Abstract
Abstract. Mouse mammary epithelial cell cultures previously described bring about extensive proliferation and a cell population with the appropriate markers for luminal ductal epithelial cells, and also the ability to form normal tissue after implantation into mice. This success may result from a culture environment that resembles certain aspects of the environment in the mammary gland. Mouse mammary epithelial cells, whose proliferation is limited when plated alone, can be stimulated to multiply by contact with lethally irradiated cells of the LA7 rat mammary tumour line. Most of the proliferative stimulus is imparted by direct cell contact between LA7 and mouse mammary cells. Junctions, including adherens junctions, form among all cells in the culture, much as junctions form in the mammary gland. LA7 cells secrete TGFα and bFGF, factors found in the mammary gland, and factors to which mouse mammary cells respond in culture. Mouse mammary cells express keratins 8 and 18, markers for luminal cells of the mammary duct. LA7 cells express keratin 14 and vimentin, markers for myoepithelial cells. These facts, taken together, fit a model of cell replacement in an epithelial tissue and also imitate the relationship between luminal ductal cells and myoepithelial cells in the mammary gland. This method of culturing cells is useful, not only for in vitro–in vivo carcinogenesis studies, but also for the study of mechanisms by which growth signals are imparted from one cell to another.
INTRODUCTION
Historically, epithelial cells, compared with other cell types, have been difficult to culture. Now, even with published methods for various epithelial cells, the ones which promote a number of cell doublings approaching the expected limit, which result in a cell population with markers of the desired cell type, or which successfully function in animals or humans after implantation, is small. In this respect, our previously published unique method for culturing mouse mammary epithelial cells (MMEC) has been particularly successful. One reason for this success may be the similarity of the culture system to the in vivo condition of the cells. Here, we present findings that demonstrate some of these similarities.
Normal MMEC, taken directly from tissue, can multiply to 10–20 times the original cell number when plated by themselves in primary culture (Imagawa et al. 1982), but have resisted substantial proliferation after subculture. However, growth stimulation can be achieved if the MMEC are cultured in direct contact with lethally irradiated cells of the immortal LA7 rat mammary tumour line. By this method, MMEC can be cultured for at least 12 passages and 30 doublings in cell number (Ehmann et al. 1984), and their ultimate life span capacity in culture is not known. The cultured cells form normal‐appearing glandular structures when implanted into mammary fat pads (Ehmann et al. 1987). Here, we present evidence that they also retain markers of normal mammary epithelial cells.
This unusual culture method was conceived to be an in vitro model of cell replacement in epithelial tissue. Epithelial cells, unlike other cell types such as fibroblasts, are always connected in vivo by direct contact via tight junctions that form an intact barrier to specific ions, molecules, and solutes. The integrity of this barrier must be maintained, even when a cell within the tissue dies. Therefore, some signal(s) for the healthy cells to divide must be transmitted when a damaged cell is lost from the confluent epithelial tissue. Cells of the LA7 rat mammary line were chosen as surrogates that the MMEC might recognize as being similar and with which they might form a demi‐epithelial tissue in vitro if the two cell types were plated together at a confluent density. Indeed, as the lethally irradiated LA7 cells slowly die, the MMEC divide to occupy the spaces of the dying LA7 cells. In this way, the confluent monolayer slowly changes from one composed mostly of irradiated LA7 cells to one composed entirely of MMEC (Ehmann et al. 1984). This suggests that the culture system is a model for replacement of wounded epithelial cells.
The propagation of MMEC probably depends on both production of growth factors by the LA7 cells and on formation of physical connections between LA7 cells and MMEC. We now present data indicating that the LA7 cells produce at least two growth factors known to operate in the mammary gland, namely, transforming growth factor α (TGFα) and basic fibroblast growth factor (bFGF). Close juxtaposition of the two cell types provides the opportunity for stimulation by membrane‐bound growth factors, and confluency of the mixed MMEC‐LA7 culture is necessary for maximum proliferation of MMEC (Ehmann 1992). LA7 cells also form junctional complexes with MMEC, including tight junctions and desmosomes (Ehmann et al. 1998). The junctional complexes and growth factors involved here are those found in the intact mammary gland.
The usefulness of this culture system is two‐fold. First, the ability to transfer MMEC from culture into the animal facilitates studies of proliferation, carcinogenesis and cell aging. Secondly, that the in vitro environment nurturing MMEC proliferation mimics the in vivo situation allows extrapolation of conclusions reached about cell–cell interactions in culture to cell–cell interactions in the mammary gland.
MATERIALS AND METHODS
Mouse mammary epithelial cell culture
The culture of MMEC has been previously described (Ehmann et al. 1984). MMEC cultures were prepared from glands excised aseptically from 6‐ to 8‐week‐old female Balb/c mice (Simonson Laboratories, Gilroy, CA, USA), minced, and digested overnight with 0.1% collagenase (Worthington Biochemicals, Malvern, PA, USA) in culture medium (see below) at 37 °C. The tissue suspension was poured through a 65‐µm nytex filter to capture the epithelial organoids, but allow most single cells (including fibroblasts) to pass through. The organoids were introduced into tissue culture flasks whose growth surface was covered with lethally irradiated LA7 cells. The cells were fed with fresh medium three times per week until the growth surface was covered with a confluent monolayer of the normal epithelial cells, usually ∼5 weeks. The cultures were maintained in a 1 : 1 combination of Dulbecco's modified Eagle's medium (DMEM) and Ham's F12 medium (Hazleton Biologics, Lenexa, KS, USA) supplemented with 10 µg/ml insulin, 5 µg/ml transferrin, 0.2 mm (120 U/ml) penicillin G, 0.05 mm streptomycin sulphate (Sigma, St Louis, MO, USA), 20 mm HEPES buffer, 2.0 g/l NaHCO3, and 0–10% fetal bovine serum (Sterile Systems, Logan, UT, USA) or new‐born calf serum (Sigma), and a supplement containing the trace elements Se, Zn, Cu, V, Si, Mn, Sn, and Ni (Hammond et al. 1984).
MMEC subcultured to passages 3–5 from primary cultures were used in most experiments. To prepare subcultures, the cells were dislodged from the plastic by incubation in a trypsin‐EDTA phosphate‐buffered saline (PBS) solution. The cells were introduced into a new flask at the desired dilution, usually 1 : 5–1 : 20, from which they again proliferated to cover the flask surface in about 5 weeks if fresh irradiated LA7 cells were provided.
LA7 cells
Rat mammary cells of the LA7 line were originally obtained from Dr Renato Dulbecco (The Salk Institute, San Diego, CA, USA). These cells are a clonal derivative of the Rama 25 line, which came from a Sprague‐Dawley rat treated with dimethylbenz(a)‐anthracene (Dulbecco et al. 1979). Before plating the normal epithelial cells, the LA7 cells were proliferatively inactivated by a single dose of 60 Gy (6000 rads) from a 137Cs source. Culture medium for the LA7 cells alone consisted of 1 : 1 DMEM/F12 base supplemented with 10 µg/ml insulin, 50 nm hydrocortisone, 0.2 mm penicillin G, 0.05 mm streptomycin sulphate, and 5% new‐born calf serum. MDA‐MB‐231 cells, from a breast adenocarcinoma, used as a positive control known to secrete TGFα, were cultured and treated the same as the LA7 cells.
Conditioned media
LA7 or MDA‐MB‐231 cells were diluted into tissue culture flasks in passages 30–35 and fed three times per week with culture medium as described above. When the cells became nearly confluent, the medium was changed to include only 1.0 µg/ml insulin, and the trace elements mentioned above without any serum. At each feeding, the medium was collected and frozen and replaced with fresh medium. After several collections, the proteins in the pooled media were concentrated by a factor of 30 in an Amicon 600‐ml thin channel system fitted with a 3000 molecular weight cut‐off filter. This concentrate was sterilized by passage through a 0.20‐µ filter and mixed with fresh medium to achieve a final concentration of proteins of 20 times that in the original conditioned media.
MMEC growth curves
MMEC were removed from a confluent secondary culture by trypsin/EDTA and plated in medium with 10% new‐born calf serum plus insulin, transferrin, antibiotics and trace elements. About 5 × 104 cells/35‐mm dish were plated in experiments to which the growth factors TGFα and bFGF were added, and ∼105 cells/35‐mm dish were plated in experiments to study the effects of irradiated LA7 cells or conditioned medium from them. The next day, the dishes were divided into experimental groups, and growth factors, conditioned media, or feeder cells, as appropriate, were added along with the appropriate growth factors and 0.5% serum plus all other supplements. All cultures were fed three times per week with the appropriate media, and a dish from each group was removed every 3–4 days for analysis. The cells for analysis were trypsinized and a portion counted on a Coulter Counter.
In co‐cultures the remainder were analysed for mouse/rat, i.e. MMEC/LA7, cell ratio (Fig. 1). The sample was incubated with an anti‐mouse serum raised in Sprague‐Dawley rats injected with dead MMEC (Josman Laboratories, Napa, CA). Subsequently, the sample was incubated with an FITC‐conjugated rabbit anti‐rat IgG serum. After fixation with 1% paraformaldehyde, the fraction of fluorescently labelled mouse cells was determined by analysis on a Becton Dickenson FACScan (Fig. 1). To obtain the number of epithelial cells in the sample, the fraction of fluorescently labelled cells was multiplied by the total cell count.
Figure 1.
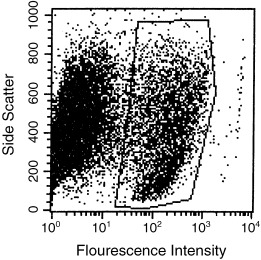
FACScan analysis of percentages of MMEC in co‐culture with LA7 feeder cells on day 7 after plating. Polygon defines population of MMEC, i.e. those that reacted with the anti‐MMEC serum. Twenty‐two per cent of the cell population were MMEC.
Immunocytochemistry
LA7 cells were plated into T25 tissue culture flasks in passage 54 and fed for 1–2 days until they were confluent. After irradiation of the LA7 cells, 1000 MMEC were seeded onto the monolayer. After regular feeding, MMEC colonies appeared after about 6 weeks. Before fixation and between every step of immunocytochemical staining, the cells were washed thoroughly with PBS. The cells were fixed with methanol (10 min, −80 °C), and then permeabilized with 0.5% triton‐X‐100 in PBS. The first stage antisera (Table 1) were applied to the monolayers and incubated at 37 °C for 1.5 h, followed by 1 h. incubation with the second stage sera (Table 1). The cells were viewed by epifluorescence on a Nikon microscope and photographed with a Nikon 35‐mm N2000 camera and 400 ASA black‐and‐white film.
Table 1.
Antibodies for immunocytochemistry
| Antigen | First antibody | Vendor or reference | Second antibody b | Vendor | ||
|---|---|---|---|---|---|---|
| Source a | Dilution | Source | Dilution | |||
| TGFα | Rabbit p | 1 : 100 | Research & Diagnostic Ab | Goat | 1 : 160 | Sigma |
| bFGF | Rabbit p | 1 : 50 | Sigma | Goat | 1 : 160 | Sigma |
| Keratin 8 | Rat m | 1 : 40 | DSHB c | Rabbit | 1 : 20 | Vector |
| Keratin 14 | Mouse m | 1 : 20 | Neomarkers | Horse | 1 : 100 | Vector |
| Keratin 18 | Rabbit p | 1 : 25 | Ku, et al. (1995) | Goat | 1 : 100 | Sigma |
| Vimentin | Mouse m | 1 : 30 | Sigma | Horse | 1 : 100 | Vector |
| E‐cadherin | Rat m | 1 : 250 | Sigma | Rabbit | 1 : 20 | Vector |
| Epithelial membrane antigen | Mouse m | 0 | Dako | Primary FITC‐ conjugated | ||
m, monoclonal; p, polyclonal.
b All second‐stage antibodies were FITC‐conjugated.
c Developmental Studies Hybridoma Bank, University of Iowa.
Immunoblotting
The 30 × conditioned medium described above was further concentrated to 230 ×. Proteins in the concentrated medium were separated by sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS–PAGE) (Laemmli 1970). The samples prepared for detection of TGFα, EGF, and bFGF were separated on a tricine gel consisting of 16.5% T and 3% C resolving gel, 10% T and 3% C spacer gel, and 4% T and 3% C stacking gel, where T denotes total percentage concentration of acrylamide and bisacrylamide and C denotes percentage concentration of the crosslinker relative to total concentration of T (Schagger & von Jagow 1987). The proteins were transferred from the gels to a nitrocellulose membrane, and then probed with mouse monoclonal anti‐TGFα (2 µg/ml), anti‐bFGF (1 : 1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or rabbit anti‐TGFα (1 : 1000), or anti‐bFGF (1 : 1500) (both from Sigma). The reacted bands were visualized with enhanced chemiluminescence.
RESULTS
MMEC did not proliferate noticeably when plated onto tissue culture plastic alone, even in medium containing insulin and transferrin (Fig. 2). However, if they were surrounded by a confluent monolayer of irradiated cells of the LA7 rat mammary tumour line, they multiplied and gradually replaced the dying LA7 cells (1, 2). LA7 rat mammary tumour cells produced factors that stimulated MMEC proliferation because MMEC numbers increased when medium conditioned by LA7 cells was concentrated and applied to MMEC cultures (Fig. 2). The resulting increase was not as great as when MMEC were co‐cultured with the LA7 cells themselves, but was greater than that of MMEC cultured in unconditioned medium, where the cell number decreased with time (Fig. 2).
Figure 2.
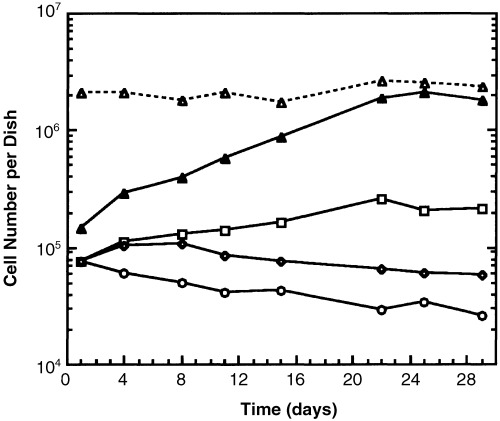
Proliferation of MMEC with conditioned media or LA7 feeder cells. (○) MMEC alone in usual culture medium; (◆) MMEC alone in medium conditioned by MDA‐MB‐231 cells, which secrete TGFα; (□) MMEC alone in medium conditioned by LA7 cells; (▴) MMEC co‐cultured with lethally irradiated LA7 cells; (Δ) total number of MMEC + LA7 feeders in co‐culture. All data points were obtained by FACs analysis similar to that of Figure 1. This experiment is representative of all experiments of this type. Each curve on the graph differs significantly from each other curve at P < 0.01.
We have identified two growth factors produced by LA7 cells, namely, TGFα and bFGF. Antisera to both TGFα and bFGF labelled cultured, fixed LA7 cells, whether they were unirradiated (Fig. 3) or irradiated (not shown). The antiserum to TGFα also labelled cultured, fixed MDA‐MB‐231 breast carcinoma cells, which are known to secrete TGFα (not shown) (Dickson et al. 1986). To determine whether the growth factors were secreted into the medium, proteins from concentrated LA7 conditioned media were separated by SDS–PAGE and probed with antisera. Both TGFα and bFGF proteins were detected on the blots from these separations (Fig. 4). A major band reacted with anti‐TGFα serum and co‐migrated with synthetic TGFα (5.6 kDa), and several other higher molecular weight bands, a wide one of which was 55 kDa, were also visible (Fig. 4). A weak band reacted to anti‐bFGF serum at 12 kDa, the same molecular weight as that of the minor band of synthetic bFGF, and a heavy band was visible at ∼31 kDa.
Figure 3.
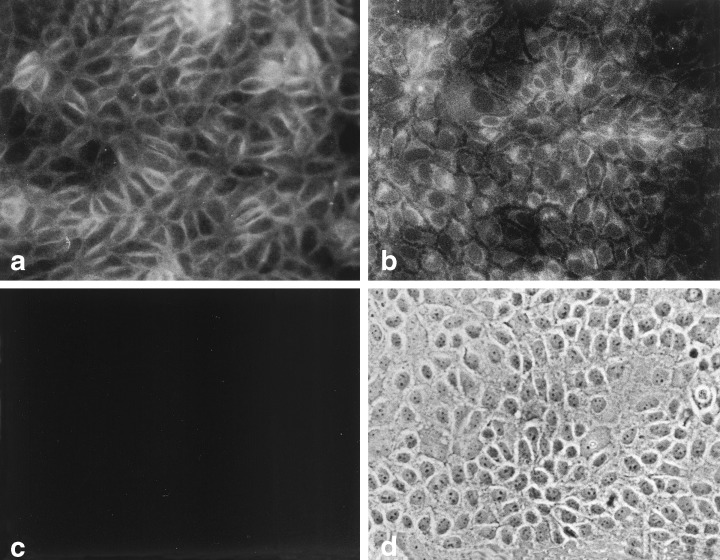
Immunocytochemistry of LA7 cells for growth factors. (a) Fluorescent image of cells incubated with rabbit anti‐TGFα. (b) Fluorescent image of cells incubated with rabbit anti‐bFGF. (c) Fluorescent image of cells incubated with rabbit pre‐immune serum and the FITC‐conjugated second stage antibody. (d) Phase contrast image of cells in field (c). 200 ×.
Figure 4.
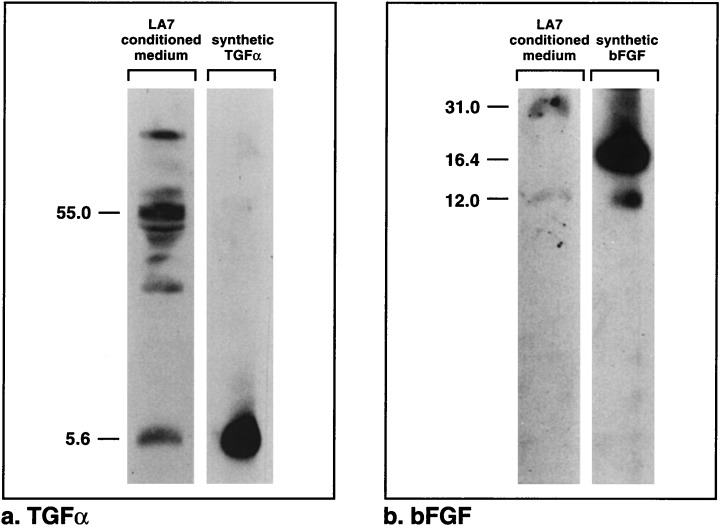
Growth factors from LA7 conditioned medium separated by SDS–PAGE. (a) Proteins reacting with antibodies to TGFα. One band co‐migrates with the synthetic TGFα marker, and several bands are evident at higher molecular weights. (b) Proteins reacting with antibodies to bFGF. One faint band co‐migrates with a minor band of the bFGF standard, and another band of ∼31 kDa is visible.
Both TGFα and bFGF elicited proliferative responses in MMEC plated alone and fed with media to which each growth factor had been added (Fig. 5). Addition of both growth factors together elicited a greater response than either one alone. Although the increase in cell number was small and unsustained, the cell numbers in those cultures remained higher than in MMEC cultures fed with control medium. The proliferative response brought about by synthetic TGFα was mirrored by increased MMEC proliferation in medium conditioned by TGFα‐secreting MDA‐MB‐231 cells (Fig. 2).
Figure 5.
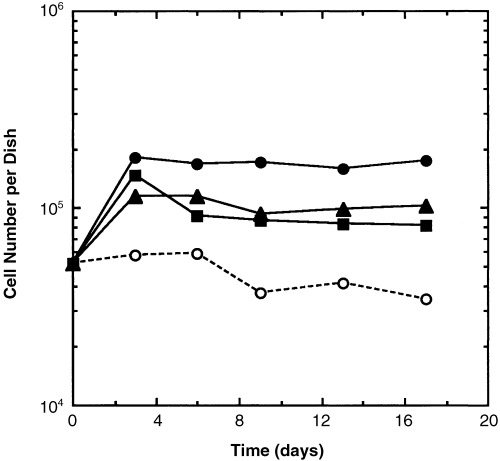
MMEC proliferation stimulated by growth factors. (○) Control culture medium with insulin, transferrin, and 0.5% new‐born calf serum. (▴) Control medium plus 10 ng/ml TGFα (▪) Control medium plus 50 ng/ml bFGF. (•) Control medium plus TGFα and bFGF. The control curve without TGFα and bFGF differs significantly from the other curves at P < 0.05, as does the curve with both TGFα and bFGF. The curves representing cell numbers from cultures to which either TFGα or bFGF were added do not differ significantly from each other. The results are from one experiment which is representative of three experiments.
The physical connections between MMEC and LA7 feeders were explored in a previous communication (Ehmann et al. 1998). At that time, we were not able to detect adherens junctions by immunocytochemistry even though β‐catenin, which normally binds cadherins, was visible, and E‐cadherin was present in LA7 cell lysates. In more recent experiments, E‐cadherin between some LA7 cells and adjacent MMEC was detectable by immunocytochemistry (Fig. 6). These appeared dimmer than junctions among MMEC themselves. That they have not always been visible is probably related to the hybrid nature of the adherens junctions formed between the two cell types and the specificity of the antibody.
Figure 6.
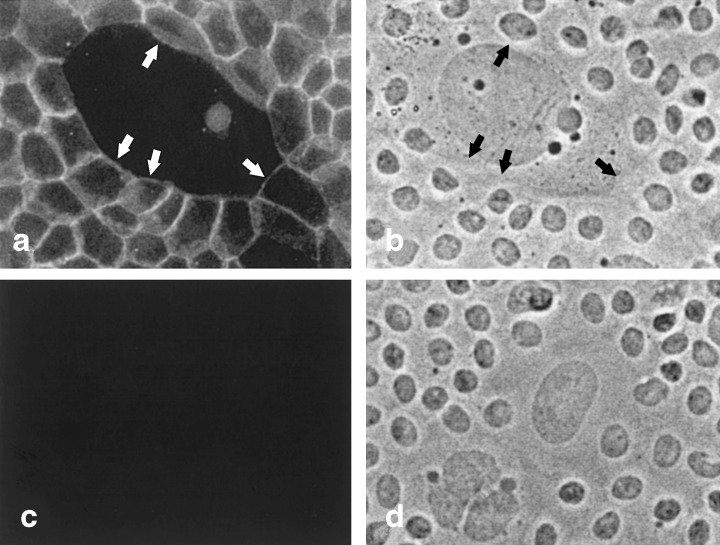
Adherens junctions between MMEC and an irradiated feeder LA7 cell. (a) Fluorescent image of cells incubated with antibody to E‐cadherin. Adherens junctions line borders between MMEC on the edges of the field. Arrows in (a) and (b) point to sections of the junctions that line the border between MMEC and the large LA7 feeder cell in the centre of the field. (b) Phase contrast image of cells in (a). (c) Fluorescent image of a field of cells incubated with control rat serum. (d) Phase contrast image of cells in (c). 200 ×.
Even though both the MMEC and LA7 cells originated in rodent mammary glands, they expressed antigens characteristic of different cell types. The MMEC strongly expressed markers of luminal ductal epithelial cells, namely, keratins 8 (Fig. 7) and 18, and they moderately expressed epithelial membrane antigen. LA7 cells, on the other hand, did not express those keratins, but, instead, expressed keratin 14 (Ehmann & Terris 2002) and vimentin (Fig. 8), both of which are characteristic of myoepithelial cells.
Figure 7.
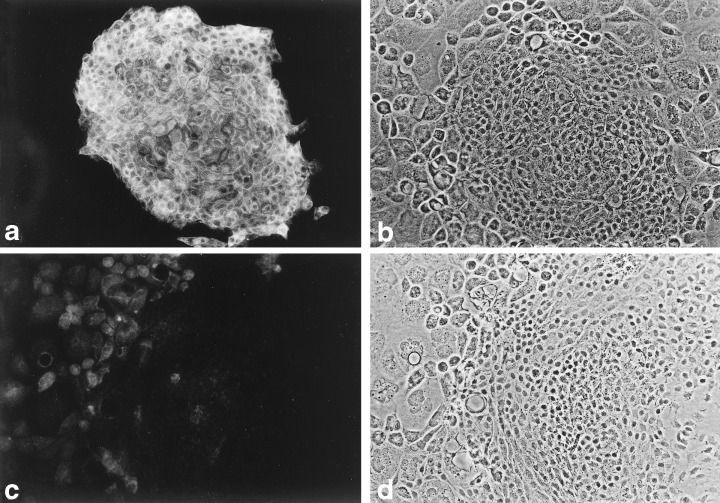
Keratin 8 in MMEC. (a) Fluorescent image of an MMEC colony surrounded by dying feeder LA7 cells, after exposure to a rat anti‐keratin 8 serum and visualized with an FITC‐conjugated antibody to rat IgG. (b) Phase contrast image of field (a). (c) Fluorescent image of control cells exposed to pre‐immune rat serum. (d) Phase contrast view of field (c). An MMEC colony is on the right and feeder cells on the left. 100 ×.
Figure 8.
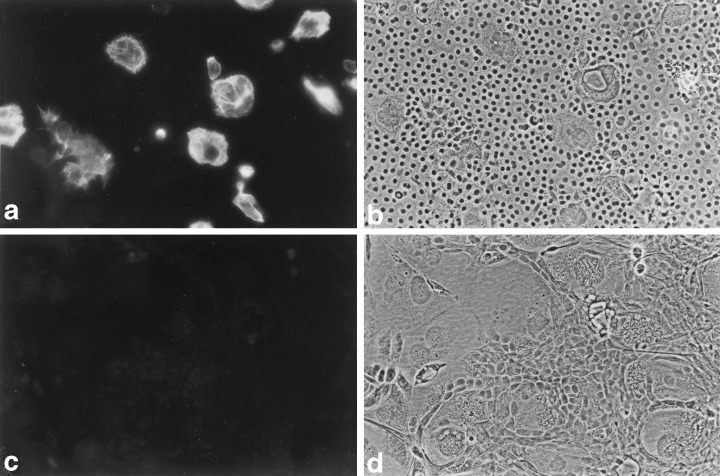
Vimentin in LA7 feeders. (a) A field of nearly confluent MMEC which surround a few remaining LA7 feeders, exposed to mouse anti‐vimentin serum. The LA7 cells, but not the MMEC, stain brightly. (b) Phase contrast view of field (a). (c) Control field of cells exposed to mouse pre‐immune serum. (d) Phase contrast view of field (c). 100 ×.
DISCUSSION
Many elements of the in vivo environment from which MMEC derive are present in the culture system described here. The growth factors, TGFα and bFGF, which stimulate mammary cells in vivo are also presented to these cells in culture. The cultured cells form desmosomes, tight junctions and adherens junctions with their neighbours (Ehmann et al. 1998), just as in vivo (Monaghan & Moss 1996; Nguyen & Neville 1998; Locke et al. 2000; Nanba, Nakanishi & Hieda 2001; Nguyen, Parlow & Neville 2001; Runswick et al. 2001). Cultured MMEC express the keratins and connexins of luminal cells of the mammary ducts. In the mammary gland, as in other epithelial tissues, a mechanism must exist for maintaining an intact epithelial cell sheet by proliferation of cells to fill in for wounded or dying cells. In culture, dying LA7 cells being replaced by MMEC provides a model for maintenance of epithelial integrity. Together, these features suggest that the cells in vitro form an epithelial tissue similar to that found in vivo.
The growth factors operating in this culture system are known to be important in vivo. Insulin and transferrin, both components of the culture medium used here, are necessary for mammary cells in vivo (Li et al. 1998). Both TGFα and bFGF, produced by LA7 cells, are active in the mammary gland. TGFα is present in terminal end buds of developing mammary glands (Smith et al. 1989; 1990, 1991). In adults it is produced mostly by the myoepithelial population (Smith et al. 1989), but is also detectable in glandular and ductal mammary cells (Yasui et al. 1992). It belongs to a family of growth‐stimulating peptides that includes epidermal growth factor (EGF), cripto‐1, amphiregulin, heregulin α and β, and β‐cellulin. Several of these are known to affect mammary cell proliferation, and more of these factors are produced in malignant than in normal tissue (Normanno et al. 1994; Qi et al. 1994; Li et al. 1998). The EGF receptor, to which most growth factors in this family, including TGFα, bind, is overexpressed in 50–60% of human breast tumours (Salomon et al. 1990). It has intrinsic tyrosine kinase activity and is located on the basolateral surfaces of many types of epithelial cells. One response to oestrogen stimulation in the mammary gland is secretion by epithelial cells of TGFα, which binds to receptors of the secreting or neighbouring cells, resulting in proliferation (Liu et al. 1987). TGFα precursors (∼20 kDa) on one cell can directly stimulate the EGF receptor on neighbouring cells, even without cleavage of the TGFα itself (5.6 kDa) from the precursor (Brachman et al. 1989; Wong et al. 1989; Higashiyama et al. 1995). Thus, unsecreted TGFα can be at least as important in cell stimulation as the secreted form.
It is worth noting that others have demonstrated that radiation of a human mammary cell line resulted in increased TGFα transcription (Schmidt‐Ullrich et al. 1992), and that TGFα‐containing medium from irradiated cells activated the EGF receptor pathway on unirradiated cells (Dent et al. 1999). Perhaps irradiation of LA7 cells, as in this study, also stimulates their TGFα production.
bFGF, a member of the fibroblast growth factor family (Rubin et al. 1989; Manetti et al. 2000; Powers et al. 2000), is expressed in all cells of growing neonatal mammary ducts at all stages of development (Rudland et al. 1993; Jackson et al. 1997; Lavendero et al. 1998), but in adults is secreted mostly by the myoepithelial cell population (Fernig et al. 1990; Gomm et al. 1991; Li et al. 1998). Myoepithelial cells also express bFGF receptors and synthesize DNA in response to bFGF (Barraclough et al. 1990; Gomm et al. 1991; Fernig et al. 1993; Ke et al. 1993; Rudland et al. 1993). bFGF is probably delivered to receiving cells by the extracellular matrix, where much of it is found in the mammary gland (Li et al. 1998; Barraclaugh et al. 1990). Others have demonstrated that mammary cells in vitro respond to bFGF and that this response is additive to that of EGF (Levay‐Young et al. 1989), similar to our experience that synthetic bFGF and TGFα stimulate MMEC proliferation in an additive manner. In our experiments, the cells did not continue to respond to the growth factor stimuli after a few days. The same lack of continued stimulation occurred with conditioned medium from MDA‐MB‐231 cells (Fig. 2), which are known to secrete TGFα. Whether the growth factor receptors are down‐regulated or whether additional factors are required for continued stimulation is not known. Also, the culture conditions may not perfectly simulate the in vivo environment. For example, we were not able to detect oestrogen receptor mRNA in cultured MMEC (data not presented), even though the cells later multiplied to form glandular structures in vivo (Ehmann et al. 1987). Thus, the ability to respond to oestrogen was retained by the cultured cells even though they did not respond in the culture environment.
That LA7 cells secrete both TGFα and bFGF, as we demonstrated, suggests that they are part of the growth stimulus in LA7‐conditioned medium. We also observed bands of higher molecular weights than purified TGFα and bFGF upon immune blotting of LA7 conditioned medium. It is not unusual for proteins to be secreted in forms different from those predicted. For example, two rat mammary cell lines secrete bioactive TGFα of 50 kDa as well as at 5.6 kDa (Li et al. 1998), and a human line secretes TGFα of 7 and 30 kDa (Bates et al. 1988). Several human mammary lines secrete bFGF of 18, 24, 26, and 27 kDa (Li & Shipley 1991; Ke et al. 1993). These higher molecular weight forms might represent post‐translational modification of the secreted factors or may represent antibody cross‐reaction to unrelated or related protein species.
Physical contact seems to be of paramount importance in growth stimulation of MMEC (Fig. 2 and Ehmann 1992). Whether this juxtaposition of cells facilitates cell multiplication by changing the physical conformation of MMEC, whether it facilitates the action of membrane bound growth factors on LA7 cells (Brachman et al. 1989; Wong et al. 1989; Higashiyama et al. 1995), or whether it merely subjects the MMEC to high concentrations of growth factors secreted by LA7 cells is not clear. The importance of ‘tensegrity’ as a growth stimulus has been discussed (Huang & Ingber 2000), and direct transmission of growth factors, especially TGFα, from one cell membrane to another, has been well documented (Brachman et al. 1989; Wong et al. 1989; Higashiyama et al. 1995). Each of these modes of cell stimulation could contribute to a system that ensures replacement of a wounded cell in an epithelial sheet. That irradiated LA7 cells also support multiplication of normal mammary cells from the rat (Ehmann et al. 1991), the same species from which the LA7 cell line was derived, supports this model of cell replacement.
Cultured MMEC express markers of the luminal ductal population, which are thought to comprise the main proliferative population of the mammary gland. In vivo, some of these differentiate in stages to myoepithelial cells that surround the ducts (Li et al. 1998). Cultured MMEC express keratins 8 and 18, and epithelial antigen, which mark luminal ductal epithelial cells in vivo. They do not express keratin 14 or vimentin, markers of myoepithelial cells (Stampfer et al. 2002). Cultured MMEC express gap junction proteins Cx26 (Ehmann et al. 2000), which is known to connect luminal cells in vivo, and Cx43, which has so far been described connecting rodent myoepithelial cells (Pozzi et al. 1995). Therefore, MMEC represent the in vitro counterpart of luminal ductal cells in vivo with the possibility that some of them have undergone small steps towards differentiation into myoepithelial cells. LA7 cells, on the other hand, express markers of myoepithelial cells, namely, keratin 14 and vimentin, as well as Cx43. In addition, LA7 cells produce growth factors known to be produced by myoepithelial cells in vivo (Gomm et al. 1991; Li et al. 1998).
In summary, MMEC and LA7 cells together in culture seem to form an epithelial tissue comparable to that found in vivo. The MMEC respond to growth factors produced by LA7 cells, as luminal epithelial cells in vivo respond to growth factors made by myoepithelial cells (Li et al. 1998). The cells are all in contact with each other and connected by junctions, as in the mammary gland. The MMEC grown in this culture system carry markers of normal luminal cells of mammary ducts. Because this culture system stimulates extensive proliferation and maintains cells in their normal state, it provides an important tool for studying normal cell–cell interactions that have significance in vivo.
REFERENCES
- Barraclough R, Fernig DG, Rudland PS, Smith JA (1990) Synthesis of fibroblast growth factor upon differentiation of rat mammary epithelial to myoepithelial cells in culture. J. Cell Physiol. 144, 333. [DOI] [PubMed] [Google Scholar]
- Bates SE, Davidson NE, Valverius EM, Freter CE, Dickson B, Tam JP, Kudlow JE, Lippman ME, Salomon DS (1988) Expression of transforming growth factor α and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significance. Mol. Endocrinol. 2, 543. [DOI] [PubMed] [Google Scholar]
- Brachman R, Lindquist PB, Nagashima M, Kohr W, Lipari T, Napier M, Derynck (1989) Transmembrane TGF‐α precursors, activate EGF/TFG‐α receptors. Cell 56, 691. [DOI] [PubMed] [Google Scholar]
- Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt‐Ullrich R (1999) Radiation‐induced release of transforming growth factor α activates the epidermal growth factor receptor and mitogen‐activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation‐induced cell death. Mol. Biol. Cell. 10, 2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R, Bates SE, Mcmanaway ME, Lippman ME (1986) Characterization of estrogen responsive transforming activity in human breast cancer cell lines. Cancer Res. 46, 1707. [PubMed] [Google Scholar]
- Dulbecco R, Bologna M, Unger M (1979) Differentiation of a rat mammary cell line in vitro . Proc. Natl Acad. Sci. USA 76, 1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann UK (1992) Feeder cells impart a growth stimulus to recipient epithelial cells by direct contact. Exp. Cell Res. 199, 314. [DOI] [PubMed] [Google Scholar]
- Ehmann UK, Terris MK (2002) Juxtacrine stimulation of normal and malignant human bladder epithelial cell proliferation. J. Urol. 167, 735. [DOI] [PubMed] [Google Scholar]
- Ehmann UK, Peterson WD Jr, Misfeldt DS (1984) To grow mouse mammary epithelial cells in culture. J. Cell Biol. 98, 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann UK, Guzman RC, Osborn RC, Young LJT, Cardiff RD, Nandi S (1987) Cultured mouse mammary epithelial cells: normal phenotype after implantation. JNCI 78, 751. [PubMed] [Google Scholar]
- Ehmann UK, Osborn RC, Guzman RC, Fajardo LF (1991) Cultured proliferating rat mammary epithelial cells. In vitro Cell Dev. Biol. 27A, 749. [DOI] [PubMed] [Google Scholar]
- Ehmann UK, Calderwood SK, Stevenson MA, Devries JT (1998) Physical connections between feeder cells and recipient normal mammary epithelial cells. Exp. Cell Res. 243, 76. [DOI] [PubMed] [Google Scholar]
- Ehmann UK, Calderwood SK, Stevenson MA (2000) Gap‐junctional communication between feeder cells and recipient normal epithelial cells correlates with growth stimulation. In Vitro Cell Dev. Biol. 37, 100. [DOI] [PubMed] [Google Scholar]
- Fernig DG, Smith JA, Rudland PS (1990) Appearance of basic fibroblast growth factor receptors upon differentiation of rat mammary epithelial to myoepithelial‐like cells in culture. J. Cell. Physiol. 142, 108. [DOI] [PubMed] [Google Scholar]
- Fernig DG, Barraclough R, Ke Y, Wilkinson MC, Rudland PS, Smith JA (1993) Ectopic production of heparin‐binding growth factors and receptors for basic fibroblast growth factor by rat mammary epithelial cell lines derived from malignant metastatic tumours. Int. J. Cancer 54, 629. [DOI] [PubMed] [Google Scholar]
- Gomm JJ, Smith J, Ryall GK, Baillie R, Turnbull L, Coombes RC (1991) Localization of basic fibroblast growth factor and transforming growth factor β1 in the human mammary gland. Cancer Res. 51, 4685. [PubMed] [Google Scholar]
- Hammond SL, Ham RG, Stampfer MR (1984) Serum‐free growth of normal human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc. Natl Acad. Sci. USA 81, 5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E (1995) The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane‐anchored heparin‐binding EGF‐like growth factor. J. Cell Biol. 128, 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Ingber DE (2000) Shape‐dependent control of cell growth, differentiation, and apoptosis: switching between attractors in cell regulatory networks. Exp. Cell Res. 261, 91. [DOI] [PubMed] [Google Scholar]
- Imagawa W, Tomooka Y, Nandi S (1982) Serum‐free growth of normal and tumor mouse mammary epithelial cells in primary culture. Proc. Natl Acad. Sci. USA 79, 4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Bresnick J, Dickson C (1997) A role for fibroblast growth factor signaling in the lobuloalveolar development of the mammary gland. J. Mammary Gland Biol. Neoplasia 4, 385. [DOI] [PubMed] [Google Scholar]
- Ke Y, Fernig DG, Wilkinson MC, Winstanley JHR, Smith JA, Rudland PS, Barraclough R (1993) The expression of basic fibroblast growth factor and its receptor in cell lines derived from normal human mammary gland and a benign mammary lesion. J. Cell Sci. 106, 135. [DOI] [PubMed] [Google Scholar]
- Ku N‐O, Michie S, Oshima RG, Omary MB (1995) Chronic hepatitis, hepatic fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J. Cell Biol. 131, 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680. [DOI] [PubMed] [Google Scholar]
- Lavendero S, Chappuzeau A, Sapag‐Hagar M, Oka T (1998) In vivo and in vitro evidence of basic fibroblast growth factor action in mouse mammary gland development. FEBS Lett. 439, 351. [DOI] [PubMed] [Google Scholar]
- Levay‐Young BK, Imagawa W, Wallace DR, Nandi S (1989) Basic fibroblast growth factor stimulates the growth and inhibits casein accumulation in mouse mammary epithelial cells in vitro . Mol. Cell. Endocrinol. 62, 327. [DOI] [PubMed] [Google Scholar]
- Li S, Shipley GD (1991) Expression of multiple species of basic fibroblast growth factor mRNA and protein in normal and tumor‐derived mammary epithelial cells in culture. Cell Growth Diff. 2, 195. [PubMed] [Google Scholar]
- Li P, Barraclaugh R, Fernig DG, Smith JA, Rudland PS (1998) Stem cells in breast epithelia. Int. J. Exp. Path. 79, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscia DS, Merlo G, Ciardiello F, Kim N, Smith G, Callahan R, Salomon DS (1990) Transforming growth factor‐α messenger RNA localization in the developing adult rat and human mammary gland by in situ hybridization. Dev. Biol. 140, 123. [DOI] [PubMed] [Google Scholar]
- Liu SC, Sanfilippo B, Perroteau I, Derynck R, Salomon DS, Kidwell W (1987) Expression of transforming growth factor α (TGFα) in differentiated rat mammary tumors: estrogen induction of TGFα production. Molec. Endocrin. 1, 683. [DOI] [PubMed] [Google Scholar]
- Locke D, Perusinghe N, Newman T, Jayatilake H, Evans WH, Monaghan P (2000) Developmental expression and assembly of connexins into homomeric and heteromeric gap junction hemichannels in the mouse mammary gland. J. Cell Physiol. 183, 228. [DOI] [PubMed] [Google Scholar]
- Manetti F, Corelli F, Botta M (2000) Fibroblast growth factors and their inhibitors. Curr. Pharmaceut. Design 6, 1897. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Moss D (1996) Connexin expression and gap junctions in the mammary gland. Cell Biol. Int. 20, 121. [DOI] [PubMed] [Google Scholar]
- Nanba D, Nakanishi Y, Hieda Y (2001) Changes in adhesive properties of epithelial cells during early morphogenesis of the mammary gland. Dev. Growth Differ. 43, 535. [DOI] [PubMed] [Google Scholar]
- Nguyen DA, Neville MC (1998) Tight junction regulation in the mammary gland. J. Mammary Gland Biol. Neoplasia 3, 233. [DOI] [PubMed] [Google Scholar]
- Nguyen DA, Parlow AF, Neville MC (2001) Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J. Endocrinol. 170, 347. [DOI] [PubMed] [Google Scholar]
- Normanno N, Ciardiello F, Brandt R, Salomon DS (1994) Epidermal growth factor‐related peptides in the pathogenesis of human breast cancer. Breast Cancer Res. Treat. 29, 11. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A (2000) Fibroblast growth factors, their receptors and signalling. Endocr. Relat. Cancer 7, 165. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Risek B, Kiang DT, Gilula NB, Kumar NM (1995) Analysis of multiple gap junction gene products in the rodent and human mammary gland. Exp. Cell Res. 220, 212. [DOI] [PubMed] [Google Scholar]
- Qi C‐F, Liscia DS, Normanno N, Merlo G, Johnson GR, Gullick WJ, Ciardiello F, Saeki T, Brandt R, Kim N, Kenney N, Salomon DS (1994) Expression of transforming growth factor α, amphiregulin and cripto‐1 in human breast carcinomas. Br. J. Cancer 69, 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson A (1989) Purification and characterization of a newly identified growth factor specific for epithelial cells. Biochemistry 86, 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland PS, Platt‐Higgins AM, Wilkinson MC, Fernig DG (1993) Immunocytochemical identification of basic fibroblast growth factor in the developing rat mammary gland: variations in location are dependent on glandular structure and differentiation. J. Histochem. Cytochem. 41, 887. [DOI] [PubMed] [Google Scholar]
- Runswick SK, O'Hare MJ, Jones L, Streuli CH, Garrod DR (2001) Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat. Cell Biol. 3, 823. [DOI] [PubMed] [Google Scholar]
- Salomon DS, Kim N, Saeki T, Ciardiello F (1990) Transforming growth factor‐α: an oncodevelopmental growth factor. Cancer Cells 2, 389. [PubMed] [Google Scholar]
- Schagger H, Von Jagow G (1987) Tricine‐sodium dodecyl sulfate‐polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Ullrich RK, Valerie K, Chan W, Wazer DE, Lin PS (1992) Expression of oestrogen receptor and transforming growth factor‐α in MCF‐7 cells after exposure to fractionated irradiation. Int. J. Radiat. Biol. 61, 405. [DOI] [PubMed] [Google Scholar]
- Smith JA, Barraclaugh R, Fernig DG, Rudland PS (1989) Identification of alpha transforming growth factor (αTGF) as a possible local trophic agent for the mammary gland. J. Cell Physiol. 141, 363. [DOI] [PubMed] [Google Scholar]
- Snedeker SM, Brown CF, Diaugustine RP (1991) Expression and functional properties of transforming growth factor α and epidermal growth factor during mouse mammary gland ductal morphogenesis. Proc. Natl Acad. Sci. USA 88, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MR, Yaswen P, Taylor‐Papadimitriou J (2002) Culture of human mammary epithelial cells In: Freshney RI, Freshney MG, eds. Culture of Epithelial Cells, p. 95 New York: Wiley‐Liss Inc. [Google Scholar]
- Wong ST, Winchell LF, McCune BK, Earp HS, Teixido J, Massague B, Herman B, Lee DC (1989) The TGF‐α precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell 56, 495. [DOI] [PubMed] [Google Scholar]
- Yasui W, Ji Z‐Q, Kuniyasu H, Ayhan A, Yokozaki H, Ito H, Tahara E (1992) Expression of transforming growth factor alpha in human tissues: immunohistochemical study and northern blot analysis. Virchows Arch. A Pathol. Anat. 421, 513. [DOI] [PubMed] [Google Scholar]


