Abstract
Objectives
Worldwide, lung cancer accounts for the majority of cancer‐related deaths. Aberrant expression of miRNAs has increasingly been reported to be associated with tumour progression. This study aimed to explore the role of miR‐301b in regulating apoptosis in lung cancer.
Materials and methods
Expression of miR‐301b was assessed by real‐time PCR in cell lines, human patient tissues and cells treated under hypoxia and DMOG. Scramble siRNA, miR‐301b inhibitor and miR‐301b mimics were transfected into lung cancer cells to determine their effects on apoptosis. Additionally, a mouse xenograft model was used to explore functions of miR‐301b on apoptosis, in vivo. Finally, relationships between Bim and miR‐301b levels were explored by luciferase reporter assay and Western blotting.
Results
We found that miR‐301b was highly expressed in lung cancer tissues and cell lines. Expression of miR‐301b was induced by hypoxia, and miR‐301b suppressed expression of Bim by targeting its 3′UTR. Functionally, ectopic expression of miR‐301b enhanced cell population growth, reduced apoptosis and reduced sensitivity of cells to chemotherapy. In the xenograft model, overexpression of miR‐301b promoted tumour growth. Additionally, miR‐301b and Bim expression were inversely correlated in clinical lung cancer samples.
Conclusions
This study provides new insights into the function of miRNA‐301b in lung cancer and suggests that miRNA‐301b could be a potential molecular target for chemotherapy.
1. Introduction
Lung cancer accounts for the majority of cancer‐related deaths worldwide in both men and women and is responsible for more deaths than colon, breast and prostate cancer.1, 2 Primarily, lung cancer consists of two main clinicopathological categories, small cell lung carcinoma (SCLC) and non‐small‐cell lung carcinoma (NSCLC), depending on the cell of origin.3 SCLC accounts for 15% of all lung cancers and is far more aggressive than NSCLC.4 NSCLC cancers are composed of three subtypes, which account for at least 85% of all cases and are more common.5 Despite improvements and advancements in medical treatments and surgery, the 5‐year survival rate of lung cancer patients remains frustratingly poor.6
MicroRNAs are a small class of noncoding, endogenous RNAs that are 18–24 nucleotides in length and play critical roles in repressing protein translation and the degradation of mRNAs by binding to complementary target mRNAs. MicroRNAs control a wide range of biological events, including development, apoptosis, growth and differentiation.7, 8, 9 They post‐transcriptionally regulate gene expression by binding to complementary sequences in the 3′ untranslated regions (3′UTR) of downstream target messenger RNA.10 An increasing body of evidence has shown that microRNAs are abnormally expressed in many different types of cancers and are involved in tumourigenesis.9, 11 However, the understanding of the expression patterns of microRNAs and their biological functions in carcinogenesis and progression, especially in lung cancer, is still largely unclear.12
An increasing amount of evidence has shown that a number of microRNAs are differentially expressed in response to hypoxia, thereby leading to drug resistance, by inhibiting apoptosis.13 For instance, hypoxia‐induced miR‐424 decreases the sensitivity of cancer cells to chemotherapy and inhibits apoptosis by regulating PDCD4.14 Additionally, microRNA‐210 targets the expression of anti‐apoptotic Bcl‐2 and mediates hypoxia‐induced apoptosis of neuroblastoma cells.15 MicroRNA‐196b inhibits cell proliferation and induces apoptosis in HepG2 cells by targeting IGF2BP1.16 HIF‐1a‐induced microRNA‐210 reduces hypoxia‐induced apoptosis of osteoblast MG‐63 cells,17 and the overexpression of microRNA‐494 up‐regulates hypoxia‐inducible factor‐1 alpha expression via the PI3K/AKT pathway and protects against hypoxia‐induced apoptosis.18
MicroRNA‐301b has been reported to be associated with vasoconstriction in pulmonary hypertension.19 Recently, the aberrant expression of microRNA‐301b has been implicated in the development of human cancers. For example, microRNA‐301b is up‐regulated in pancreatic, colorectal and oral carcinoma.20, 21, 22, 23 However, the biological function of microRNA‐301b in lung cancer cells is not fully understood.
In this study, we found that miR‐301b is highly expressed in lung cancer tissues and cell lines compared with their corresponding normal counterparts. The expression of miR‐301b can be induced by hypoxia, and miR‐301b suppresses Bim expression by targeting its 3′ untranslated region. Finally, the overexpression of microRNA‐301b enhances cell growth, reduces apoptosis and decreases the drug sensitivity.
2. Materials and methods
2.1. Cell culture, reagents and hypoxia incubation
Human lung cancer cell lines were purchased from China Center for Type Culture Collection (Wuhan, China) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum (FBS) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) in a 5% carbon dioxide incubator. Antibodies against HIF‐1a, PARP, Bim and actin were purchased from Cell Signaling Technology (Boston, MA, USA). To expose the cells to hypoxia, the cancer cells were maintained in a Billups‐Rotenburg chamber (Billups‐Rothenberg, Inc., Del Mar, CA, USA) with 1% O2, 94% N2 and 5% CO2 for the indicated durations.
2.2. Transient transfection
MicroRNA‐301b mimics, microRNA‐301b inhibitor and the scramble control were purchased from GenePharma (Shanghai, China). The cells were cultured until they reached 60% confluency, at which time they were transfected with the microRNA‐301b mimics, microRNA‐301b inhibitor and scramble control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. At 24 or 48 hours after transfection, the cells were collected for subsequent experiments.
2.3. Vector construction
To construct the luciferase reporter vector, the wild‐type 3′‐UTR of human Bim mRNA (GenBank accession number: NM_001204106.1) or mutant 3′‐UTR, which contained the putative microRNA‐301b‐binding sequence, was subcloned into the pmirGLO‐control vector.
2.4. RNA extraction and real‐time RT‐PCR
Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), according to the manufacturer's protocol. The expression of microRNAs was measured using the TaqMan MicroRNA Assay (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer's instruction. Briefly, cDNA was synthesized from 200 ng of total RNA in a 15 μl reaction. The cDNAs were then amplified by quantitative PCR using TaqMan microRNA Assays Human Panel sequence‐specific primers. The expression of RNU48 was used as an internal control.
2.5. Protein extraction and Western blots
The cells were collected and quickly washed twice with ice‐cold phosphate‐buffered saline (PBS). The cell pellets were solubilized for 15 min on ice in a lysis buffer containing a protease inhibitor mixture. Then, the cell lysates were centrifuged at 12 000 g for 10 minutes. The supernatants were retained and boiled for 10 minutes. Equal amounts of total protein were separated on 10% SDS‐PAGE gels and transferred onto polyvinylidene fluoride microporous membranes (PVDF; Bio‐Rad, Hercules, CA, USA). The membranes were blocked with 5% non‐fat milk for 1 hour at 37°C and subsequently incubated with the appropriate primary antibody. After washing, the membranes were incubated with a secondary antibody. The proteins were detected by chemiluminescence.
2.6. Luciferase reporter assay
HEK293T cells were seeded at a density of 5000 cells in a 96‐well plate. The next day, the luciferase vectors (pmirGLO‐Bim‐3′UTR‐WT or pmirGLO‐Bim‐3′UTR‐MUT) and microRNA‐301b mimics or scramble control were transfected into HEK293T cells using Lipofectamine 2000. At 48 hours after transfection, the cells were harvested and analysed using the Dual‐Luciferase Reporter Assay System (Promega, Madison, WI, USA). The transfections were performed in duplicate, and at least three independent experiments were performed.
2.7. Cell viability assays
Cells were seeded at 5000 cells per well in 96‐well plates and were transfected with microRNA‐301b mimics or a microRNA‐301b inhibitor with Lipofectamine 2000. At 1, 2, 3, 4 and 5 days post‐transfection, cells were labelled with Cell Counting kit‐8 (DOJINDO, Tabaru, Japan) for 2 hours. The absorbance was measured at 450 nm.
2.8. Apoptosis assay
Cells were transfected with miR‐301b mimics or miR‐301b inhibitor and scramble control and were incubated for 48 hours. After transfection, cells were harvested and stained with 25 ng/ml annexin V and 10 mg/ml propidium iodide and incubated for 15 minutes at room temperature in the dark. Subsequently, apoptosis was measured using flow cytometry (BD Biosciences, San Jose, CA, USA).
2.9. Tumourigenicity assay in nude mice
All experiments involving animals were undertaken in accordance with the Shandong University of Health Guide for the Care and Use of Laboratory Animals. Specifically, U2OS cells (1×106 cells) stably expressing miR‐96 or the vector were inoculated bilaterally into the armpit region of 4‐week‐old immunodeficient nude mice. The weights of the tumours were measured 30 days after inoculation.
2.10. TUNEL analysis
OCT‐embedded sections of the tumours were fixed with 4% paraformaldehyde. After permeabilization with 0.1% Triton X‐100 in PBS for 10 minutes, the slides were incubated with methanol containing 3% H2O2, followed by dUTP nick end labelling at 37°C for 1 hour.
2.11. Statistical analyses
All experiments were performed in triplicate, and the data are shown as the mean±SD, where applicable. Statistical analysis was performed with Graphpad Prism (Graphpad Software Inc., La Jolla, CA, USA). The statistical significance between compared groups was determined by Student's t test. A P<.05 was considered to be statistically significant.
3. Results
3.1. miR‐301b is up‐regulated in lung cancer
To examine the expression level of microRNA‐301b in human lung cancer, we downloaded the microRNA expression profile from a previously published data set (Gene expression omnibus accession GSE25631).24 We analysed the expression of microRNA‐301b and found that it was significantly up‐regulated in lung cancer (Fig. 1a,b). Next, to further confirm the overexpression of microRNA‐301b in lung cancer tissues, a total of five paired lung cancer tissues and normal adjacent tissues were biopsy obtained prior to any treatment. The subtype of the lung cancer is non‐small cell lung cancer, which were histological diagnosed with stage II or stage III. We performed quantitative PCR to test the miR‐301b level in this sample. The results demonstrated that microRNA‐301b is markedly increased in lung cancer compared to adjacent normal tissues (Fig. 1c). In addition, microRNA‐301b was markedly higher in lung cancer cell lines than in normal cell lines (Fig. 1d). In conclusion, these data show that microRNA‐301b is up‐regulated in human lung cancer tissues.
Figure 1.
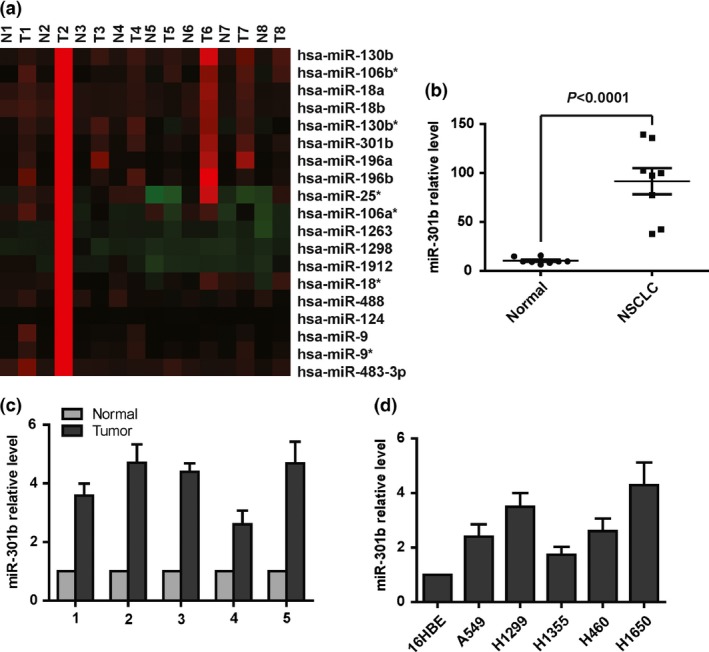
MicroRNA‐301b is overexpressed in lung cancer. (a) Heat map showing the expression of selected microRNAs in lung cancer tissues compared with normal tissues. (b) The statistical analysis of microRNA‐301b expression in lung cancer tissues compared with normal tissues. (c) Real‐time PCR analysis showing that microRNA‐301b expression is significantly higher in human lung cancer tissues than in normal tissue. (d) Real‐time PCR analysis showing that microRNA‐301b is overexpressed in lung cancer cell lines compared with a normal cell line
3.2. Hypoxia induces miR‐301b expression in lung cancer
A growing number of studies have showed that many microRNAs are differentially induced by hypoxia.14, 25, 26 However, whether microRNA‐301b is induced by hypoxia in lung cancer cells has not been reported. We treated A549 and H460 cells with 1% oxygen or with DMOG, a well‐established agent that is used to mimic hypoxic conditions,26 and measured the expression of mature microRNA‐301b by quantitative PCR. The results showed that the expression of microRNA‐301b was markedly increased under hypoxic conditions (Fig. 2a,b). As expected, Western blot showed that the level of HIF1‐a was higher in A549 and H460 cells than in control cells (Fig. 2c,d). HIF1‐a is a major transcription factor that regulates the expression of downstream target genes in response to hypoxia.27 Next, we knocked down HIF1‐a expression with shRNA under hypoxic conditions (Fig. 2e) and found that microRNA‐301b was decreased when HIF1‐a was knocked down (Fig. 2f). In conclusion, hypoxia induces the expression of microRNA‐301b in lung cancer.
Figure 2.
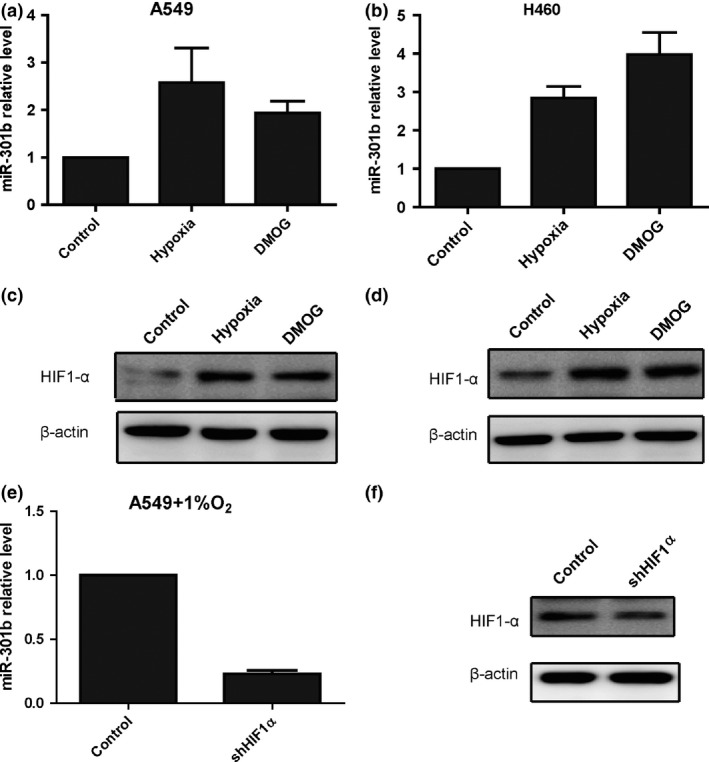
Hypoxia induces the expression of microRNA‐301b in lung cancer cells. (a) Quantitative PCR assays showing that hypoxia and DMOG treatment increase the level of mature microRNA‐301b in A549 cells. (b) Quantitative PCR assays showing that hypoxia and DMOG treatment increase the level of mature microRNA‐301b in H460 cells. (c) Western blot assays showing that the protein level of HIF1α is increased in A549 cells under hypoxic conditions and following DMOG treatment. (d) Western blot assays showing that the protein level of HIF1α is increased in H460 cells under hypoxic conditions and following DMOG treatment. (e) Quantitative PCR assays showing that the level of microRNA‐301b is decreased in A549 cells treated with shRNA‐HIF1α under hypoxic conditions. (f) Western blot assays showing that the level of HIF1α is decreased in A549 cells treated with shRNA‐HIF1α under hypoxic conditions
3.3. MicroRNA‐301b enhances cell proliferation, reduces apoptosis and increases resistance to chemotherapy
Having shown that the expression of microRNA‐301b was significantly increased in lung cancer tissues, we sought to determine the biological significance of microRNA‐301b in lung cancers. We transfected lung cancer cells with microRNA‐301b mimics or inhibitor to markedly reduce or restore, respectively, intracellular microRNA‐301b expression (Fig. 3a). Then, we assessed the effects of the modulating microRNA‐301b levels on cell proliferation using the CCK8 assay. The results showed that proliferation of the cells was suppressed following transfection with microRNA‐301b inhibitors (Fig. 3b). In contrast, the growth rate was increased in cells transfected with microRNA‐301b mimics (Fig. 3b). Next, the ability of microRNA‐301b to induce apoptosis was evaluated by co‐staining with propidium iodide (PI) and Annexin V and analysed by flow cytometry. The data showed that the overexpression of microRNA‐301b suppressed apoptosis (Fig. 3c,d). In contrast, the inhibition of microRNA‐301b decreased the rate of apoptosis (Fig. 3c,d). To further confirm the effect of microRNA‐301b on apoptosis, we performed Western blotting to detect the level of cleaved PARP, a well‐known marker of apoptosis. The data showed that the overexpression of microRNA‐301b reduced the level of cleaved PARP (Fig. 3e). In addition, the survival ratio of A549 cells treated with 10 nmol/L Dox was evaluated using the CCK8 assay. The data showed that the survival ratio was increased in cells transfected with microRNA‐301b mimics (Fig. 3f), indicating that microRNA‐301b reduced the sensitivity of these cells to drug treatment. In conclusion, these results demonstrate that microRNA‐301b increases cell growth, reduces cell apoptosis and improves the resistance of cells to drug treatment.
Figure 3.
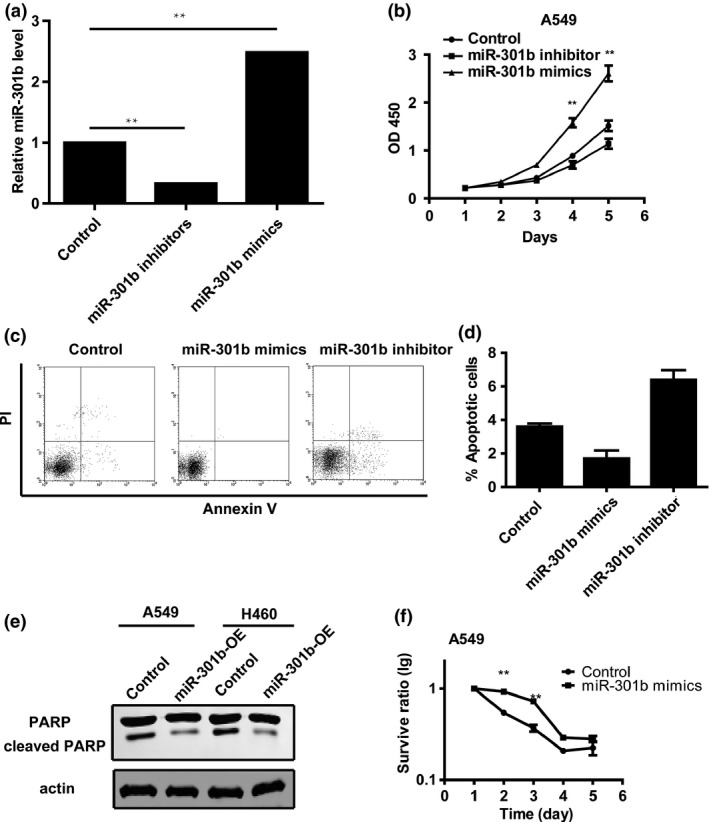
MicroRNA‐301b increases cell proliferation, reduces apoptosis and enhances drug resistance in lung cancer. (a) Real‐time PCR analysis of microRNA‐301b levels in A549 cells transfected with microRNA‐301b mimics or inhibitor. (b) CCK8 assay results showing that the proliferation rate of microRNA‐301b‐overexpressing cells is increased compared to control cells. Conversely, the knockdown of microRNA‐301b enhances cell growth. (c) A549 cells were transfected with scramble, microRNA‐301b mimics or microRNA‐301b inhibitor. The cells were stained with PI and Annexin V and analysed by FACS. (d) The annexin V‐positive populations of A549 transfected with scramble, microRNA‐301b mimics or microRNA‐301b inhibitor. (e) Western blot analysis showing that microRNA‐301b overexpression decreases the levels of cleaved PARP. (f) CCK8 assays showing that the overexpression of microRNA‐301b can increase the resistance of lung cancer cells to doxorubicin
3.4. MicroRNA‐301b directly targets the expression of Bim, a well‐known pro‐apoptotic protein
To explore the underlying mechanisms by which microRNA‐301b regulates cell proliferation and apoptosis, we used bioinformatic tools (miRanda and Targetscan) to predict potential downstream targets.28 Among these candidate targets, we focused on Bim, a well‐known pro‐apoptotic protein that is involved in cell growth and apoptosis. A potential targeting sequence for microRNA‐301B is present in the 3′UTR of Bim (Fig. 4a), and to verify whether Bim is a target of microRNA‐301b, we assessed the levels of endogenous Bim protein in lung cancer cells treated with microRNA‐301b mimics or inhibitor. As shown by Western blotting, the protein level of Bim was substantially decreased in cells transfected with microRNA‐301b mimics compared with control cells (Fig. 4b). As expected, Bim was significantly up‐regulated in cells treated with the microRNA‐301b inhibitor (Fig. 4c). Next, to assess the interaction of microRNA‐301b with Bim, we performed luciferase reporter assays. We found that microRNA‐301b caused a significant decrease in luciferase activity in cells transfected with the plasmid containing the wild‐type sequence, but not in those transfected with the plasmid containing the mutant sequence (Fig. 4d). To examine whether Bim is required for the effect of microRNA‐301b on apoptosis, we overexpressed Bim in A549 cells transfected with microRNA‐301b mimics and then measured the protein level of cleaved PARP. The results showed that the overexpression of Bim clearly restored the protein level of Bim in A549 cells transfected with microRNA‐301b mimics (Fig. 4e). As expected, the levels of cleaved PARP in the cells that were co‐treated with microRNA‐301b mimics and Bim overexpression was restored compared with cells transfected with microRNA‐301b alone (Fig. 4f). Taken together, the results showed that the effect of microRNA‐301b on apoptosis is mediated by Bim.
Figure 4.
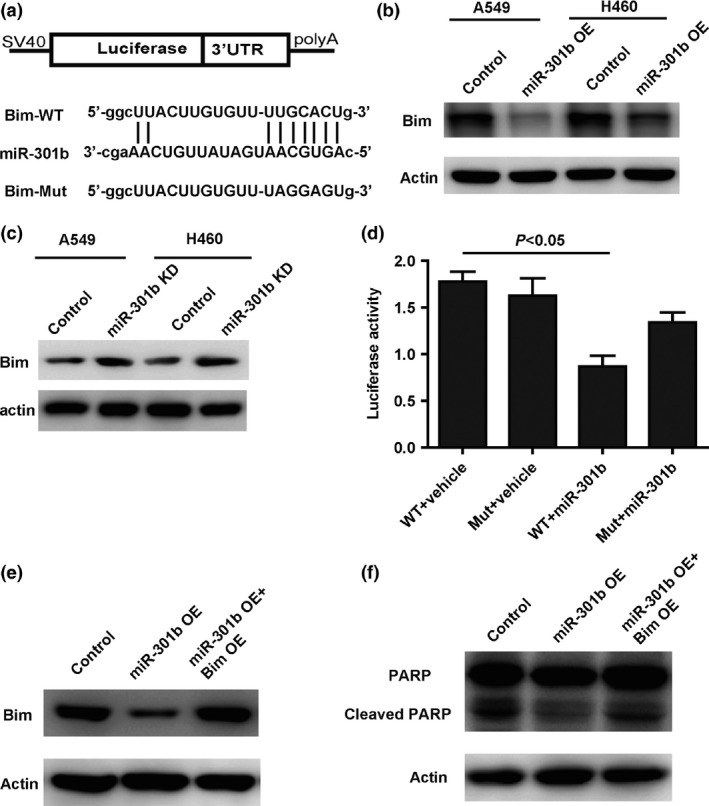
MicroRNA‐301b down‐regulates the expression of Bim by directly binding to its 3′UTR. (a) The sequences in the 3′UTR of Bim that were predicted as potential binding sites for microRNA‐301b. (b) Western blot analysis showing that the overexpression of microRNA‐301b suppresses the protein level of Bim in A549 and H460 cells. (c) Western blot analysis showing that the knockdown of microRNA‐301b increases the protein level of Bim in A549 and H460 cells. (d) A luciferase reporter activity assay showing that microRNA‐301b suppresses the luciferase activity of the plasmid with the wild‐type UTR, but not of the plasmid with the mutant UTR. (e) Western blot assay showing that overexpression of Bim rescues the protein level of Bim in cells transfected with microRNA‐301b mimics. (f) Restored expression of Bim increases the cell apoptosis
3.5. The overexpression of microRNA‐301b increases tumour growth and decreases apoptosis in vivo
To further explore the impact of microRNA‐301b on tumour growth and apoptosis in vivo, cells overexpressing microRNA‐301b or control cells were subcutaneously injected into Balb/c nude mice. After 30 days, the mice were sacrificed and the tumour weights were measured. The tumour size in the microRNA‐301b overexpression group was significantly increased compared with the control group, and the average weight of tumours in the microRNA‐301b overexpression group was significantly higher than in the control group (Fig. 5a,b). In addition, in situ TUNEL analysis on cryogenic sections from xenograft tumour demonstrated that the tumours overexpressing microRNA‐301b had fewer TUNEL‐positive cells than the control cells (Fig. 5c,d). We also performed Western blotting to assess the protein level of Bim in the tumours. As expected, microRNA‐301b significantly reduced the protein level of Bim in vivo (Fig. 5e). Finally, we performed a comprehensive analysis of the relationship between microRNA‐301b and Bim expression in clinical lung cancer cases that were downloaded from a previously published data set and found that microRNA‐301b expression showed a strong inverse correlation with Bim levels (Fig. 5f). In conclusion, these results suggest that microRNA‐301b increases tumour growth and reduces apoptosis by modulating Bim levels in vivo.
Figure 5.
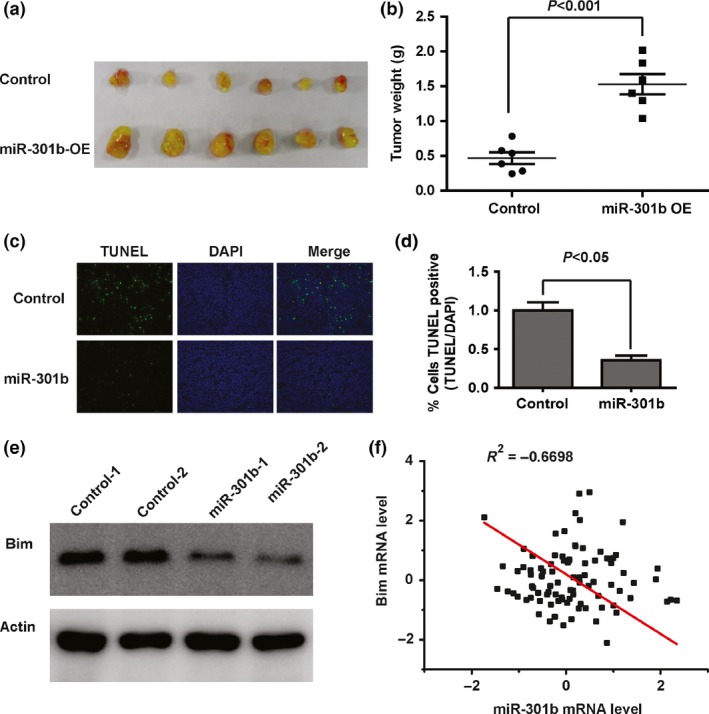
MicroRNA‐301b increases tumour growth and decreases apoptosis in vivo. (a) A representative photo showing the xenograft tumours. (b) The tumour weights were of the two groups. (c) The TUNEL‐positive cells were analysed in the tumour sections (original magnification, 20X). (d) The percentage of TUNEL‐positive cells in the two groups was quantified. (e) The expression of PDCD4 in xenograft tumours was analysed by Western blotting. (f) Correlation analysis of microRNA‐301b and Bim expression in clinical lung cancer samples
4. Discussion
A growing number of studies have reported the critical role of microRNAs as tumour suppressive or oncogenic genes that can modulate cell growth and apoptosis through the regulation of different downstream target genes in lung cancer.29 Therefore, a potential therapeutic strategy would be to target onco‐miRNAs or restore the expression of tumour suppressor miRNAs for the treatment of lung cancer. MicroRNA‐301b has been reported to be associated with vasoconstriction in pulmonary hypertension.19 Recently, the aberrant expression of microRNA‐301b has been implicated in the development of some human cancers. For example, microRNA‐301b is up‐regulated in pancreatic, colorectal and oral carcinoma.20, 21, 22, 23 However, the biological function of microRNA‐301b in lung cancer cells remained incompletely understood.
In this study, we studied the expression profile of microRNAs in lung cancer samples. The results showed that miR‐301b was up‐regulated in lung cancer, and we further confirmed this by comparing lung cancer tissues and cell lines with normal tissues and cell lines by real‐time PCR.
Hypoxia is a characteristic feature of the microenvironment in various solid cancers, and it has emerged as a pivotal factor in cancer development because it can promote cancer progression. A growing body of data have shown that hypoxia can induce the expression of many miRNAs in various cancers. In this study, we show that hypoxia can induce the expression of miR‐301b expression in lung cancer cells. We are the first to show that miR‐301b is up‐regulated in lung cancer and that its expression can be induced by hypoxia.
The role of microRNA‐301b in lung cancer is not well defined. Our results showed that the inhibition of microRNA‐301b significantly suppressed cell proliferation and increased apoptosis in lung cancer cells. By contrast, the overexpression of microRNA‐301b significantly enhanced cell growth and reduced apoptosis. We also found that the inhibition of microRNA‐301b suppressed apoptosis by directly targeting Bim and altering its expression level. Furthermore, in vivo tumour formation assays in nude mice showed that the overexpression of microRNA‐301b in lung cancer cells increased tumour growth. A previous report showed that miR‐301b may have potential as a novel therapeutic target for cancer treatment due to its effects on cell invasiveness. The data presented here show that miR‐301b may function as oncomiR by regulating cell growth and apoptosis.
Briefly, our results demonstrate that hypoxia‐induced microRNA‐301b expression enhances cell growth and reduces apoptosis by regulating Bim expression. Therefore, microRNA‐301b is a potential target for chemotherapy, and knocking it down with microRNA‐301b antagomirs may be effective in the treatment of lung cancer.
References
- 1. Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non‐small‐cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Dincklage JJ, Ball D, Silvestri GA. A review of clinical practice guidelines for lung cancer. J Thorac Dis 2013;5(Suppl 5):S607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng L, Alexander RE, Maclennan GT, et al. Molecular pathology of lung cancer: key to personalized medicine. Mod Pathol. 2012;25:347. [DOI] [PubMed] [Google Scholar]
- 4. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non‐small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rankin SC. Staging of non‐small cell lung cancer (NSCLC). Cancer Imaging. 2006;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Latimer KM, Mott TF. Lung cancer: diagnosis, treatment principles, and screening. Am Fam Physician. 2015;91:250. [PubMed] [Google Scholar]
- 7. Zhang D, Wang Y, Shi Z, et al. Metabolic reprogramming of cancer‐associated fibroblasts by IDH3alpha downregulation. Cell Rep. 2015;10:1335. [DOI] [PubMed] [Google Scholar]
- 8. Lages E, Ipas H, Guttin A, Nesr H, Berger F, Issartel JP. MicroRNAs: molecular features and role in cancer. Front Biosci (Landmark Ed). 2012;17:2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li C, Feng Y, Coukos G, Zhang L. Therapeutic microRNA strategies in human cancer. AAPS J. 2009;11:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwak PB, Iwasaki S, Tomari Y. The microRNA pathway and cancer. Cancer Sci. 2010;101:2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460. [DOI] [PubMed] [Google Scholar]
- 13. Shen G, Li X, Jia YF, Piazza GA, Xi Y. Hypoxia‐regulated microRNAs in human cancer. Acta Pharmacol Sin. 2013;34:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang D, Shi Z, Li M, Mi J. Hypoxia‐induced miR‐424 decreases tumor sensitivity to chemotherapy by inhibiting apoptosis. Cell Death Dis. 2014;5:e1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chio CC, Lin JW, Cheng HA, et al. MicroRNA‐210 targets antiapoptotic Bcl‐2 expression and mediates hypoxia‐induced apoptosis of neuroblastoma cells. Arch Toxicol. 2013;87:459. [DOI] [PubMed] [Google Scholar]
- 16. Rebucci M, Sermeus A, Leonard E, et al. miRNA‐196b inhibits cell proliferation and induces apoptosis in HepG2 cells by targeting IGF2BP1. Mol Cancer. 2015;14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun G, Peng H. HIF‐1alpha‐induced microRNA‐210 reduces hypoxia‐induced osteoblast MG‐63 cell apoptosis. Biosci Biotechnol Biochem. 2015;79:1232. [DOI] [PubMed] [Google Scholar]
- 18. Sun G, Zhou Y, Li H, et al. Over‐expression of microRNA‐494 up‐regulates hypoxia‐inducible factor‐1 alpha expression via PI3K/Akt pathway and protects against hypoxia‐induced apoptosis. J Biomed Sci. 2013;20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bertero T, Cottrill K, Krauszman A, et al. The microRNA‐130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem. 2015;290:2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miko E, Czimmerer Z, Csanky E, et al. Differentially expressed microRNAs in small cell lung cancer. Exp Lung Res. 2009;35:646. [DOI] [PubMed] [Google Scholar]
- 22. Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. J Dig Dis. 2010;11:50. [DOI] [PubMed] [Google Scholar]
- 23. Lu YC, Chen YJ, Wang HM, et al. Oncogenic function and early detection potential of miRNA‐10b in oral cancer as identified by microRNA profiling. Cancer Prev Res (Phila). 2012;5:665. [DOI] [PubMed] [Google Scholar]
- 24. Chen L, Zhang W, Yan W, et al. The putative tumor suppressor miR‐524‐5p directly targets Jagged‐1 and Hes‐1 in glioma. Carcinogenesis. 2012;33:2276. [DOI] [PubMed] [Google Scholar]
- 25. Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blick C, Ramachandran A, McCormick R, et al. Identification of a hypoxia‐regulated miRNA signature in bladder cancer and a role for miR‐145 in hypoxia‐dependent apoptosis. Br J Cancer. 2015;113:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bandara V, Michael MZ, Gleadle JM. Hypoxia represses microRNA biogenesis proteins in breast cancer cells. BMC Cancer. 2014;14:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zandberga E, Kozirovskis V, Abols A, Andrejeva D, Purkalne G, Line A. Cell‐free microRNAs as diagnostic, prognostic, and predictive biomarkers for lung cancer. Genes Chromosom Cancer. 2013;52:356. [DOI] [PubMed] [Google Scholar]


