Abstract
Objectives
Restorative properties of medicinal plants such as Genista sessilifolia DC. have often been suggested to occur, in epidemiological studies. However, full characterization of effective principles responsible for this action has never previously been performed. Here, we have characterized G. sessilifolia's anti‐cancer effects and identified the chemical components involved in this anti‐tumour action.
Materials and methods
Cell cycle, apoptosis, necrosis, differentiation analyses, high‐performance liquid chromatography, western blotting, RNA extraction, real‐time PCR and primers have all been observed/used in the study.
Results
We report that G. sessilifolia methanol extract has anti‐cancer activity on solid and haematological cancer cells. G. sessilifolia extract's anti‐proliferative action is closely bound to induction of apoptosis, whereas differentiation is only weakly modulated. Analysis of G. sessilifolia extract, by high‐performance liquid chromatography, identifies fraction 18–22 as the pertinent component for induction of apoptosis, whereas fractions 11–13 and 27–30 both seem to contribute to differentiation. G. sessilifolia extract induces apoptosis mediated by caspase activation and p21, Rb, p53, Bcl2‐associated agonist of cell death (BAD), tumour necrosis factor receptor super‐family, member 10 (TRAIL) overexpression and death receptor 5 (DR5). Accordingly, fraction 18–22 inducing apoptosis was able to induce TRAIL.
Conclusions
Our results indicate that G. sessilifolia extract and its fraction 18–22 containing genistin and isoprunetin, were able to induce anti‐cancer effects supporting the hypothesis of a pro‐apoptotic intrinsic content of this natural medicinal plant.
Introduction
The rational design of chemical compounds to target specific molecules often draws on components already existing in nature 1, 2. Thus, improving and understanding biological effects of natural compounds represents a significant opportunity in biomedical sciences. As yet, emerging fields such as molecular biology and combinatorial chemistry have not appeared to be able to satisfy the requirements of the pharmaceutical industries. For example, by exploiting their natural properties, validation of natural target compounds might become useful for treatment of human diseases such as cancer 3, 4, 5, 6, 7, 8.
In search of such beneficial compounds, a crucial prerequisite is their non‐toxicity to normal cells, and as a consequence, there has been a focus on investigating components of traditional medicine for possible anti‐cancer therapeutic use. Natural compounds, and particularly plant derivatives 9, are the subject of an increasing number of studies and growing interest is being directed to plants that are well known for general medicinal properties. Indeed, we (and others) have previously shown potential anti‐cancer effects of components of Psidium guajava 10 and Feijoa sellowiana 11.
The genus Genista L. (Leguminosae, GL) consists of 87 species predominantly distributed in the Mediterranean area 12. Composition of GL plants is characterized by presence of flavones, quinolizidine alkaloids 13, glycoflavones, and above all, of isoflavones, particularly substituted‐isoflavones such as 5‐methylgenistein and O‐glucosylated isoflavones 14. Daidzein, genistein and isoprunetin are the most representative compounds of the genus 15 and several Genista species have interesting biological properties, such as being hypoglycaemic, anti‐inflammatory, anti‐ulcer, spasmolytic, antioxidant, oestrogenic and cytotoxic in different human cancer cell lines 16, 17, 18. Genista sessilifolia DC. (syn. Cytisus sessilifolius L., GS) is a shrub with sessile or sub‐sessile leaves and yellow flowers collected in racemes. GS occurs in two rather separate areas, one through Anatolia, and the other in south‐eastern European countries such as Bulgaria, southern Romania, Macedonia, northern Greece 19, 20 and Italy, where it spreads though woods up to 800 m above sealevel 12. Previous reports indicate anabolic and anti‐inflammatory properties of flavonoids extracted from the plant 21. Recently, we have studied effects of methanolic extracts from the aerial parts of G. sessilifolia DC. and G. tinctoria L., on pBR322 DNA cleavage induced by hydroxyl radicals ( OH) generated from UV‐photolysis of hydrogen peroxide (H2O2), and by nitric oxide (NO), and cell population growth inhibitory activity of these natural products, to human melanoma cell line (M14) 22. Previous results have shown that isoflavonoid components of extracts of G. sessilifolia and G. tinctoria, have prevented UV light and nitric oxide‐mediated plasmid DNA damage and attenuated population growth of malignant melanoma cells, probably triggering apoptotic processes 22. Here, we report biological effects of G. sessilifolia DC. extracts, which include induction of cell cycle arrest and apoptosis. In fractionation experiments, we have been able to identify fraction 18–22 content of genistin and isoprunetin as active against malignant cells.
Materials and methods
Preparation of methanol extracts of G. sessilifolia
Aerial parts of G. sessilifolia were collected at full flowering stage in April 2005, from plants growing in the ‘Parco Nazionale del Cilento’ (Salerno, Italy). A voucher specimen (SN 033) was deposited at the Herbarium Neapolitanum (NAP), Faculty of Pharmacy, University of Naples ‘Federico II’. For preparation of the extract, G. sessilifolia aerial parts (667 g) were air‐dried, cut into small pieces, then sequentially extracted by cold maceration with petroleum ether (40–60 °C) (3 × 2.5 l), CHCl3 (3 × 2.5 l) and CH3OH (3 × 2.5 l). After filtration, the methanol solution was concentrated under reduced pressure to provide 72.8 g gum. Samples of extracts were used for biological assays.
High‐performance liquid chromatography
Methanolic extract of G. sessilifolia was chromatographed in 2 g portions on a Sephadex LH‐20 (Pharmacia, Uppsala, Sweden) column, eluting with CH3OH to afford 35 fractions of 20 ml each. Fractions were analysed by TLC using n‐BuOH/CH3COOH/H2O (60:15:25, v/v) as eluent and Ce(SO4)2 in H2SO4 as spray reagent, and gathered according TLC analysis. Fractions 18–19 (1.28 g), 20–22 (590 mg), 23–26 (369.3 mg) and 27–30 (72 mg), shown to be the most active in preliminary biological assays (data not shown), were further purified by HPLC on a C18 μ‐Bondapak column, with the exception of fraction 23–26 that was constituted entirely of genistein (3) 15. Fraction 18–19 was eluted with CH3OH/H2O 50:50 to yield pure genistin (1; 33.1 mg; Rt = 7.5 min) 23. Fraction 20–22 was eluted with CH3OH/H2O 50:50 to yield pure isoprunetin (2; 3.4 mg; Rt = 15.5 min) 15. Fraction 27–30 was eluted with CH3OH/H2O 70:30 to yield pure orobol (4; 7.7 mg; Rt = 9.5 min) 15. Structures of these compounds were determined by comparison of their spectroscopic data (NMR and MS) with values found in the literature 15, 23.
Cell lines and culture conditions
NB4 cells (human acute promyelocitic leukaemia) were obtained by DSMZ and were routinely cultured, while MCF‐7, HeLa, MDA‐MB231, LnCaP and U937 cell lines were obtained from ATCC and routinely cultured. MCF‐7 and HeLa cells were grown at 37 °C in 5% CO2 atmosphere in Dulbecco's modified Eagle's medium (DMEM; Gibco, Paisley, NY, USA), supplemented with 5% foetal bovine serum (FBS; Gibco), 1% l‐glutamine, 1% ampicillin/streptomycin and 0.1% gentamicin. U937, MDA‐MB231, LnCaP and NB4 cells were grown at 37 °C in 5% CO2 atmosphere in RPMI‐1640 medium (Gibco), supplemented with 10% heat‐inactivated foetal bovine serum (FBS), 1% L‐glutamine, 1% ampicillin⁄streptomycin and 0.1% gentamicin. SAHA (kindly provided by Merck) and MS275 (Alexis, Vinci‐Biochem srl, Vinci, Italy) were resuspended in DMSO and used at final concentration of 5 μm. Cell morphological analysis (for all cell lines used) was performed using bright field light microscopy (20×).
Cell cycle analysis
Samples were applied to a FACS‐Calibur flow cytometer and analysed following standard procedures using Cell Quest software (Becton Dickinson, Milan, Italy) and ModFit LT version 3 Software (Verity Software House, Topsham, ME, USA), as previously reported 24.
FACS analysis of apoptosis
Apoptotic levels were measured as pre‐G1, analysed by FACS with Cell Quest software (Becton Dickinson), as previously reported 25, 26. As second assays, caspases 8, 9 and 7–3 detection (B‐Bridge) were performed as recommended by the suppliers and quantified using FACS (Becton Dickinson) analysis. Discrimination between necrosis and apoptosis was performed according to the manufacturer's instructions (ENZ‐51002). Briefly, 2 × 105 cells were treated with the compound of interest for 24 h. Cells were washed in cold PBS and resuspended in dual detection reagent, provided with the kit. After 15 min incubation at RT, samples were analysed using flow cytometry.
Granulocyte differentiation assay
Granulocyte differentiation analysis was carried out as previously described 25, 27. Briefly, NB4 cells were harvested and resuspended in 10 μl phycoerythrineconjugated CD11c (CD11c‐PE) (Pharmingen, San Diego, California, US). Control samples were treated with 10 μl PE conjugated mouse IgG1, incubated for 30 min at 4 °C in the dark, washed in phosphate‐buffered saline (PBS 1 × with 0.1% BSA) and resuspended in 500 μl PBS containing PI (0.25 μg⁄ml). Samples were analysed by FACS with Cell Quest software (Becton Dickinson). PI positive cells were excluded from the analysis.
Western blot analysis
Forty micrograms total protein extracts were separated on polyacrylamide gel and blotted as previously described 28. Western blots were performed for p21 (dilution 1:500; Transduction Laboratories, BD group), Rb and p53 (dilution 1:500; Santa Cruz, Santa Cruz, CA, USA). Total mitogen‐activated protein kinases (ERKs) (dilution 1:1000; Santa Cruz) were used to normalize for equal loading. To quantify TRAIL, 40 μg total protein extract was separated on 12% polyacrylamide gel and blotted. Western blots were performed of TNF‐related apoptosis inducing ligand (TRAIL) (dilution 1:200; Abcam, Cambridge, UK), ERKs (dilution 1:1000; Santa Cruz) being used for equal loading. For determination of BAD and Bcl2 expression levels, 40 μg total protein extracts were separated on 12% polyacrylamide gel and blotted. Antibodies used were: anti‐BAD (dilution 1:500; Cell Signaling #9292, Danvers, MA, USA) and anti‐Bcl2 (dilution 1:500, Bcl2 (Ab‐1); Oncogene Science, Cambridge, MA, USA). Total ERKs were used to normalize for equal loading.
RNA extraction
Cells were collected by centrifugation and resuspended in 1 ml of TRIzol reagent (Invitrogen ‐ Life Technologies, Monza, Italy), vigorously shaken, and stored at −20 °C overnight. The following day, samples were supplemented with 100 μl of 2‐bromo‐3‐chloro propane (Sigma Aldrich, St Louse, MO, USA), shaken gently and incubated for 15 min at RT. After centrifugation for 15 min, 16000 g at 4 °C, supernatants were collected and dispensed into fresh tubes and supplemented with 500 μl cold isopropylicalcohol. RNA precipitation reaction was carried out for 1 h at −80 °C and followed by centrifugation for 15 min, 13 000 rpm at 4 °C. Pellets were then resuspended in 1 ml cold 75% ethanol and samples were centrifuged again for 10 min, 5300 g at 4 °C. Pellets were subsequently dried at 42 °C for several minutes and resuspended in DEPC‐treated H2O. RNA samples were quantified using a Nanodrop 1000 spectrophotometer.
Real‐time PCR and primers
RNA samples were converted into c‐DNA using VILO Invitrogen kit: 2 μg RNA was mixed with 1x VILO reaction mixture, 1x Super‐Script Mix and DEPC‐H2O; samples were then incubated for 10 min at 25 °C, 60 min at 42 °C and 5 min at 85 °C. RT‐PCR experiments for TRAIL, DR5, p21 and GAPDH were then performed. Thermal protocol was as follows: 95 °C for 5 min plus 35 cycles at 95 °C for 30 s, 60 °C for 30 s and 72 °C for 45 s, with final elongation of 10 min at 72 °C. For amplification, the following primers were used: TRAIL forward (5′‐CAA CTC CGT CAG CTC GTT AGA‐3′) and reverse (5′‐TTA GAC CAA CAA CTA TTT CTA‐3′); DR5 forward (5′‐TGC AGC CGT AGT CTT GAT TG‐3′) and reverse (5′‐TCC TGG ACT TCC ATT TCC TG‐3′); p21 forward (5′‐GAC AAC CTCA CTC GTC AAA TC‐3′) and reverse (5′‐ACA GCA CTG TAA GAA TGA GC‐3′); GAPDH forward (5′‐GGA GTC AAC GGA TTT GGT‐3′) and reverse (5′‐CTT CCC GTT CTC AGC CTT‐3′).
Results
Total GS extract induced anti‐malignant effects in several cell models of cancer
Genista sessilifolia had anti‐malignant cell activity and this property was initially confirmed by using total GS extract at different concentrations, for times of 24 and 48 h, on NB4 human acute promyelocitic leukaemia cells (APL) (Fig. 1a). Interestingly, effects of GS total extract were linear and dose‐dependent (Fig. 1a). In agreement, morphological experiments clearly showed anti‐proliferative effects on the NB4 cells after 24 h, suggesting that with GS incubation, they underwent proliferative arrest and apoptosis (Fig. 1b). When cell cycle effects were analysed, treatment with total GS extract (within ranges of biologically active doses) induced G1 cell cycle block (Fig. 1c) of around 70% at concentrations of 0.5/0.75/1.50 mg/ml. To establish whether GS extract was also able to induce differentiation, presence of CD11c, a specific marker for granulocytic differentiation, was evaluated on the NB4 cells after 48 h treatment. CD11c expression increased, even at concentration of 0.5 mg/ml, (Fig. 1d). Nevertheless, the differentiative effect was lower than that of MS (MS275, Entinostat), a well‐known anti‐tumour and pro‐differentiative agent 25, 26, 27, 29. To corroborate these data in different leukaemia models, we tested anti‐cancer activity of GS on U937 cells after 48 h treatment. Both morphology and apoptosis evaluation indicate that action of GS was not restricted to NB4 cells, but was a more general anti‐leukaemic cell property(Fig. 2a). To verify whether solid cancer model cell types would also respond to GS, with arrest of proliferation and apoptosis, we tested it on breast cancer (MCF7, MDA‐MB231), cervical cancer (Hela) and prostate cancer (LNCaP) cell lines. As shown in Fig. 2b–e, all cancer cells displayed arrest of proliferation, and cell death, after 48 h GS incubation, despite some differences in sensitivity, MDA‐MB231 and U937 cells being more sensitive than MCF7, Hela and LNCaP cells.
Figure 1.
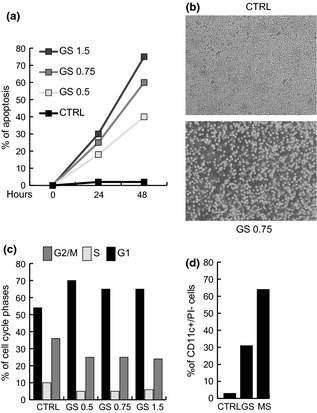
Genista sessifolia methanolic extract exerts anti‐proliferative and differentiative action in leukaemia NB 4 cells. (a) Apoptotic curve of NB4 cells at 24 and 48 h after treatment with G. sessifolia methanolic extract at reported concentrations. (b) Morphological analysis of NB4 cells after 24 h treatment with G. sessifolia methanolic extract at reported concentration. (c) Cell cycle analysis of NB4 cells at 24 h after treatment with G. sessifolia methanolic extract at reported concentrations. Results represent average of triplicates. (d) CD11c expression levels measured by FACS after 48 h treatment with indicated amounts of G. sessifolia methanolic extract, on NB4 cells. Note: PI positive cells were excluded from the analysis. MS (MS‐275) used as positive pro‐differentiative compound.
Figure 2.
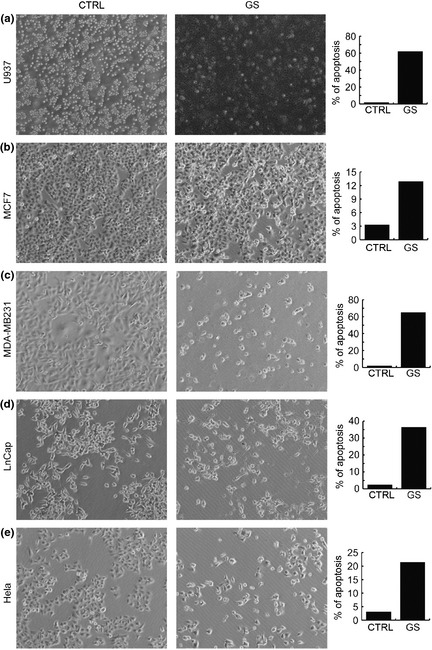
Genista sessifolia methanolic extract exerts anti‐cancer action in different cancer cell line models. (a) Morphological analysis and percentage of apoptosis in U937 cells after 48 h treatment with G. sessifolia methanolic extract at indicated concentration. (b) Morphological analysis and percentage apoptosis, in MCF7 breast cancer cells after 48 h treatment with G. sessifolia methanolic extract at indicated concentration. (c) Morphological analysis and percentage of apoptosis of MDA‐MB‐231 breast cancer cells after 48 h treatment with G. sessifolia methanolic extract at indicated concentration. (d) Morphological analysis and percentage of apoptosis in LNCaP prostate cancer cells after 48 h treatment with G. sessifolia methanolic extract at indicated concentration (e) Morphological analysis and percentage of apoptosis in Hela cervical cancer cells after 48 h treatment with G. sessifolia methanolic extract at indicated concentration.
GS extract mediated caspase activation and apoptotic molecular events
To investigate molecular events underlying GS‐induced cell death, caspase assays (caspase 8, 9 and caspase 3/7) were performed. As shown in Fig. 3a, caspases 8, 9 and 3/7 were mainly activated, suggesting that cell death was due to apoptosis of NB4 cells. To evaluate which molecular players might be involved in this anti‐cancer action of GS total extract, expression levels of different known key factors in cell cycle progression and apoptosis were analysed by western blot analysis, in NB4 cells. As shown in Fig. 3b, after 48 h induction, total GS extract induced expression of p21, a known cell cycle inhibitor, and Rb, recognized to negatively regulate G1‐S transition. Taken together, these data confirm the observation on cell cycle block (Figs 3b,1b). Under similar experimental conditions, expression levels of pro‐ and anti‐apoptotic proteins were analysed. Interestingly, whereas Bcl2 remained unchanged, p53, TRAIL and BAD appeared to be induced after treatment with total GS extract (Fig. 3b), validating the pro‐apoptotic action of the compound. In particular, that both mitochondrial (intrinsic) and death receptor (extrinsic) apoptotic pathways were activated strengthens the evidence suggested by caspase 8 and 9 activation (Fig. 2a).
Figure 3.
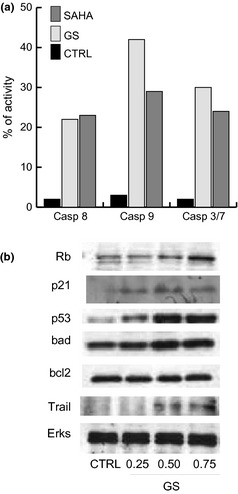
Genista sessifolia methanolic extract induced apoptosis by caspase activation in NB 4 cells. (a) Apoptosis measured by caspase activation with FACS analysis in NB4 cells after 24 h induction with G. sessifolia methanolic extract. Data show average of triplicates. (b) Western blot evaluation of Rb, p21, p53, Bad, Bcl2 and TRAIL in NB4 cells after 24 h treatment with indicated concentrations of GS. Total Erks for equal loading.
Selective GS fractions induced anti‐malignant effects on acute promyelocitic leukaemia NB4 cells
To investigate different components of the GS total extract involved in its anti‐cancer effects, the extract was chromatographed to afford 35 fractions; these were then analysed for their action with respect to apoptosis, showing that fractions from 18 to 22 18, 19, 20, 21, 22 gave rise to strong increase in pre‐G1 phase (apoptotic fraction), whereas fractions 6–17 and 27–30 caused weaker increases in cell death (Fig. 4a). When CD11c was measured as a marker of differentiation, alone fractions 11–13 and 27–30 induced only a weak increase in CD11c expression at 48 h treatment (Fig. 4b). In comparison to total extract, effects of fractions 11–13 and 27–30 were much lower, suggesting that both fractions might contribute to the effect of GS total extract on induction of differentiation. Finally, in agreement with biological analyses shown in Fig. 4a, when fractions 18–22 had been tested by western blot analyses, stronger induction of effectors of apoptosis such as TRAIL and DR5 were detected at 0.2 mg/ml concentration at 48 h treatment (Fig. 4c), thus corroborating that components contained in these fractions accounted for cell death property of GS. Strongest apoptotic response triggered by fractions 18–19 and 20–22 were also confirmed by cytofluorimetric analysis using annexin V (Fig. 4d). Moreover, qPCR analyses of TRAIL, its death receptor DR5 and p21 indicate that there was modulation in fractions used (Fig. 4d). In particular, TRAIL was highly expressed in fraction 20–22 already at 0.2 mg/ml, while DR5 and p21 induction were more detectable at higher concentrations.
Figure 4.
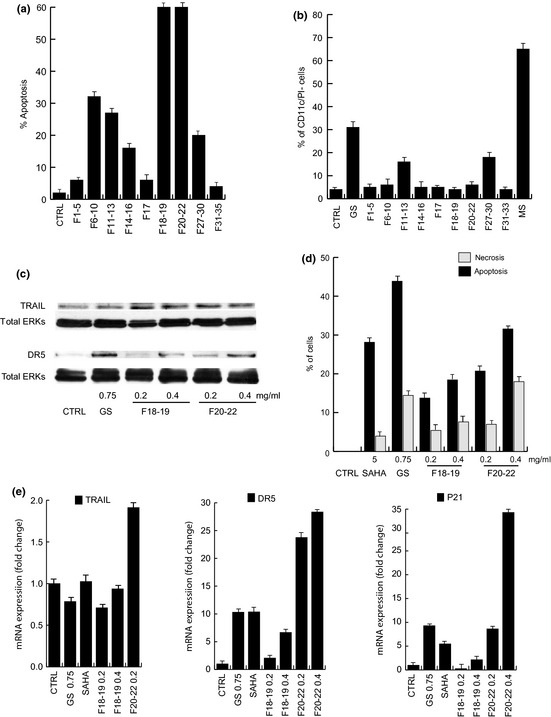
Genista sessifolia fraction induced apoptosis in NB 4 cells. (a) Apoptotic curve of NB4 cells at 24 h after treatment with G. sessifolia fractions at concentration of 0.2 mg/ml. (b) CD11c expression levels on treatment with G. sessifolia fractions at concentration of 0.2 mg/ml for 48 h in NB4 cells. MS (MS‐275) was used as positive pro‐differentiative compound. (c) Western blot analysis of fraction 18–22 actions on TRAIL and DR5 induction at indicated concentrations, for 24 h. Total Erks account for equal loading. (d) Percentage of apoptotic and necrotic cells by GFP‐certified apoptosis/necrosis detection kit using flow cytometry. SAHA was used as positive control for apoptotic induction. (e) Real‐time PCR for TRAIL, DR5 and p21 for 18–19 and 20–22 fractions at the indicated concentrations.
Quantification of isoflavonoids
To determine major constituents, methanolic extract of G. sessilifolia was fractionated over Sephadex LH‐20, yielding as the main constituents, genistin (1) 3, 30, 31, 32, isoprunetin (2), genistein (3) and orobol (4) 22 (Fig. 5). These compounds were quantified using HPLC‐PAD. Results of quantitative analyses of the isoflavones in methanolic extracts of G. sessilifolia, are shown in Table 1. Compounds were identified by comparison of their retention times (t R), UV spectra and MS, with reference samples (Table 1); purity of peaks was checked by PAD (200–400 nm). UV spectra recorded at three different points per peak (up‐slope, apex and down‐slope) were compared with UV spectrum of the reference standard. Quantitative determination of genistin, isoprunetin, genistein and orobol was performed directly by HPLC‐PAD using five‐point regression curves in the range 1–25 μg/ml; all samples were injected three times at each level. Calibration curves for each compound, made by linear regression by graph, reporting area ratio of external standard against known concentration of external standard, were linear in the range 1–25 μg/ml for all isoflavones. Three aliquots of the methanol extract of G. sessilifolia were analysed to quantify genistin, isoprunetin, genistein and orobol. Results of quantitative analyses are shown in Table 1. Four isoflavones occurred as major constituents of G. sessilifolia (by methanolic extraction), in particular genistin and genistein were the most abundant compounds.
Figure 5.
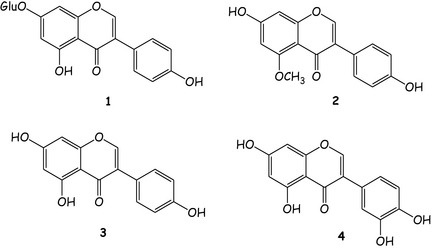
Active components of Genista sessifolia fractions: (1) Genistin; (2) Isoprunetin; (3) Genistein; (4) Orobol.
Table 1.
Results of quantitative analysis
| Compound | r 2 | t R (SD) | mg of compound/g of Genista sessifolia MeOH extract (SD) |
|---|---|---|---|
| Genistin (1) | 0.9999 | 18.66 (0.08) | 286.64 (3.41) |
| Isoprunetin (2) | 0.9997 | 25.45 (0.13) | 42.31 (0.13) |
| Genistein (3) | 0.9993 | 35.98 (0.18) | 123.54 (2.61) |
| Orobol (4) | 0.9997 | 29.01 (0.16) | 33.98 (2.41) |
r = linear regression; SD = standard deviation.
Discussion
Plants are an appreciated and natural font of therapeutic agents 33, 34, 35, 36. At present, it is generally documented that intake of a number of native herbs and vegetables significantly contributes to improvement in human health, in terms of prevention and treatment of disease 37, 38, 39, 40, 41, 42. In the region of 80% of existing drugs originate from medicinal plants, including several anti‐tumoural agents as Paclitaxel (taxol) from Taxus brevifolia 43, 44, 45, 46. Frequently in industrialized countries, raw ingredients used to synthesize pure chemicals originate from plants. The genus Genista L. (Leguminosae, GL), consisting of 87 species, is mostly found spread across the Mediterranean area 12. Genista L. is characterized by the presence of flavones, glycoflavones, and isoflavones, particularly substituted‐isoflavones such as 5‐methylgenistein and O‐glucosylated isoflavones 14, whil daidzein, genistein and isoprunetin are the most represented of such compounds of the genus 15. Several Genista species have medical properties such as being hypoglycaemic, anti‐inflammatory, anti‐ulcer, spasmolytic, antioxidant, oestrogenic and as having cytotoxic activities against different human cancer cells 16, 17. Recently, we have studied effects of extracts from aerial parts of G. sessilifolia DC. and Genista tinctoria L. on pBR322 DNA cleavage induced by hydroxyl radicals (OH) generated from UV‐photolysis of hydrogen peroxide (H2O2) and by nitric oxide (NO) and cell population growth inhibitory activity of these natural products against human malignant melanoma cells 22.
Although a variety of components are present within them, the main one connected to anti‐cancer action is genistin. Experimental indication that crude methanol extract was able to induce cell cycle arrest and cell death in NB4 cells confirmed our hypothesis that some components of the plant have anti‐cancer activity. Methanol extract from G. sessilifolia was also capable of inducing CD11c expression (taken as ability to differentiate) in acute promyelocytic leukaemia (APL) cells, despite lower levels than of known differentiating agents such as MS (MS‐275, Entinostat). Apoptosis evaluation in different cancer cell line models indicated that anti‐malignant action of GS is not restricted to NB4 cells, but is a general anti‐cancer property, active in additional leukaemia models (U937) and against solid cancer cells (LnCaP, MDA‐MB231, MCF7 and Hela). All cancer cell models tested here with GS methanol extract showed sensitivity (Fig. 2b–e); differential effects shown by different cancer cell lines. MDA‐MB231 and U937 cells were more sensitive than MCF7, Hela and LNCaP cells, suggesting both some cell‐selectivity and very low toxic effect of the extract.
Interestingly, we demonstrated that cell death, occurring on GS treatment, was caspase‐mediated and able to activate both cell cycle and molecular death programmes (Fig. 3). Both initiator caspases (caspases 8 and 9) and effectors (caspases 3/7) were active after GS treatment, accompanied by induction of p21, Rb, p53, Bad and TRAIL (Fig. 3). These lines of evidence strongly suggest involvement of death receptors and mitochondrial‐mediated death pathways in regulation of apoptosis induced by GS extract. Intrigued by these data, we set out to determine the most biologically active fraction and identified fractions 18–22 as responsible for apoptotic action of GS extracts in cancer cells tested. In agreement, fractions 18–22 were able to induce apoptosis as demonstrated by FACS analysis and by induction of TRAIL and DR5 (Fig. 4). In contrast, fractions 11–13 and 27–30 were the only ones able to slightly alter differentiation capability (as CD11c expression), indicating that multi‐component action was needed to modulate CD11c expression levels in these settings. Interestingly, fractions 18–19 main component was genistin (Fig. 5) whose anti‐cancer potential has already been recognized 31, 47, 48, 49. Interestingly, fractions 20–22 main component was isoprunetin whose anti‐cancer 50 and anti‐oestrogenic 51 potentials have also been suggested.
Taken together, these data provide a new perspective for analysis and use of natural products in treatment of human pathologies, and indicate that plant components exert anti‐cancer activities. Furthermore, identification of two components, genistin and isoprunetin, that account for the anti‐cancer potential of GS, corroborates the knowledge of genistin action against cancer and strongly support further investigations of isoprunetin anti‐cancer action. Given the need for new approaches into anti‐cancer treatments, in‐depth investigation of properties of natural products with minor side effects and targeted action represent a new approach for the future development of cancer‐selective drugs and prevention strategies.
Acknowledgements
The authors thank Dr. Diego Di Novella for kindly providing the plant. Thanks are also to the C.S.I.A.S of University ‘Federico II’ of Naples for technical assistance. This study was supported by EU (‘Blueprint contract no. 282510), by AIRC (Associazione Italiana per la Ricerca sul Cancro project no. 4625), by MIUR (PRIN_2009PX2T2E_004). We acknowledge Dr C. Fisher for kindly revising the manuscript. The authors declare that they have no conflict of interests.
References
- 1. Camp D, Davis RA, Campitelli M, Ebdon J, Quinn RJ (2012) Drug‐like properties: guiding principles for the design of natural product libraries. J. Nat. Prod. 75, 72–81. [DOI] [PubMed] [Google Scholar]
- 2. Hann MM, Keserü GM (2012) Finding the sweet spot: the role of nature and nurture in medicinal chemistry. Nat. Rev. Drug Discov. 11, 355–365. [DOI] [PubMed] [Google Scholar]
- 3. Cragg GM, Newman DJ (2005) Plants as a source of anti‐cancer agents. J. Ethnopharmacol. 1–2, 72–79. [DOI] [PubMed] [Google Scholar]
- 4. Ahmad A, Sakr WA, Rahman KM (2012) Novel targets for detection of cancer and their modulation by chemopreventive natural compounds. Front. Biosci. (Elite Ed) 4, 410–425. [DOI] [PubMed] [Google Scholar]
- 5. Mans DRA, da Rocha AB, Schwartsmann G (2000) Anti‐cancer drug discovery and development in Brazil: targeted plant collection as a rational strategy to acquire candidate anti‐cancer compounds. Oncologist 5, 185–198. [DOI] [PubMed] [Google Scholar]
- 6. Qurishi Y, Hamid A, Majeed R, Hussain A, Qazi AK, Ahmed M et al (2011) Interaction of natural products with cell survival and signaling pathways in the biochemical elucidation of drug targets in cancer. Future Oncol. 7, 1007–1021. [DOI] [PubMed] [Google Scholar]
- 7. Russo P, Nastrucci C, Cesario A (2011) From the sea to anticancer therapy. Curr. Med. Chem. 18, 3551–3562. [DOI] [PubMed] [Google Scholar]
- 8. Shynu M, Gupta PK, Saini M (2011) Antineoplastic potential of medicinal plants. Recent Pat. Biotechnol. 5, 85–94. [DOI] [PubMed] [Google Scholar]
- 9. Verma PK, Bala M, Kumar N, Singh B (2012) Therapeutic potential of natural products from terrestrial plants as TNF‐α antagonist. Curr. Top. Med. Chem. 12, 1422–1435. [DOI] [PubMed] [Google Scholar]
- 10. Bontempo P, Doto A, Miceli M, Mita L, Benedetti R, Nebbioso A et al (2012) Psidium guajava L. anti‐neoplastic effects: induction of apoptosis and cell differentiation. Cell Prolif. 1, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bontempo P, Mita L, Miceli M, Doto A, Nebbioso A, De Bellis F et al (2007) Feijoa sellowiana derived natural Flavone exerts anti‐cancer action displaying HDAC inhibitory activities. Int. J. Biochem. Cell Biol. 10, 1902–1914. [DOI] [PubMed] [Google Scholar]
- 12. Pignatti S (1982) Edagricole (Ed.). Flora d'Italia (Bologna) 1, 636. [Google Scholar]
- 13. Martins A, Wink M, Tei A, Brum‐Bousquet M, Tillequin F, Rauter AP (2005) A phytochemical study of the quinolizidine alkaloids from Genista Tenera by gas chromatography‐mass spectrometry. Phytochem. Anal. 16, 264–266. [DOI] [PubMed] [Google Scholar]
- 14. Harborne JB (1969) Chemosystematics of the Leguminosae. Flavonoid and isoflavonoid patterns in the tribe Genisteae. Phytochemistry 8, 1449–1456. [Google Scholar]
- 15. Harborne JB, Turner BL (1984) Plant Chemosystematics. London, UK: Academy Press. [Google Scholar]
- 16. Ilarionov I, Rainova L, Nakov N (1979) Antinflammatory and antiulcer effect of some flavonoide isolated from the genus Genista. Farmatsiya 29, 39–46. [Google Scholar]
- 17. Raja S, Ahamed HN, Kumar V, Mukerjee K, Bandyopadhyay A, Mukherjee PK (2007) Exploring the effect of Cytsius Scoparius on markers of oxidative stress in rats. Iranian J. Pharmacol. Ther. 6, 15–21. [Google Scholar]
- 18. Rigano D, Russo A, Formisano C, Cardile V, Senatore F (2010) Antiproliferative and cytotoxic effects on malignant melanoma cells of essential oils from the aerial parts of Genista sessilifolia and G. tinctoria . Nat. Prod. Commun. 5, 1127–1132. [PubMed] [Google Scholar]
- 19. Gibbs PE (1966) A revision of the genus Genista L. Notes Royal Botanic Garden Edinburgh 27, 11–99. [Google Scholar]
- 20. Greuter W, Burdet HM, Long G (1989) Conservatoire et Jardin botaniques, Genève. Med‐Checklist 4, 91–105. [Google Scholar]
- 21. Ilarinov I (1988) Pharmacologic effects on the reproductive system and anti‐inflammatory action of the total flavonoid mixtures contained in Genista tintoria and Genista sessilifolia . Farmatsiya 38, 47–51. [Google Scholar]
- 22. Rigano D, Cardile V, Formisano C, Maldini MT, Piacente S, Bevilacqua J et al (2009) Genista sessilifolia DC. and Genista tinctoria L. inhibit UV light and nitric oxide‐induced DNA damage and melanoma cell growth. Chem. Biol. Interact. 180, 211–219. [DOI] [PubMed] [Google Scholar]
- 23. Rainova L, Nakov N, Bogdanova S, Minkov E, Staneva‐Stoicheva D (1988) Ulceroprotective activity of the flavonoide of Genista rumelica Vel. Phytother. Res. 2, 137–139. [Google Scholar]
- 24. De Luca A, Baldi A, Russo P, Todisco A, Altucci L, Giardullo N et al (2003) Coexpression of Helicobacter pylori's proteins CagA and HspB induces cell proliferation in AGS gastric epithelial cells, independently from the bacterial infection. Cancer Res. 63, 6350–6356. [PubMed] [Google Scholar]
- 25. Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P et al (2005) Tumor‐selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 11, 77–84. [DOI] [PubMed] [Google Scholar]
- 26. Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H (2001) Retinoic acid‐induced apoptosis in leukemia cells is mediated by paracrine action of tumor‐selective death ligand TRAIL. Nat. Med. 7, 680–686. [DOI] [PubMed] [Google Scholar]
- 27. Altucci L, Gronemeyer H (2001) The promise of retinoids to fight against cancer. Nat. Rev. Cancer 1, 181–193. [DOI] [PubMed] [Google Scholar]
- 28. Mai A, Massa S, Rotili D, Simeoni S, Ragno R, Botta G et al (2006) Synthesis and biological properties of novel uracil‐containing histone deacetylase inhibitors. J. Med. Chem. 49, 6046–6056. [DOI] [PubMed] [Google Scholar]
- 29. Altucci L, Wilhelm E, Gronemeyer H (2004) Leukemia: beneficial actions of retinoids and rexinoids. Int. J. Biochem. Cell Biol. 36, 178–182. [DOI] [PubMed] [Google Scholar]
- 30. Walter ED (1941) Genistin (an isoflavone glucoside) and its aglucone, genistein, from soybeans. J. Am. Chem. Soc. 62, 3273–3276. [Google Scholar]
- 31. Coward L, Barnes NC, Setchell KDR, Barnes S (1993) Genistein, daidzein, and their β‐glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J. Agric. Food Chem. 41, 1961–1967. [Google Scholar]
- 32. Du H, Huang Y, Tang Y (2010) Genetic and metabolic engineering of isoflavonoid biosynthesis. Appl. Microbiol. Biotechnol. 86, 1293–1312. [DOI] [PubMed] [Google Scholar]
- 33. Cragg GM, Newmann DJ, Snader KM (1997) Natural products in drug discovery and development. Nat. Prod. 60, 52–60. [DOI] [PubMed] [Google Scholar]
- 34. Harvey AL (1999) Medicines from nature: are natural products still relevant to drug discovery? Trends Pharmacol. Sci. 20, 196–198. [DOI] [PubMed] [Google Scholar]
- 35. Farnsworth NR (1984) The role of medicinal plants in drug development In: Krogsgaard‐Larsen P, Christensen SB, Kofod H, eds. Natural Products and Drug Development, pp. 8–98. London, UK: Ballière, Tindall, and Cox. [Google Scholar]
- 36. Cox PA (1994) The ethnobotanical approach to drug discovery: strengths and limitations In: Chadwick DJ, Marsh J, eds. CIBA Foundation Symposium 185—Ethnobotany and the Search for New Drugs, pp. 25–47. Chichester, UK: John Wiley & Sons. [PubMed] [Google Scholar]
- 37. Kapiszewska M (2006) A vegetable to meat consumption ratio as a relevant factor determining cancer preventive diet. The Mediterranean versus other European countries. Forum Nutr. 59, 130–153. [DOI] [PubMed] [Google Scholar]
- 38. Shukla Y, George J (2011) Combinatorial strategies employing nutraceuticals for cancer development. Ann. N. Y. Acad. Sci. 1229, 162–175. [DOI] [PubMed] [Google Scholar]
- 39. Amin AR, Kucuk O, Khuri FR, Shin DM (2009) Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 27, 2712–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khan N, Afaq F, Mukhtar H (2008) Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid. Redox Signal. 10, 475–510. [DOI] [PubMed] [Google Scholar]
- 41. Kris‐Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF et al (2002) Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 113, 71S–88S. [DOI] [PubMed] [Google Scholar]
- 42. Trovato GM (2012) Behavior, nutrition and lifestyle in a comprehensive health and disease paradigm: skills and knowledge for a predictive, preventive and personalized medicine. EPMA J. 3, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gotaskie GE, Andreassi BF (1994) Paclitaxel: a new antimitotic chemotherapeutic agent. Cancer Pract. 2, 27–33. [PubMed] [Google Scholar]
- 44. Ferguson PJ, Phillips JR, Selner M, Cass CE (1984) Differential activity of vincristine and vinblastine against cultured cells. Cancer Res. 44, 3307–3312. [PubMed] [Google Scholar]
- 45. Cormio G, Loizzi V, Gissi F, Camporeale A, De Mitri P, Leone L et al (2011) Long‐term topotecan therapy in recurrent or persistent ovarian cancer. Eur. J. Gynaecol. Oncol. 32, 153–155. [PubMed] [Google Scholar]
- 46. Sinkule JA (1984) Etoposide: a semisynthetic epipodophyllotoxin. Chemistry, pharmacology, pharmacokinetics, adverse effects and use as an antineoplastic agent. Pharmacotherapy 4, 61–73. [DOI] [PubMed] [Google Scholar]
- 47. Hooshmand S, Khalil DA, Murillo G, Singletary K, Kamath SK, Arjmandi BH (2008) The combination of genistin and ipriflavone prevents mammary tumorigenesis and modulates lipid profile. Clin. Nutr. 27, 643–648. [DOI] [PubMed] [Google Scholar]
- 48. Choi EJ, Kim T, Lee M‐S (2007) Pro‐apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK‐OV‐3 cells. Life Sci. 80, 1403–1408. [DOI] [PubMed] [Google Scholar]
- 49. Russo A, Cardile V, Lombardo L, Vanella L, Acquaviva R (2006) Genistin inhibits UV light‐induced plasmid DNA damage and cell growth in human melanoma cells. J. Nutr. Biochem. 17, 103–108. [DOI] [PubMed] [Google Scholar]
- 50. Feng S, Hao J, Xu Z, Chen T, Qiu SX (2011) Polyprenylated isoflavanone and isoflavonoids from Ormosia henryi and their cytotoxicity and anti‐oxidation activity. Fitoterapia 83, 161–165. [DOI] [PubMed] [Google Scholar]
- 51. Pinto B, Bertoli A, Noccioli C, Garritano S, Reali D, Pistelli L (2008) Estradiol‐antagonistic activity of phenolic compounds from leguminous plants. Phytother. Res. 22, 362–366. [DOI] [PubMed] [Google Scholar]


