Abstract
Objectives: Keratinocyte stem/progenitor cells (KSCs) are known to regenerate epidermal tissue which they perform through to their great regenerative capacity.
Materials and methods: Because stimulation of resident KSCs may regenerate epidermal tissue, we devised a strategy to find an appropriate KSC activator from natural products and to develop it as a skin‐rejuvenating agent.
Results: Ent‐16α, 17‐dihydroxy‐kauran‐19‐oic acid (DHK) isolated from Siegesbeckia pubescens exhibited a KSC‐stimulating effect during screening of natural products. DHK increased proliferation and migration of KSCs using the Akt/ERK pathway. We further examined the mechanism of KSC stimulation and found that phosphorylation of Y1068 epithelial growth factor receptor (EGFR) was significantly increased. Functional inhibition of EGFR using neutralizing antibody and a chemical inhibitor, AG1478, attenuated DHK‐induced KSC stimulation. In a 3D culture model of KSCs, DHK treatment significantly induced establishment of fully stratified epidermis and increased numbers of p63‐positive cells. Likewise, DHK treatment significantly accelerated healing of epidermal wounds created by laser and dermatome, and increased p63‐positive cells, in animal models.
Conclusion: Collectively, these results indicate that DHK regenerates epidermal tissue mainly through EGFR phosphorylation. As DHK has diverse advantages over recombinant growth factors for commercialization (that is long‐term stability and skin permeability), DHK might be applied to wound‐healing agents and to a basic materials used in cosmetics.
Introduction
Epidermis, the outermost component of the skin, is the primary barrier that protects the body from dehydration, mechanical trauma and microbial insult. This layer regenerates throughout life to replace dead sloughed‐off skin. The epidermis is ectodermal in origin and is comprised of four sub‐layers of epidermal cells, including keratinocyte stem/progenitor cells (KSCs), transit‐amplifying cells and terminally differentiated keratinocytes (1, 2, 3). KSCs reside in at least two locations, a bulge region of the hair follicle and in non‐random distributions within the basal layer of inter‐follicular epithelium (4, 5, 6). KSCs are generally quiescent with low tendency to divide, under normal conditions, but they exhibit an extensive self‐renewal capacity upon injury. During epidermal regeneration processes, KSCs undergo self‐renewal and generate transit‐amplifying cells that rapidly divide to provide skin with new epidermal cells. Thus, several lines of evidence have demonstrated an important role for KSCs in skin‐regeneration and wound‐healing processes (2, 7, 8). For example, Li et al. (7) have reported that KSCs readily regenerated fully stratified epidermis in short‐term organotypic culture systems. In addition, KSCs exhibit rapid in vivo epidermal regeneration compared to unfractionated human foreskin keratinocytes or transit amplifying cells (7). Skin rejuvenation by activation of resident KSCs is a promising strategy for development of new wound‐healing agents and a new basic materials for cosmetic use.
Conventional growth factors such as epithelial growth factor (EGF) have been extensively developed for wound‐healing agents and cosmetics, however, they are unstable and expensive thus limit their wide‐scale application. Thus, we decided on a strategy to search and develop natural products that activate KSCs and replace conventional growth factors. We screened KSC activators using natural products and found that the methanol extract of Siegesbeckia pubescens increases proliferation of KSCs. Therefore, we identified chemical components of this methanol fraction and isolated them from S. pubescens. Of these products, one major compound, ent‐16α, 17‐dihydroxy‐kauran‐19‐oic acid (DHK) exhibited stimulatory effects on KSCs (Fig. 1). In the present study, we report the KSC stimulatory effect of DHK and mechanism of its action for epidermal regeneration. Human primary KSCs were used to measure the effect of DHK on proliferation and migration of KSCs. In addition, epidermal regeneration by DHK has been demonstrated in 3D culture of KSCs, which was followed by a laser‐induced wound model and an excision wound model. Mechanism of KSC activation was further characterized by western blot analysis and inhibition studies.
Figure 1.
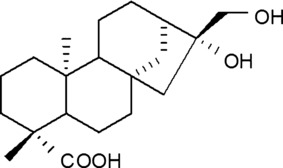
Chemical structure of ent‐16α, 17‐dihydroxy‐kauran‐19‐oic acid (DHK).
Materials and methods
Isolation of DHK from S. pubescens
Dried leaves of S. pubescens (Compositae) were purchased from the Kyungdong traditional herbal market (Seoul, Korea). The plant material (5 kg) was ground and extracted with 80% methanol (3 h × 4) by ultrasonication at room temperature. The methanol extract (433.0 g) was then suspended in H2O and partitioned successively with CH2Cl2 and n‐butanol. Resultant CH2Cl2 extract (237.6 g) was suspended in 90% methanol and extracted with n‐hexane. Residual 90% methanol fraction (110.7 g) was chromatographed on a silica gel column using a CHCl3‐MeOH gradient as mobile phase, to afford eleven fractions (M1 ∼ 11). A white powdery compound (961.0 mg) was purified by recrystallization in MeOH and this isolated compound was identified as DHK from spectral data (Figs S2 and S3).
KSC culture and characterization
KSCs were obtained from CELLnTEC (Bern, Switzerland) and grown in CnT‐57 medium (CELLnTEC) at 37 °C in a humidified incubator in an atmosphere of 5% CO2 and 95% air. KSCs were characterized by fluorescence‐activated cell‐sorting (FACS) analysis using antibodies to integrin α6 and CD71.
KSC proliferation
Cells were seeded at density of 5000 cells/cm2 in 48‐well plates. After 24‐h incubation, culture medium was replaced by new medium containing a range of concentrations of DHK (1, 5, 10, 50 and 100 μm), and cells were then incubated for 72 h to allow them to proliferate. A cell proliferation assay was performed using a Cell‐Counting Kit‐8 (CCK‐8; Dojindo Laboratories, Kumamoto, Japan) and CCK‐8 solution (150 μl) was added and each well was incubated for 2 h. After incubation, absorbance was measured at 450 nm using a microplate reader (Tecan, Grodig, Austria). In addition, inhibition studies were expanded using DHK and examples of inhibitors (U0126, LY204002, AG1478 and EGF receptor (EGFR) neutralizing antibody).
KSC migration
For measurement of cell migration, confluent KSCs were incubated in serum‐free medium for 24 h and ‘wounded’ with a plastic micropipette tip with a large orifice. After washing, medium was replaced by new medium containing DHK. Photographs of the wounded area were taken every 24 h by phase‐contrast microscopy. For evaluation of migration distance, four randomly selected points along each wound were marked, and horizontal distance of migrating cells from the initial wound was measured. In addition, inhibition studies were expanded using DHK and inhibitors.
Western blot analysis
Cells were lysed with RIPA buffer (50 mm Tris–HCl, 0.15 m NaCl, 1 mm EDTA, 1% Triton X‐100, 1% SDS, 50 mm NaF, 1 mm Na3VO4, 5 mm dithiothreitol, 1 mg/ml leupeptin and 20 mg/ml PMSF, pH 7.4). Fifty micrograms of protein was separated on an 8–10% SDS–polyacrylamide gel, by electrophoresis. Proteins were transferred to PVDF membranes and were incubated with antibodies to phospho‐ERK1/2, total‐ERK1/2, phospho‐Akt, total‐Akt, phospho‐EGFR(Tyr1068), EGFR (Cell Signaling Technology, Danvers, MA, USA) and β‐actin (Santa Cruz, St. Louis, MO, USA). Then, membranes were washed and incubated with horseradish peroxidase‐conjugated anti‐rabbit IgG antibody (Santa Cruz). Blots were reacted with immunobilon western reagent and exposed to X‐ray film.
Protein tyrosine phosphatase inhibition assay
KSCs were washed twice in ice‐cold hypotonic buffer (20 mm Tris–HCl, pH 7.6 with 10 mm NaCl) and supplemented with 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstain A and 1 mm phenylmethylsulfonyl fluoride. Then cells were disrupted in a homogenizer. Lysates were centrifuged at 500 g for 10 min and supernatants were centrifuged at 25 000 g for 30 min and membrane fractions were extracted with 0.5 m NaCl to remove loosely associated proteins. Cell membrane extract (10 μg) in a buffer containing 50 mm citrate (pH 6.0), 0.1 m NaCl, 1 mm EDTA, 1 mm dithiothreitol and 10 mm pNPP was added to each of the 96 wells with or without DHK (final volume: 100 μl), followed by incubation at 37 °C for 60 min. Amounts of p‐nitrophenol produced were determined by measuring the absorbance at 405 nm.
3D culture
Millicell‐PCF culture plate inserts (0.4 μm; Millipore, Billerica, MA, USA) were used for 3D culture. Early passage (passage 2) human KSCs were seeded at density of 50 000/insert in 0.2 ml expansion medium (CNT‐07; CELLnTEC) and inserts were then placed in a six‐well plate with addition of 1 ml CNT‐07 medium. Cultures were maintained for 2–3 days at 37 °C and 5% CO2 until 100% confluence, and were then maintained in a 3D medium (CNT‐02‐3DP5; CELLnTEC). After 16 h, culture medium inside inserts was aspirated and culture medium outside the inserts was replaced with fresh 3D Prime differentiation medium (with or without DHK), to the level of the membrane. 3D culture was then maintained for 7, 11 and 14 days by changing medium every 2–3 days. Samples were then collected for H&E staining and immunohistochemistry.
Animal experimentation
Principles of laboratory animal care were followed, and our Medikinetics‐Institutional Animal Care and Use Committee (M‐IACUC) approved protocols for the present study. Three adult (10 months of age) female miniature pigs (Medi Kinetics micropigs®, Pyungtaek, Kyunggido, Korea) were obtained from a Medikinetics closed‐barrier facility and were housed in single pens under controlled environmental GLP conditions. Animals were allowed 2 weeks for pre‐assessment and acclimatization before initiation of experimental procedures. Food was withheld for at least 6–8 h prior to anaesthesia. Atropine (0.05 mg/kg) and half‐dose of combination of tiletamine‐zolazepam (5–25 mg/kg, Zoletil®; Virbac Animal Health, St. Louis, MO, USA) and xylazine (2 mg/kg, Narcosyl®; Merial, Duluth, GA, USA) were administered intramuscularly for pre‐anaesthesia. General anaesthesia was induced using the remaining half‐dose of tiletamine‐zolazepam and xylazine combination, through intramuscular or intravenous injection. After positioning the animal at ventral recumbency, an appropriate size (6.5 mm) of endotracheal tube was inserted for maintenance of anaesthesia, using gas anaesthetic apparatus. Anaesthesia was maintained by isoflurane (Ifran®; Hana Pharm. Co. Ltd., Seoul, Korea) with oxygen at 1:1 (5–10 ml/kg/min) ratio. Intravenous hydration with normal saline was maintained via the superficial auricular vein (25 ml/h). For final preparation, animals were shaved over experimental area and sterilized with 20% povidone solution (Betadine solution).
The animal study included two experiments. For the first experiment, twelve sites comprising 1 × 1 cm squares were demarcated by surgical marker on the cephalic portion of the back. Sites were treated with pulsed CO2 laser (Union Medical, Gyeonggi‐do, South Korea) using a 0.3 mm spot size, 10.6 μm wavelength and 7.5 W power. After several laser passes, partially desiccated tissue was completely removed using saline‐soaked applicator, and de‐epithelialized wounds were inflicted. In the first experiment, most cephalic raw sites (four sites, control group) were treated with Vaseline (control), middle raw sites (four sites) were treated with a 0.1% DHK mixture (100 μg DHK suspended and homogenized in 100 mg Vaseline base), and the most caudal raw sites (four sites) were treated with 1% DHK mixture (1 mg DHK was suspended and homogenized in 100 mg of a Vaseline base). Each site was covered with 3M Micropore following treatment (St. Paul, MN, USA). Wounds were cleansed, photographs were taken using a Canon 450D (Canon Inc, Tokyo, Japan) and dressings were changed daily. After evaluating re‐epithelialization, samples of skin tissue were collected for H&E staining and immunohistochemistry.
The second experiment was performed on the caudal portion of the back. The study design was similar to that of the first experiment, but the number of lesions was 9, and size of lesions was 2 × 1.5 cm square, and skin defects were made using a dermatome (Aesculap B Braun Korea Co. Ltd., South Korea), to a depth of 0.5 mm. In the second experiment, the most cephalic raw sites (three sites, control) were treated with Vaseline (control), middle raw sites (three sites) were treated with a 0.1% DHK mixture (100 μg DHK suspended and homogenized in 100 mg of a Vaseline base), and the most caudal raw sites (three sites) were treated with a 1% DHK mixture (1 mg DHK was suspended and homogenized in 100 mg Vaseline base). Each site was covered with MediFoam (Ildong Pharmacy, Seoul, South Korea). Wounds were cleansed, photographs were taken using a Canon 450D (Canon Inc), and dressings were changed daily. After evaluating re‐epithelialization, skin tissues were collected for H&E staining and immunohistochemistry.
Statistical analysis
Statistical significance was determined using one‐way ANOVA followed by paired t‐test. Data were considered to be statistically significant if probability had a value of 0.05 or less.
Results
KSC characterization
FACS analysis to define cell‐surface markers for KSCs has shown that KSCs express high levels of α6 integrin and low levels of CD71 (3, 9, 10). In the present study, those markers confirmed that KSCs express high α6 integrin and low CD71 compared to normal keratinocytes (Fig. S1). Expression of α6 integrin decreased with passage number, which is a unique characteristic of KSCs. In addition, examination of different growth factor secretions between KSCs and keratinocytes showed that KSCs secreted high amounts of amphiregulin, insulin‐like growth factor binding proteins 2 and 4, platelet‐derived growth factor ‐AA and vascular endothelial growth factors (data not shown).
Proliferative effect of DHK
Because methanol extract of S. pubescens showed stimulatory effects on KSCs, we isolated and characterized chemical components of kaurane diterpenes form this plant; DHK is one of the major compounds of this fraction and showed the proliferative potential, so we further expanded the functional and mechanistic study. Compared to control (Fig. 2a), DHK treatment (50 μm concentration) significantly increased proliferation of KSCs (Fig. 2b), which increased in a dose‐dependent manner (Fig. 2c). To clarify signal pathways involved in proliferation of KSCs, a phosphokinase chip array was carried out and it revealed that phospho‐Erk1/2, ‐Akt signals were significantly increased by DHK1 treatment (50 μm concentration, data not shown). Western blot analysis also detected that phosphorylation of ERK1/2 and Akt was significantly higher after DHK treatment (Fig. 2d). Chemical inhibitors against ERK1/2 (U0126, 5 μm concentration) and Akt (LY294002, 5 μm concentration) significantly attenuated DHK‐enhanced proliferation of KSCs (Fig. 2e). These results indicated that DHK increased proliferation of KSCs via Akt and ERK pathways.
Figure 2.
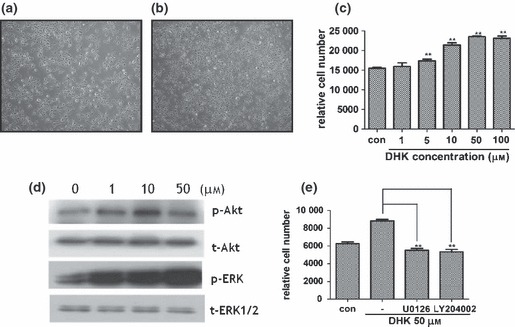
Effect of DHK on proliferation of KSCs. Note that DHK significantly increased proliferation of KSCs via Akt and ERK pathways. Compared to control (a), DHK treatment (50 μm concentration, b) increased proliferation of KSCs and relative cell number was measured (c). Expression of phosphorylated Akt and ERK1/2 were detected by western blot analysis (d). DHK‐induced KSC proliferation was attenuated by specific Akt (LY294002, 5 μm) and ERK1/2 (U0126, 5 μm) inhibitors (e). **P < 0.01.
Increased KSC migration
The effect of DHK on migration of KSCs was examined by wound migration assay. Compared to controls (Fig. 3a), DHK treatment significantly increased migration of KSCs (approximate 2‐fold increase at concentration of 50 μm, Fig. 3b) and migration increased in a dose‐dependent manner (Fig. 3c). In addition, chemical inhibitors against ERK1/2 (U0126, 5 μm concentration) and Akt (LY294002, 5 μm concentration) significantly attenuated DHK‐induced migration of KSCs (Fig. 3d).
Figure 3.
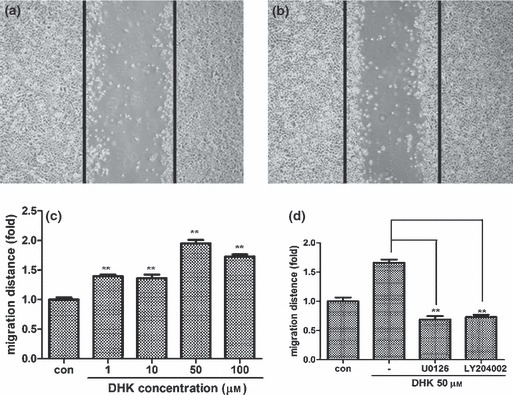
Effect of DHK on migration of KSCs. Note that DHK significantly increased migration of KSCs. Photographs were taken 24 h after wounding (a: control, b: 50 μm DHK treatment). Black lines indicate the initial wound after scraping. Migration distance was increased by DHK treatment in a dose‐dependent manner (c). Increased cell migration was reversed by specific Akt (LY294002, 5 μm) and ERK1/2 (U0126, 5 μm) inhibitors (d). **P < 0.01.
EGFR involvement
Phosphorylation of multiple receptor tyrosine kinases (EGFR) has reportedly been affected by kaurane diterpenes such as oridonin (11, 12, 13). Thus, membrane receptors involved in activation of KSCs by DHK were examined, using a phosphor‐receptor kinase array. Spot density of phosphorylated EGFR was significantly increased after DHK treatment (50 μm concentration, data not shown). In addition, phosphorylation of EGFR was examined by western blot analysis using 3 phospho‐EGFR (Y1068, Y992 and Y1045) antibodies. Y1068‐phosphorylated EGFR was significantly higher compared to total EGFR expression (Fig. 4a) and protein expression of Y1068‐phosphorylated EGFR increased in a dose‐dependent manner (Fig. 4b). Considering the structural difference between EGF and DHK, they may not act as substrates for EGFR. Therefore, we hypothesized that DHK may act as a protein tyrosine phosphatase (PTPase) inhibitor and further examined PTPase activity using plasmamembrane and cytoplasmic fractions of KSCs. As Fig. 4c shows, DHK treatment significantly inhibited activity of PTPase in the membrane fraction and decreased production of p‐nitrophenol. However, cytoplasmic PTPase was not inhibited by DHK (data not shown). Because it is reasonable to assume that DHK stimulates KSCs via EGFR phosphorylation, inhibition studies using EGFR neutralizing antibody (1 μg total protein) and chemical inhibitor (AG1478, 1 μm concentration) were investigated. The EGFR neutralizing antibody and chemical inhibitor significantly decreased phosphorylation of Akt and ERK1/2 signal pathways (Fig. 4d). In addition, DHK‐induced proliferation (Fig. 4e) and migration (Fig. 4f) of KSCs were significantly attenuated by neutralizing antibody and inhibitor treatment. These results collectively indicate a pivotal role for EGFR phosphorylation in DHK‐induced KSC activation.
Figure 4.
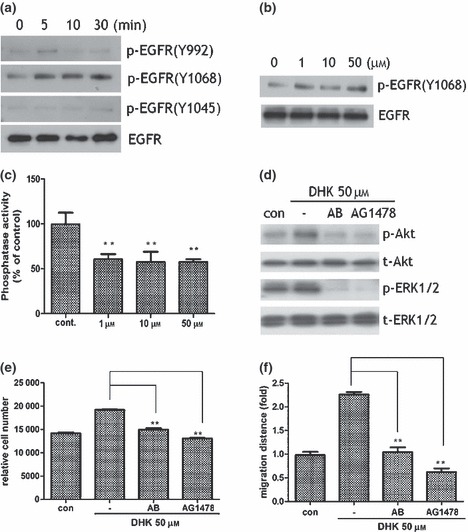
EGFR involvement in KSC stimulation. Note that DHK increased phosphorylation of EGFR. DHK significantly increased EGFR phosphorylation as shown in western blot analysis. Phosphorylation of Y1068‐EGFR increased just a few minutes after DHK treatment (a). Expression of Y1068‐phosphorylated EGFR increased in a dose‐dependent manner (b). DHK treatment significantly inhibited activity of protein tyrosine phosphotase activity in the membrane fraction of KSCs (c). EGFR neutralizing antibody (1 μg total protein) and chemical inhibitor (AG1478, 1 μm concentration) significantly attenuated phosphorylation of Akt and ERK1/2 signal pathways (d). In addition, proliferation (e) and migration (f) of KSCs were significantly reduced by antibody and inhibitor treatment. **P < 0.01.
DHK induced replenishment of fully stratified epidermis
We further examined effects of DHK on differentiation of KSCs and on establishment of fully stratified epidermis, using a 3D culture model of KSCs on a porous membrane. Multilayered epidermal tissues with a basal layer, suprabasal layers and terminally differentiated cornified layers were detected by H&E staining at 11 and 14 days after adding differentiation medium. KSCs cultured in this system generated morphologically normal epithelial tissue within 2 weeks. However, epithelium produced by DHK treatment (at day 11 and 14) showed regular stratification of thick epidermis, whereas the control group produced relatively thin epidermis (Fig. 5a). In addition, DHK treatment produced a more compact basal layer and cells had a more cubal appearance compared to controls. We also analyzed phenotypes of skin equivalents by immunohistochemistry. Numbers of p63 positive cells, putative intracellular KSC markers, was significantly increased after DHK treatment in the epidermis at days 11 and 14 (Fig. 5b).
Figure 5.
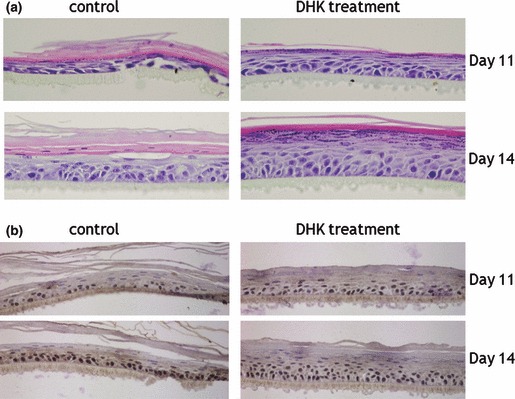
3D culture of KSCs. Note that DHK treatment induced establishment of fully stratified epidermis. Epithelium produced by DHK treatment had regular stratification of thick epidermis, whereas control groups produced only relatively thin epidermis, as shown in H&E staining (a). Expression of p63‐positive cells, a putative intracellular KSC marker, was also detected by immunohistochemistry (b) and was significantly increased by DHK treatment.
DHK accelerated healing of epidermal wounds
DHK stimulated KSCs and induced epidermal remodelling in 3D culture. Therefore, we further examined the epidermal healing effect of DHK in animal models. Epidermal wounds were created by laser burn and dermatome. First, a CO2 laser was used for de‐epithelialization of minipig skin. DHK was mixed with a Vaseline base and topically applied to burn wounds. As Fig. 6a shows, 0.1% and 1% DHK treatment significantly accelerated wound healing and increased re‐epithelialization. For quantitative analysis, we calculated the re‐epithelialized area of each group at day 4, and DHK treatment significantly promoted epithelialization compared to the control group (Fig. 6b). Although there was no significant histopathological difference between H&E staining (data not shown), p63‐positive cells (well‐known KSC marker) were stained in basal epithelial layers (Fig. 6c) and were significantly increased after DHK treatment (Fig. 6d). The second experiment was conducted using dermatome for de‐epithelialization. Similar to the laser burn, DHK treatment significantly accelerated wound healing in excision wounds (Fig. 7a) and increased re‐epithelialization at day 9 (Fig. 7b). In addition, p63‐positive cells stained (Fig. 7c) in basal epithelial layers significantly increased as shown by immunohistochemistry (Fig. 7d).
Figure 6.
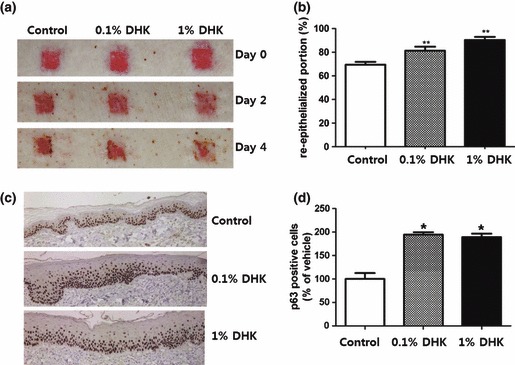
Healing of laser‐induced epidermal wound. Note that DHK accelerated healing of laser burn in micropigs. Epidermal wounds were created by laser burn (n = 4), and DHK was topically applied with Vaseline base. DHK treatment significantly accelerated wound healing of laser burns (a) and increased re‐epithelialization at day 4 (b). p63 positive cells were primarily stained in basal epithelial layers (c) and significantly increased after DHK treatment (d). *P < 0.05, **P < 0.01.
Figure 7.
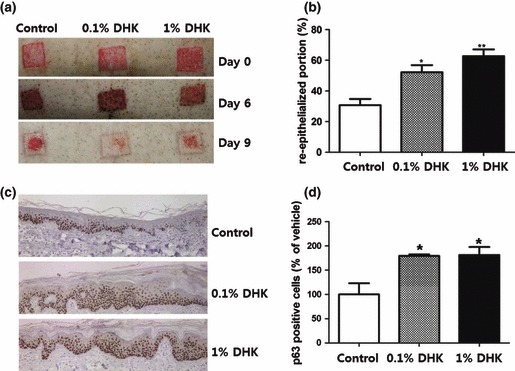
Healing of excision wound. Note that DHK accelerated healing of epidermal wounds created by a dermatome (n = 3). DHK treatment significantly accelerated wound healing in excision wounds (a) and increased re‐epithelialization at day 9 (b). In addition, p63 positive cells were primarily found to be stained in basal epithelial layers (c) and significantly increased after DHK treatment (d). *P < 0.05, **P < 0.01.
Discussion
We were interested in searching for and developing natural products that activate KSCs to develop wound‐healing agents that could also have cosmetic applications. We screened KSC activators using a chemical library of natural products and found that the methanol extract of S. pubescens increased proliferation of KSCs. Therefore, chemical components of S. pubescens were isolated, and identified and DHK, one of the major active compounds, accelerated wound healing in laser‐induced epidermal wounds and in excision wounds of micropigs. DHK promoted epidermal remodelling and maintenance of basal epithelium layers in 3D culture models. In addition, DHK significantly increased expression of p63‐positive cells (KSC marker) in 3D culture as well as in animal models. Thus, we characterized the mechanism of KSC activation and found that DHK increased proliferation and migration of KSCs via EGFR phosphorylation. A neutralizing antibody against EGFR and a chemical inhibitor (AG 1478) significantly attenuated DHK‐induced proliferation and migration of KSCs. In short, these results indicate that DHK isolated from S. pubescens accelerate epidermal regeneration mainly through EGFR phosphorylation and KSC activation.
Siegesbeckia pubescens is an annual herb indigenous to Korean mountainous regions. The aerial part and root of this plant have traditionally been used for treatment of rheumatoid arthritis and hypertension, in Korean medicine. Siegesbeckia pubescens has been shown to have anti‐inflammatory and analgesic activities and it has been used to inhibit hypersensitivity (14, 15, 16, 17, 18, 19, 20). Recently, Wang et al., isolated kaurane diterpenes from S. pubscens and found that ent‐16β, 17‐dihydroxy‐kauran‐19‐oic acid showed anti‐platelet and anti‐thrombotic effects in rats (21). With regard to chemical composition, a series of primarane‐ and kaurane‐type diterpenes were isolated from this plant (22, 23).
Kaurane diterpenes are known to have an inhibitory effect on proliferation of various cancer cell lines. For example, Wang et al. demonstrated that oridonin induced apoptosis in cancer cells by inhibiting insulin‐like growth factor receptors (11, 24). Li et al. reported the inhibitory effect of oridonin in A431 cell proliferation by down‐regulation of EGFR expression (12, 13). In addition, oridonin suppresses ERK and Akt signalling pathways in multiple cancer cell lines to reduce cell proliferation (25, 26). However, the cell proliferative effect of kaurane diterpenes has not been fully demonstrated yet. In the present study, we first report the KSC stimulatory effect of kaurane diterpene and its potential for epidermal regeneration. DHK increased proliferation and migration of KSCs by EGFR phosphorylation. In addition, DHK stimulated vasculogenic progenitor cells (our unpublished data). Therefore, DHK might be used as a novel stem cell activator and replace conventional growth factors.
EGFR is the cell‐surface receptor for members of the EGF‐family, which includes EGF, transforming growth factor‐α, amphiregulin and heparin‐binding‐EGF. EGFRs are largely present on epidermal basal cells (major localization of KSCs), sebaceous glands, eccrine sweat glands, and within the capillary system in the skin (27, 28, 29). EGFR is activated by binding of its specific ligands. Upon activation by its growth factor ligands, EGFR undergoes a transition from inactive monomeric form to active homodimer, and autophosphorylates several tyrosine (Y) residues at the C‐terminal domain of EGFR (30, 31, 32). This autophosphorylation elicits downstream activation and initiates several signal transduction cascades, principally those of ERK and Akt pathways, leading to DNA synthesis and cell proliferation and migration (33, 34). In the present study, DHK treatment clearly increased EGFR phosphorylation in a phospho‐receptor chip array and increased phosphorylation of EGFR (Y1068), as shown in our western blot analysis. In addition, ERK and Akt phosphorylation were significantly increased by DHK treatment. Inhibition studies against EGFR, using a neutralizing antibody and a chemical inhibitor, attenuated the enhanced function. Thus, these results collectively indicate that DHK activates KSCs via an EGFR‐mediated signal pathway, and it may substitute for EGF and other EGFR ligands.
Recently, a wound healing effect of S. pubscens has been reported by Wang et al., and that methanol extract increased viability and proliferation of fibroblasts and accelerated wound healing in excision and incision wound models (35). In excision wounds, animals treated with methanol extract exhibited significant increases in rate of wound contraction, period of epithelialization and content of hydroxyproline. In incision wounds, treatment with methanol extract showed an increase in breaking strength. However, this report is focused on dermal regeneration by methanolic extract of S. pubscens. In our study, we first demonstrated that DHK, one of the major components of S. pubscens, regenerates the epidermal layer and accelerates epidermal wound‐healing.
In summary, we tried to develop a novel skin rejuvenator by screening natural products using KSCs, and found that DHK from S. pubescens is effective for KSC stimulation. DHK increased proliferation and migration of KSCs by activating EGFR, and accelerated epidermal regeneration in 3D culture and in an animal model. Because natural products have diverse advantages over recombinant growth factors in commercial use (long‐term stability and skin permeability), DHK might be applied to new wound‐healing agents and to new basic materials for cosmetics.
Supporting information
Fig. S1 FACS analysis of KSCs. Compared with mature keratinocytes (b), KSCs (a) express bright integrin α6 but dim CD71.
Fig. S2 The 1H‐, and 13C‐ spectra of DHK.
Fig. S3 The HMBC spectra of DHK.
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
This research was supported by a grant (C10102410) from the Gyeonggi Technology Development Program funded by Gyeonggi Province and by a grant from CHA University (CHAIACF‐2009‐A008). The authors declare no competing financial interests.
References
- 1. Gangatirkar P, Paquet‐Fifield S, Li A, Rossi R, Kaur P (2007) Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat. Protoc. 2, 178–186. [DOI] [PubMed] [Google Scholar]
- 2. Pouliot N, Redvers RP, Ellis S, Saunders NA, Kaur P (2005) Optimization of a transplant model to assess skin reconstitution from stem cell‐enriched primary human keratinocyte populations. Exp. Dermatol. 14, 60–69. [DOI] [PubMed] [Google Scholar]
- 3. Kim DS, Cho HJ, Choi HR, Kwon SB, Park KC (2004) Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell. Mol. Life Sci. 61, 2774–2781. [DOI] [PubMed] [Google Scholar]
- 4. Kaur P (2006) Inter‐follicular epidermal stem cells: identification, challenges, potential. J. Invest. Dermatol. 126, 1450–1458. [DOI] [PubMed] [Google Scholar]
- 5. Webb A, Li A, Kaur P (2004) Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 72, 387–395. [DOI] [PubMed] [Google Scholar]
- 6. Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM et al. (2003) Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 120, 501–511. [DOI] [PubMed] [Google Scholar]
- 7. Li A, Pouliot N, Redvers R, Kaur P (2004) Extensive tissue‐regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J. Clin. Invest. 113, 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abbas O, Mahalingam M (2009) Epidermal stem cells: practical perspectives and potential uses. Br. J. Dermatol. 161, 228–236. [DOI] [PubMed] [Google Scholar]
- 9. Kaur P, Li A, Redvers R, Bertoncello I (2004) Keratinocyte stem cell assays: an evolving science. J. Investig. Dermatol. Symp. Proc. 9, 238–247. [DOI] [PubMed] [Google Scholar]
- 10. Lavker RM, Sun TT (2000) Epidermal stem cells: properties, markers, and location. Proc. Natl. Acad. Sci. USA 97, 13473–13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang HJ, Li D, Yang FY, Tashiro S, Onodera S, Ikejima T (2008) Oridonin induces human melanoma A375‐S2 cell death partially through inhibiting insulin‐like growth factor 1 receptor signaling. J. Asian Nat. Prod. Res. 10, 787–798. [DOI] [PubMed] [Google Scholar]
- 12. Li D, Wu LJ, Tashiro S, Onodera S, Ikejima T (2008) Oridonin induces human epidermoid carcinoma A431 cell apoptosis through tyrosine kinase and mitochondrial pathway. J. Asian Nat. Prod. Res. 10, 77–87. [DOI] [PubMed] [Google Scholar]
- 13. Li D, Wu LJ, Tashiro S, Onodera S, Ikejima T (2007) Oridonin inhibited the tyrosine kinase activity and induced apoptosis in human epidermoid carcinoma A431 cells. Biol. Pharm. Bull. 30, 254–260. [DOI] [PubMed] [Google Scholar]
- 14. Huh JE, Baek YH, Lee JD, Choi DY, Park DS (2008) Therapeutic effect of Siegesbeckia pubescens on cartilage protection in a rabbit collagenase‐induced model of osteoarthritis. J. Pharmacol. Sci. 107, 317–328. [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Cai Y, Wu Y (2008) Anti‐inflammatory and analgesic activity of topical administration of Siegesbeckia pubescens. Pak. J. Pharm. Sci. 21, 89–91. [PubMed] [Google Scholar]
- 16. Kim JY, Lim HJ, Ryu JH (2008) In vitro anti‐inflammatory activity of 3‐O‐methyl‐flavones isolated from Siegesbeckia glabrescens. Bioorg. Med. Chem. Lett. 18, 1511–1514. [DOI] [PubMed] [Google Scholar]
- 17. Kim HM, Lee JH, Won JH, Park EJ, Chae HJ, Kim HR et al. (2001) Inhibitory effect on immunoglobulin E production in vivo and in vitro by Siegesbeckia glabrescens. Phytother. Res. 15, 572–576. [DOI] [PubMed] [Google Scholar]
- 18. Kang BK, Lee EH, Kim HM (1997) Inhibitory effects of Korean folk medicine ‘Hi‐Chum’ on histamine release from mast cells in vivo and in vitro. J. Ethnopharmacol. 57, 73–79. [DOI] [PubMed] [Google Scholar]
- 19. Park HJ, Kim IT, Won JH, Jeong SH, Park EY, Nam JH et al. (2007) Anti‐inflammatory activities of ent‐16alphaH,17‐hydroxy‐kauran‐19‐oic acid isolated from the roots of Siegesbeckia pubescens are due to the inhibition of iNOS and COX‐2 expression in RAW 264.7 macrophages via NF‐kappaB inactivation. Eur. J. Pharmacol. 558, 185–193. [DOI] [PubMed] [Google Scholar]
- 20. Kim HM (1997) Effects of siegesbeckia pubescens on immediate hypersensitivity reaction. Am. J. Chinese Med. 25, 163–167. [DOI] [PubMed] [Google Scholar]
- 21. Wang JP, Xu HX, Wu YX, Ye YJ, Ruan JL, Xiong CM et al. (2011) Ent‐16beta,17‐dihydroxy‐kauran‐19‐oic acid, a kaurane diterpene acid from Siegesbeckia pubescens, presents anti‐platelet and anti‐thrombotic effects in rats. Phytomedicine 18, 873–878. [DOI] [PubMed] [Google Scholar]
- 22. Wang R, Chen WH, Shi YP (2010) ent‐kaurane and ent‐pimarane diterpenoids from Siegesbeckia pubescens. J. Nat. Prod. 73: 17–21. [DOI] [PubMed] [Google Scholar]
- 23. Kim S, Na M, Oh H, Jang J, Sohn CB, Kim BY et al. (2006) PTP1B inhibitory activity of kaurane diterpenes isolated from Siegesbeckia glabrescens. J. Enzyme Inhib. Med. Chem. 21, 379–383. [DOI] [PubMed] [Google Scholar]
- 24. Wang K, Zhang ZQ (2007) [Determination of oridonin and rosemarinic acid in Rabdosia rubescens by HPLC]. Zhong yao cai = Zhongyaocai = J. Chinese Med. Mater. 30, 1396–1398. [PubMed] [Google Scholar]
- 25. Li D, Wu LJ, Tashiro S, Onodera S, Ikejima T (2007) Oridonin‐induced A431 cell apoptosis partially through blockage of the Ras/Raf/ERK signal pathway. J. Pharmacol. Sci. 103, 56–66. [DOI] [PubMed] [Google Scholar]
- 26. Hu HZ, Yang YB, Xu XD, Shen HW, Shu YM, Ren Z et al. (2007) Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol. Sin. 28, 1819–1826. [DOI] [PubMed] [Google Scholar]
- 27. Wenczak BA, Lynch JB, Nanney LB (1992) Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor‐mediated repair. J. Clin. Invest. 90, 2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pierard‐Franchimont C, Colige A, Arrese Estrada J, Lapiere CM, Pierard GE (1991) Immunohistochemical expression of epidermal growth factor receptors in nuclei of a subpopulation of keratinocytes and sweat gland cells. Dermatologica 183, 7–9. [DOI] [PubMed] [Google Scholar]
- 29. Saga K, Jimbow K (2001) Immunohistochemical localization of activated EGF receptor in human eccrine and apocrine sweat glands. J. Histochem. Cytochem. 49, 597–602. [DOI] [PubMed] [Google Scholar]
- 30. Kim ES, Khuri FR, Herbst RS (2001) Epidermal growth factor receptor biology (IMC‐C225). Curr. Opin. Oncol. 13, 506–513. [DOI] [PubMed] [Google Scholar]
- 31. Downward J, Waterfield MD, Parker PJ (1985) Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J. Biol. Chem. 260, 14538–14546. [PubMed] [Google Scholar]
- 32. Downward J, Parker P, Waterfield MD (1984) Autophosphorylation sites on the epidermal growth factor receptor. Nature 311, 483–485. [DOI] [PubMed] [Google Scholar]
- 33. Oda K, Matsuoka Y, Funahashi A, Kitano H (2005) A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 1, 2005.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filardo EJ (2002) Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G‐protein‐coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J. Steroid Biochem. Mol. Biol. 80, 231–238. [DOI] [PubMed] [Google Scholar]
- 35. Wang JP, Ruan JL, Cai YL, Luo Q, Xu HX, Wu YX (2011) In vitro and in vivo evaluation of the wound healing properties of Siegesbeckia pubescens. J. Ethnopharmacol. 134, 1033–1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 FACS analysis of KSCs. Compared with mature keratinocytes (b), KSCs (a) express bright integrin α6 but dim CD71.
Fig. S2 The 1H‐, and 13C‐ spectra of DHK.
Fig. S3 The HMBC spectra of DHK.
Supporting info item
Supporting info item
Supporting info item


