signalling, is a physiological protein secreted by MSCs and multiple myeloma cells 18. Dkk1 can bind to a receptor or interact with transmembrane receptor Kremen to induce rapid endocytosis and downregulate expression of low‐density lipoprotein receptor‐related protein 5/6 (LRP‐5/6), which blocks intracellular delivery of Wnt signalling 19, 20. Related experiments have indicated that heterozygous Dkk1‐deficient mice have increased bone formation and a high bone mass phenotype. Moreover, number of osteoblasts, mineral apposition and bone formation rate were all increased in these mice 21. In contrast, experiments on human fracture non‐union stromal cells found that senescent cells secreted more Dkk1 22. Whether higher secretion of Dkk1 in senescent cells is the result of senescence or its cause, has not been clearly explained. With regard to dose effect of Dkk1, from 25 ng/ml it inhibited non‐phosphorylated β‐catenin, with maximal inhibition at 50 ng/ml in C2C12 18. Furthermore, 50 ng/ml is the physiological secretion dose 22.
Thus, we hypothesized that Wnt signalling may be the link in processes by which Bmal1 affects in changes in MSC ageing. Our preliminary studies demonstrated that in aged mice, reduction in proliferation and osteogenic ability were positively related to reduction in Bmal1 expression 23. In a further piece of research, Zhang et al. showed that activation of Wnt signalling in osteoblasts, specially expression level of β‐catenin (cytoplasmic protein of the Wnt signalling pathway), reduces with age 24; furthermore, cell proliferation and expression of β‐catenin increase by enhancing Bmal1 expression 25. However, effects of Wnt signalling on expression of Bmal1 have remained unknown. To clarify the relationship between Bmal1 and Wnt signalling in osteo‐induction, we have explored the underlying mechanism of Bmal1 expression in osteo‐differentiation during ageing, using Wnt signalling inhibitor Dkk1 and Bmal1 transfection.
Materials and methods
Cell culture
Four week old C57/BL6 male mice with good BMSC activity and no interference by oestrogen, obtained from the Animal Centre of Sichuan University, were used in this study; all protocols used were approved by the University Bioethics Committee. The mice were sacrificed, and metaphyses from both ends of femora and tibias were obtained and marrow cavities were flushed with sterile medium, using a #25‐gauge needle. After washing in phosphate‐buffered saline (PBS) by centrifugation, cells were cultured in α‐MEM (Gibco, Life Technologies, Carlsbad, CA, USA) with 100 U/ml penicillin, 10% foetal bovine serum (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA) and 100 mg/ml streptomycin (as basic medium). They were then maintained at 37 °C in a humidified atmosphere with 5% CO2. After 36 h, non‐adherent cells were removed by gentle rinsing in sterile PBS prewarmed to 37 °C. Until confluence, cells were maintained in basic medium, which was changed two to three times per week. When confluent, MSCs were detached with 0.25% trypsin in 0.01% ethylenediaminetetraacetic acid then passaged. MSCs were identified by immunohistochemistry as CD29(+), Stro‐1(+), CD45(−) and CD34(−), as suggested in other studies 4. Osteo‐induction medium (OS medium) used was basic medium supplemented with 10 mm β‐glycerophosphate, 10 nm dexamethasone, and 50 mg/l l‐ascorbic acid (Sigma‐Aldrich, St. Louis, MO, USA). Dkk1 protein powder from recombinant mouse Dkk1 (R&D Systems, Inc., Minneapolis, MN, USA) was added into the α‐MEM medium, final concentration 50 ng/ml 26.
Flow cytometry of MSCs after addition of Dkk1
Mesenchymal stem cells in basic medium with and without Dkk1 were passaged. At passage 4, when cells reached 70% confluence, they were detached with trypsin and ethylenediaminetetraacetic acid; after washing twice in PBS and centrifuging at 500 g for 5 min, they were fixed in 70% alcohol and frozen to below −20 °C. Two hours before detection, cells were centrifuged to remove alcohol and were washed in PBS. After incubating in RNase for 30 min, propidium iodide (PI) was infused into them. Cell density was approximately 1 × 106 cells/ml for detection. One tube was incubated in steam buffer only as control, to eliminate interference of residual green fluorescent protein (GFP) fluorescence. DNA content of cells was detected by flow cytometry (Beckman Coulter, Brea, CA, USA), S‐phase fraction and DNA proliferation index of total cells in each sample was calculated according to the formula:
Assessment of senescence‐associated β‐galactosidase (SA‐β‐gal) staining
Passage 4 MSCs were digested and seeded into six‐well plates, 1 × 105 cells/well. After 24 h culture in a humidified atmosphere with 5% CO2, medium was discarded and cells were washed once in PBS. Then, 1 ml fixative per well was added for 15 min, and cells were rinsed three times. They were then incubated in 1 ml per well working solution of β‐galactosidase with X‐Gal, overnight, at 37 °C (SA‐β‐gal staining kit obtained from Beyotime, Chengdu, China). Senescent cells were observed using an optical microscope and counted in three random fields of vision (200 cells per field used to calculate level of positivity).
Transfection of overexpression lentiviral vector for Bmal1
Passage 2 MSCs were cultured in 12‐well plastic plates in Dkk1‐conditioned medium (Dkk1 final concentration 50 ng/ml) at initial density of 5 × 104 cells/well. In the order of 48 h after plating, cells were transfected using Bmal1 overexpressed lentiviral vector (synthesised by Genechem, Shanghai, China) at multiplicity of infection (MOI) MOI = 20; this is called the Bmal group, with the lentiviral vector expressing EGFP as positive control group. Expression of GFP was observed using an inverted fluorescence microscope 2 days later (Fig. S1a); transfection efficiency was calculated. Western blotting assay was used to test transfection effect of Bmal1 (Fig. S1b). As rhythmic expression of Bmal1 should not be induced without serum shock in vitro, no rigorous time point was followed 27.
Real‐time reverse transcription polymerase chain reaction (RT‐PCR)
Passage 2 MSCs were divided into three groups for culture, one in OS medium without Dkk1 (control group), one in OS medium with Dkk1 (final concentration Dkk1 50 ng/ml) – Dkk1 group, and the final group with Bmal1 transfection in Dkk1‐conditioned OS medium as the Dkk1 + Bmal1 group. Cells were sampled and maintained in RNA preservation solution (RNA safeguard; Keygen Biotech, Nanjing, China) after 7 days and after 14 days culture. A simple P total RNA extraction kit (Bioer, Hangzhou, China) was used to extract total RNA; this was quantified by spectrophotometry at absorbance (A) of 260 nm. RNA samples had A260:A280 ratio of 2.0 to ensure high purity., Each real‐time PCR was performed in triplicate using ABI PRISM 7300 real‐time PCR system in 20‐μl reaction mixture. The PCR program was conducted according to the manufacturer's instruction manual. Starting copy numbers of unknown samples were calculated by 7300 System SDS Software (Applied Biosystems, Foster City, CA, USA) from their standard curve. Table 1 shows primers used for RT‐PCR analysis.
Table 1.
Primers used for real‐time PCR analysis
| Target gene | Primers | Fragment size (bp) |
|---|---|---|
| P‐actin |
F:5′‐GGGCTGTATTCCCCTCCATCG‐3′ R:5′‐GCAGCTCATTGTAGAAGGTGTGGTG‐3′ |
201 |
| Osx |
F:5′‐TATGCTCCGACCTCCTCAAC‐3′ R:5′‐AATAGGATTGGGAAGCAGAAA‐3′ |
120 |
| Ocn |
F:5′‐AAGCAGGAGGGCAATAAGGT‐3′ R:5′‐CCGTAGATGCGTTTGTAGGC‐3′ |
167 |
| Bmall |
F:5′‐AACCTTCCCGCAGCTAACAG‐3′ R:5′‐AGTCCTCTTTGGGCCACCTT‐3′ |
79 |
| Wnt3a |
F:5′‐CTTAGTGCTCTGCAGCCTGA‐3′ R:5′‐GAGTGCTCAGAGAGGAGTACTGG‐3′ |
92 |
| P‐catenin |
F:5′‐GGGTCCTCTGTGAACTTGCTC‐3′ R:5′‐TGTAATCCTGTGGCTTGTCCTC‐3′ |
165 |
| Tcfl |
F:5′‐CTACAGCGACGAGCACTTTTCTC‐3′ R:5′‐GTAGAAGGTGGGGATTTCAGGAG‐3′ |
115 |
Quantitative alkaline phosphatase (ALP) and protein assays
Passage 2 MSCs were grouped as described for RT‐PCR analysis. For quantitative analysis of ALP activities, after 7 days and after 14 days osteo‐induction, cells were washed in PBS, according to instructions of the ALP assay kit (Merit Choice, China). Cells were lysed in 10 mm Tris‐HCl and 0.1% Triton X‐100 (pH 7.4) and subjected to repeat freeze‐thaw cycles three times. After sonication, lysed cells were assayed in microtitre plates with p‐nitrophenyl phosphate as substrate. Absorbance of p‐nitrophenol formed by hydrolysis of p‐nitrophenyl phosphate, and catalysed by ALP, was measured at 405 nm using a microplate reader. Total protein content was measured using the Bradford method at 595 nm and calculated according to bovine gamma globulin standards. ALP activity was expressed as U/g protein.
Western blot assay for Bmal1and β‐catenin protein
Passage 2 MSCs were grouped as described for RT‐PCR analysis, and glyceraldehyde‐ 3‐phosphate dehydrogenase (GAPDH) was used as internal reference. To obtain whole‐cell protein extracts after 7 days and after 14 days culture, cells were washed twice in ice‐cold PBS then lysed in lysis buffer (Keygen total protein extraction kit; Keygen Biotech). Supernatants were collected after centrifugation at 14 000 g at 4 °C for 15 min, and quantitatively assayed using the BCA method. Total protein extracts were conducted using standard sodium dodecylsulphonate‐polyacrylate gel electrophoresis procedures and subsequently transferred to polyvinylidene fluoride membranes. After blocking, membranes were probed with anti‐Bmal1 protein primary antibodies (Abcam, Cambridge, UK) or anti‐β‐catenin primary antibodies (Cell Signaling, Danvers, MA, USA) followed by addition of 1:5000 horseradish peroxidise‐conjugated secondary antibody. Immunoreactive proteins were visualized employing a chemiluminescence kit (Millipore, Billerica, MA, USA) and band intensities were determined using the ChemiDoc XRS Gel documentation system and Quantity One software (Bio‐Rad, Berkeley, CA, USA).
Statistical analysis
Measurements were expressed as mean ± SD. Statistical comparisons were conducted using factorial analysis of variance (ANOVA), followed by multiple comparisons utilizing the Student–Newman–Keuls test. Values of P < 0.05 were considered statistically significant.
Results
MSC proliferation after Dkk1 addition
Proliferation of passage 4 MSCs was detected by flow cytometry, to determine effects of Dkk1 during the process. However, no obvious differences were found between results in the control group and those of the Dkk1 group (Fig. 1). PI and S‐phase fraction indices were detected but differences also were not statistically significant (P > 0.1). These data indicate that addition of Dkk1 had no effect on MSC proliferation during early passages. As culture‐induced senescence begins at passage 4–6 cells in vitro, no additional cells were cultured after passage 4 19.
Figure 1.
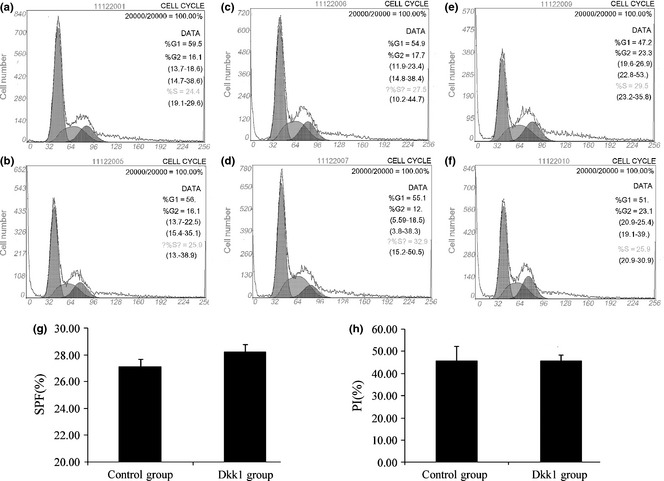
Proliferation of mesenchymal stem cells ( MSC s) after addition of Dkk1. (a, c, e) Cell cycle of control group by flow cytometric assay; (b, d, f) cell cycle of Dkk1 group; a and b, c and d, e and f obtained under the same conditions. No clear differences could be seen. (g) Propidium iodide (PI) of two groups and (h) S‐phase fraction (SPF). Data represent mean ± SD (n = 3).
SA‐β‐gal staining results of MSCs
As indicated above, the exact role of Wnt signalling in cell senescence remains unclear but as the main antagonists of Wnt, the role of Dkk1 in senescence needs to be understood. Due to complex variations in ageing cells, many interference factors are difficult to control. Thus, we used a physiological dose of Dkk1 in passage 4 MSCs and expected to simulate its effects in physiological senility 22. In the order of 50 ng/ml Dkk1 was added to the osteo‐induction medium as Dkk1‐conditioned OS medium. Results of SA‐β‐gal staining conducted on passage 4 MSCs are shown in Fig. 2. Groups showed no obvious differences in ageing, demonstrating that the physiological dose of Dkk1 did not affect MSC senescence during the early passages.
Figure 2.
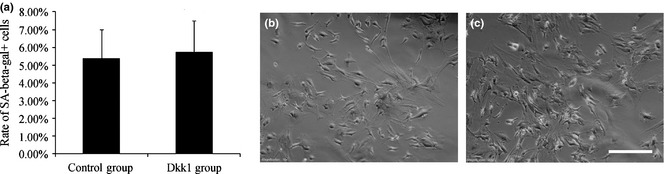
Images of SA‐β‐gal staining detected in passage 4 mesenchymal stem cells ( MSC s). (a) SA‐β‐ga+ of the two groups. Data were not statistically significant; cells were counted from three different fields to compose the average. (b) Control group was cultured in basic medium, (c) Dkk1 group (50 ng/ml), 200× scale bar = 150 μm.
Effects of MSC osteogenesis in Dkk1‐conditioned osteo‐induction medium after Bmal1 transfection
Considering that Wnt signalling plays an important role in MSC osteogenesis, we anticipated that its inhibition would affects MSC osteogenesis. Our data showed that successive stages of MSC osteogenesis were markedly suppressed by Dkk1. Meanwhile, overexpression of Bmal1 rescued the reduction in osteogenesis by Dkk1 to some extent (Fig. 3). Moreover, MSC osteogenesis with Bmal1 overexpression was slightly enhanced (Fig. S2). Osteogenic markers were detected as follows: osterix (Osx), marker of osteoblast progenitors; ALP activity level, an early osteoblast marker; and osteocalcin (Ocn), a late osteogenic marker.
Figure 3.
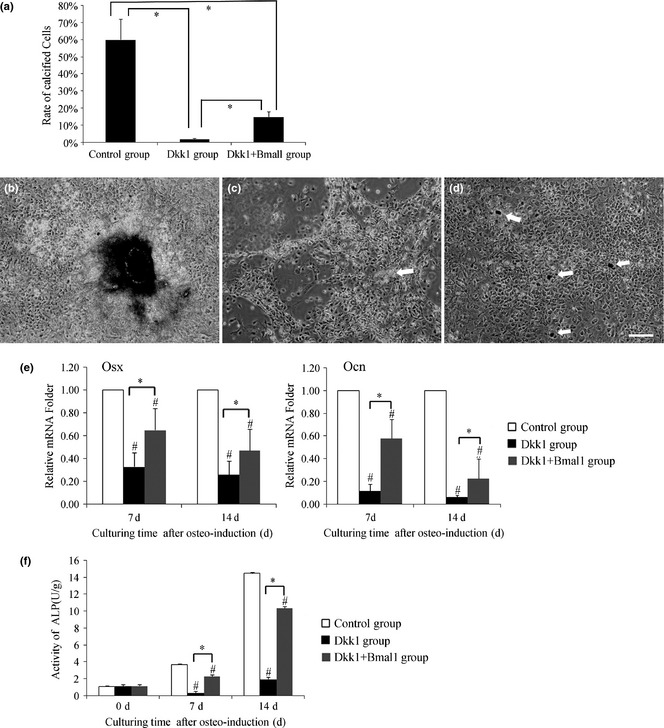
Effect of mesenchymal stem cells ( MSC s) osteogenesis in Dkk1‐conditioned osteo‐induction medium after Bmal1 transfection. (a) Level of calcified cells among the three groups. Calcified cells were counted using light microscopy from three different fields, to obtain the average. (b) The control group had clear calcifications. (c) Cell shape in the Dkk1 group slightly changed and a necrotic mass (arrow) occurred. (d) Dotted calcification (arrow) could be seen in the Dkk1 + Bmal1 group. (b, c, d) = 100×, scale bar = 150 μm. (e) Osx and Ocn mRNA expression among the groups during osteo‐ induction. (f) Alkaline phosphatase (ALP) activity among the groups after 7 and 14 days induction. Data represent mean ± SD (n = 3), *P < 0.05 indicates significant difference between them; #P < 0.05 compared to control group.
Cells were observed using light microscopy on the 14th day after osteo‐induction. Results showed that the control group was clearly calcified and Dkk1 + Bmal1 group exhibited dotted calcification; however, Dkk1 group had no sign of calcification (Fig. 3a–d). Meanwhile, mRNA levels of osteo‐factors with RT‐PCR provided more significant results. mRNA expression of Osx and Ocn was detected after 7 days and after 14 days osteo‐induction. Compared to the control group, Osx and Ocn mRNA was obviously lower in the Dkk1 group. In particular, Ocn mRNA decreased by 6% compared to the control group after 14 days osteo‐induction. However, in the Dkk1 + Bmal1 group, more moderate decline of Osx and Ocn mRNA was observed, with Ocn mRNA reaching 23% of control group level by 14 days of osteo‐induction (Fig. 3e). As shown in Fig. 3f, ALP activity was markedly suppressed in the Dkk1 group below levels of pre‐induction, but increased slightly by 14 days induction. However, was still much lower than level of the control group. Comparatively speaking, the declining trend was not very remarkable in the Dkk1 + Bmal1 group.
Transcriptional change in Bmal1 and Wnt signalling after overexpression of Bmal1 in Dkk1‐conditioned medium during osteo‐induction
To investigate whether Wnt signalling is related to the effect of Bmal1 on MSC ageing, Wnt signalling was inhibited by Dkk1 to detect any changes in Bmal1 level. Synchronously, effects of Wnt signalling were detected after overexpression of Bmal1, by transfection, in osteogenesis with added Dkk1.
After 7 days osteo‐induction, mRNA level of Wnt 3a, β‐catenin and TCF1 in the Dkk1 group decreased, and continued to decrease over 14 days osteogenesis. Interestingly, at low levels after 7 days osteo‐induction, mRNA level of Bmal1 in the Dkk1 group increased by 40% ± 40% (although not statistically significant) compared to the control group after 14 days osteo‐induction (Fig. 4a). On the other hand, with Bmal1 overexpression, mRNA levels of Wnt 3a, β‐catenin and TCF1 in the Dkk1 + Bmal1 group had a certain increase compared with to the Dkk1 group under the same conditions. Among them, increased expression of β‐catenin was most significant (β‐catenin protein level shown in Fig. 4b,c). Bmal1 protein level exhibited a similarly changing trend to that of Bmal1 mRNA. Western blotting, after 14 days osteo‐induction, exhibited a reduction in Bmal1 protein level in the Dkk1 group, although also not statistically significant. Furthermore, in Dkk1 + Bmal1 group, increase in Bmal1 protein after 14 days osteo‐induction was more obvious than after 7 days (Fig.4d,e). These data demonstrate that expression of Bmal1 changed with inhibition of Wnt signalling in osteogenesis, but the change in trend was not synchronous.
Figure 4.
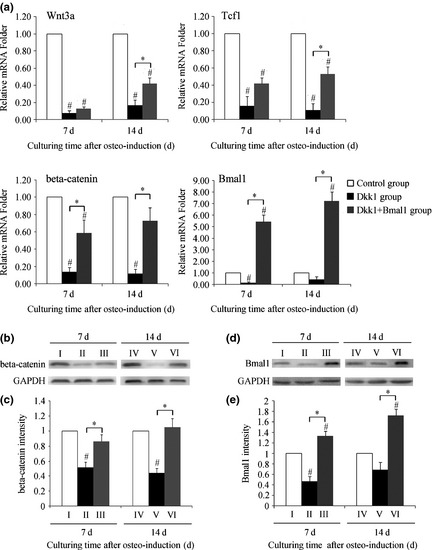
Transcriptional change in Wnt signalling and Bmal1 after transfection in Dkk1‐conditioned medium during osteo‐induction. (a) Difference in Wnt 3a, TCF1, β‐catenin and Bmal1 mRNA levels after 7 and 14 days osteo‐induction among the groups. (b) Western blotting result of β‐catenin protein changes among the groups during osteo‐induction. (c) Quantified using densitometry by Quantity One software, ratio of β‐catenin relative to GAPDH expressed as relative content of β‐catenin protein. (d) Western blotting result of Bmal1 protein changes among the groups during osteo‐induction. (e) Quantified using densitometry by Quantity One software, ratio of Bmal1 relative to GAPDH is expressed as relative content of Bmal1 protein. I: 7‐day induction of control group, II: 7‐day induction of Dkk1 group, III: 7‐day induction of Dkk1 + Bmal1 group, IV: 14‐day induction of control group, V: 14‐day induction of Dkk1 group, VI: 14‐day induction of Dkk1 + Bmal1 group. Data represent mean ± SD (n = 3), *P < 0.05 indicates significant difference between them; #P < 0.05 compared to control group.
Discussion
Preliminary studies in our laboratory have revealed that the core circadian gene Bmal1 is possibly involved in regulation of MSC senescence with age, but its mechanism of action had not been investigated 23, 25. Bmal1 may act in MSC osteo‐differentiation in two ways. First, it may affect MSC proliferation by affecting numbers of osteoblasts originating from MSCs 5, 28. Second, Bmal1 may act directly on one or several stages of osteogenesis. Both mechanisms can cause bone loss due to MSC senescence. Our preliminary results supported the first mechanism involving Bmal1 promotion of MSC proliferation through Wnt signalling 25. On the other hand, reduced osteogenic ability of MSCs is positively related to reduction in Bmal1 expression during ageing 23. However, no evidence regarding interaction between Bmal1 and Wnt signalling in MSC osteo‐differentiation had been previously reported.
Considering the circadian rhythm, many studies have shown that osteogenic markers ALP, Ocn and type I collagen fluctuate based on day and night, demonstrating effects of clock genes in regulation of the balance between bone formation and cell proliferation 29, 30. Fu et al. verified that both period (Per) and cryptochrome (Cry), the negative regulating factors of Bmal1, had an effect on bone metabolism. When Per or cryptochrome was knocked out, their mice appeared to experience excessive bone formation and loss of osteogenic response caused by leptin activity 31. Calvaria research studies conducted by Zvonic et al. have shown that 26% TCFs in osteogenesis exhibit volatility between day and night. Such factors may be independent of central control factors or further control factors such as adipose tissue, and acted by multiple signals to regulate bone metabolism 32.
More evidence directly linking Bmal1, the core gene of circadian rhythm, and bone metabolism, have been discussed. Miyamoto et al. found reduced expression of bone sialoprotein and dentin matrix protein I after removing retinoic acid‐related orphan receptor α (RORα), a positive regulating factor of Bmal1, in human MSCs, by RNA interference. This is downstream of Wnt signalling in osteogenesis, and participates in expression of bone sialoprotein and dentin matrix protein I during osteo‐differentiation and bone formation 33. Thus, RORα seemed to directly combine Bmal1 and Wnt signalling in osteo‐differentiation. Hinoi et al. studied the cartilage cell line ATDC5, and their results demonstrated that the Bmal1/Clock dimer is linked to E‐box, coded by type II collagen gene and plays the role of a reporting gene. Meanwhile, linkage is inhibited by transcription of Per gene, another important member of the clock gene family 34.Moreover, a recent study by Janich and colleagues has reported lower expression of Wnt‐related genes including Lef1 and Wnt10a in Bmal1 KO mice 27. These findings further suggest the importance of clock genes and Bmal1 in osteogenesis.
In our study, we found that after 7 days osteo‐induction with Wnt signalling blocked by Dkk1, Bmal1 mRNA and protein levels fell significantly. However, after 14 days osteo‐induction, no significant difference was found compared to the control group. These result showed that Bmal1 participated at this particular stage of osteo‐induction; however, the mechanism remained unclear. Considering variation of Wnt signalling, specially β‐catenin, in these processes, some evidence may be considered. A recent study by ChIP analysis on the interaction between Bmal1 and Wnt signalling in adipogenesis, demonstrated that β‐catenin is the target gene of Bmal1 35. Thus, reduction in β‐catenin, as the target gene of Bmal1, may have a negative feedback effect such as RORα (positive regulating factor of Bmal1), which has a slight promotional effect on Bmal1 expression at lower protein levels. Moreover, with low expression of Bmal1, target genes, including β‐catenin, had lower mRNA and protein levels. The intrinsic mechanism may be attributed to initial downregulation of β‐catenin protein level by Dkk1, which eventually changes β‐catenin mRNA level. Hence, overexpression of Bmal1 in the Dkk1 + Bmal1 group, and expression of β‐catenin, both at mRNA and protein levels, increased as expected. These results indicate that Bmal1 most likely had a synergistic effect on Wnt signalling during MSC osteogenesis, specially after 7 days osteo‐induction, critical time of Bmal1 activity in MSC proliferation and differentiation. Considering that ALP expression with circadian rhythm activity had the same changing trend with Bmal1, ALP could be the target gene of Bmal1 just as the case with β‐catenin. Further studies need to be performed to test this hypothesis.
Results here have shown comprehensive inhibition of Dkk1 on Wnt signalling from ligand Wnt3a to nuclear transcription factor TCF1. The mechanism of Dkk1‐mediated suppression of Wnt signalling by blocking LRP5/6 binding Wnt ligands has been verified 36. As the downstream target gene of Wnt signalling, it is easy to understand reduced expression of TCF1 mRNA in the Dkk1 group. That Wnt3a is the target gene of Wnt signalling has been suggested, but needs to be tested 37.The result of Wnt3a mRNA being lower in the Dkk1 group may provide evidence of this.
The ability for MSC to undergo osteogenesis falls with increasing age 23. Given that the first stage of osteoblastic differentiation in vitro is proliferation, we initially tested effects of a physiological dose of Dkk1 on proliferation and senescence. No difference was found during early passages. This finding may be caused by reduction in concentration of Dkk1 in the culture medium due to its short half life. Subsequently, this may allow Wnt signalling to recover to its normal state and cause normal levels of cell population growth and proliferation. Meanwhile, studies on proliferation of hMSCs in vitro have demonstrated that at early passages, as cells begin to proliferate rapidly, high levels of Dkk1 are secreted 19. This finding indicates that Dkk1 is a promoter of proliferation at early passages. In this respect, effects of Dkk1 on MSC proliferation in our experiment in vitro was probably reasonable.
No significant enhancement of MSC osteogenesis has been shown with Bmal1 overexpression. This might due to the good osteogenic capability of passage 2 cells and poorer transfection effect of MSCs, compared to other cells and cell lines 38. In contrast, effects of Dkk1 protein on osteogenesis in vitro were clear, MSCs did not form calcium nodules, and expression of osteo‐related factors fell greatly. Meanwhile, enhanced expression of Bmal1 rescued decreasing trend of osteogenesis in the Dkk1 group to some extent, and activity of Wnt signalling (expression of Wnt 3a, β‐catenin and TCF1) in Dkk1 + Bmal1 group slightly increased, indicating that Bmal1 might be positively regulated in osteogenesis, by Wnt signalling. Considering that under normal circumstances ageing progresses along with reducing osteogenetic ability, these results demonstrate the positive role of Bmal1 in preventing ageing during MSC differentiation. However, inhibition of Wnt signalling by Dkk1 was not reversed completely by Bmal1 transfection. This result reveals that effects of osteogenic inhibition with a physiological dose of Dkk1 was more powerful. For mechanisms of bone ageing, efforts to explore interactions between clock genes and signalling of development may yield more positive results. Moreover, molecular regulation of osteogenesis in MSCs may provide us with better understanding of bone ageing and may guide development of novel treatments for osteoporosis.
Supporting information
Fig. S1. Overexpression of Bmal1 in MSCs.
Fig. S2. Effect of MSC osteogenesis after Bmal1 transfection.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 30801206).
References
- 1. Sethe S, Scutt A, Stolzing A (2006) Aging of mesenchymal stem cells. Ageing Res. Rev. 5, 91–116. [DOI] [PubMed] [Google Scholar]
- 2. Grob GN (2011) From aging to pathology: the case of osteoporosis. J. Hist. Med. Allied Sci. 66, 1–39. [DOI] [PubMed] [Google Scholar]
- 3. Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS et al (2008) Age‐related intrinsic changes in human bone‐marrow‐derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 7, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bianco P, Riminucci M, Gronthos S, Robey PG (2001) Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19, 180–192. [DOI] [PubMed] [Google Scholar]
- 5. Moerman EJ, Teng K, Lipschitz DA, Lecka‐Czernik B (2004) Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR‐γ2 transcription factor and TGF‐β/BMP signaling pathways. Aging Cell 3, 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M et al (2005) Brain and muscle Arnt‐like protein‐1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. USA 102, 12071–12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDearmon EL, Patel KN, Ko CH, Walisser JA, Schook AC, Chong JL et al (2006) Dissecting the functions of the mammalian clock protein BMAL1 by tissue‐specific rescue in mice. Science 314, 1304–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeCarolis NA, Wharton KA Jr, Eisch AJ (2008) Which way does the Wnt blow? Exploring the duality of canonical Wnt signalling on cellular aging. Bioessays 30, 102–106. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman J, Kuhnert F, Davis CR, Kuo CJ (2004) Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle 3, 554–557. [PubMed] [Google Scholar]
- 10. Jia L, Miao C, Cao Y, Duan EK (2008) Effects of Wnt proteins on cell proliferation and apoptosis in HEK293 cells. Cell Biol. Int. 32, 807–813. [DOI] [PubMed] [Google Scholar]
- 11. Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J et al (2007) Augmented Wnt signalling in a mammalian model of accelerated aging. Science 317, 803–806. [DOI] [PubMed] [Google Scholar]
- 12. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C et al (2007) Increased Wnt signalling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810. [DOI] [PubMed] [Google Scholar]
- 13. Ling L, Nurcombe V, Cool SM (2009) Wnt signaling controls the fate of mesenchymal stem cells. Gene 433, 1–7. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Liu B, Gu S, Liang J (2012) Effects of Wnt/β‐catenin signalling on proliferation and differentiation of apical papilla stem cells. Cell Prolif. 45, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosoi T (2010) Cytokines in bone diseases. Wnt signal and excessive bone formation. Clin. Calcium 20, 1526–1531. [PubMed] [Google Scholar]
- 16. Caronia G, Wilcoxon J, Feldman P, Grove EA (2010) Bone morphogenetic protein signaling in the developing telencephalon controls formation of the hippocampal dentate gyrus and modifies fear‐related behavior. J. Neurosci. 30, 6291–6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Boer J, Siddappa R, Gaspar C, van Apeldoorn A, Fodde R, van Blitterswijk C (2004) Wnt signalling inhibits osteogenic differentiation of human mesenchymal stem cells. Bone 34, 818–826. [DOI] [PubMed] [Google Scholar]
- 18. Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD Jr (2008) Dkk1‐induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone 42, 669–680. [DOI] [PubMed] [Google Scholar]
- 19. Gregory CA, Singh H, Perry AS, Prockop DJ (2003) The Wnt signaling inhibitor dickkopf‐1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J. Biol. Chem. 278, 28067–28078. [DOI] [PubMed] [Google Scholar]
- 20. Kong XB, Zhang C (2009) Dickkopf (Dkk) 1 promotes the differentiation of mouse embryonic stem cells toward neuroectoderm. In Vitro Cell. Dev. Biol. Anim. 45, 185–193. [DOI] [PubMed] [Google Scholar]
- 21. Morvan F, Boulukos K, Clément‐Lacroix P, Roman Roman S, Suc‐Royer I, Vayssière B et al (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 21, 934–945. [DOI] [PubMed] [Google Scholar]
- 22. Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WE (2009) Decreased osteogenesis, increased cell senescence and elevated Dickkopf‐1 secretion in human fracture non union stromal cells. Bone 45, 726–735. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Xu X, Tan Z, Ye C, Zhao Q, Chen Y (2012) Age‐related BMAL1 change affects mouse bone marrow stromal cell proliferation and osteo‐differentiation potential. Arch. Med. Sci. 8, 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang H, Lu W, Zhao Y, Rong P, Cao R, Gu W et al (2011) Adipocytes derived from human bone marrow mesenchymal stem cells exert inhibitory effects on osteoblastogenesis. Curr. Mol. Med. 11, 489–502. [DOI] [PubMed] [Google Scholar]
- 25. Lin F, Chen Y, Li X, Zhao Q, Tan Z (2013) Over‐expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH‐3T3 cells. Cell Biochem. Funct. 31, 166–172. [DOI] [PubMed] [Google Scholar]
- 26. Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B et al (2003) The role of the Wnt‐signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 349, 2483–2494. [DOI] [PubMed] [Google Scholar]
- 27. Janich P, Pascual G, Merlos‐Suárez A, Batlle E, Ripperger J, Albrecht U et al (2011) The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 480, 209–214. [DOI] [PubMed] [Google Scholar]
- 28. Aranda‐Anzaldo A, Dent MA (2007) Reassessing the role of p53 in cancer and ageing from an evolutionary perspective. Mech. Ageing Dev. 128, 293–302. [DOI] [PubMed] [Google Scholar]
- 29. Takeda S (2009) Osteoporosis: a neuroskeletal disease? Int. J. Biochem. Cell Biol. 41, 455–459. [DOI] [PubMed] [Google Scholar]
- 30. Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116, 2627–2634. [DOI] [PubMed] [Google Scholar]
- 31. Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G (2005) The molecular clock mediates leptin‐regulated bone formation. Cell 122, 803–815. [DOI] [PubMed] [Google Scholar]
- 32. Zvonic S, Ptitsyn AA, Kilroy G, Wu X, Conrad SA, Scott LK et al (2007) Circadian oscillation of gene expression in murine calvarial bone. J. Bone Miner. Res. 22, 357–365. [DOI] [PubMed] [Google Scholar]
- 33. Miyamoto S, Cooper L, Watanabe K, Yamamoto S, Inoue H, Mishima K et al (2010) Role of retinoic acid‐related orphan receptor‐alpha in differentiation of human mesenchymal stem cells along with osteoblastic lineage. Pathobiology 77, 28–37. [DOI] [PubMed] [Google Scholar]
- 34. Hinoi E, Ueshima T, Hojo H, Iemata M, Takarada T, Yoneda Y (2006) Up‐regulation of per mRNA expression by parathyroid hormone through a protein kinase A‐CREB‐dependent mechanism in chondrocytes. J. Biol. Chem. 281, 23632–23642. [DOI] [PubMed] [Google Scholar]
- 35. Guo B, Chatterjee S, Li L, Kim JM, Lee J, Yechoor VK et al (2012) The clock gene, brain and muscle Arnt‐like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 26, 3453–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A (2008) Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta‐catenin signaling. Dev. Cell 15, 37–48. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Z, Deb A, Zhang Z, Pachori A, He W, Guo J et al (2009) Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J. Mol. Cell. Cardiol. 46, 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang F, Wu LA, Li W, Yang Y, Guo F, Gao Q et al (2013) Immortalized mouse dental papilla mesenchymal cells preserve odontoblastic phenotype and respond to bone morphogenetic protein 2. In Vitro Cell. Dev. Biol. Anim. 49, 626–637. Doi: 10.1007/s11626-013-9641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Overexpression of Bmal1 in MSCs.
Fig. S2. Effect of MSC osteogenesis after Bmal1 transfection.


