Abstract
Intervertebral disc degeneration (IDD) is a chronic, complex process associated with low back pain; mechanisms of its occurrence have not yet been fully elucidated. Its process is not only accompanied by morphological changes, but also by systematic changes in its histological and biochemical properties. Many cellular and molecular mechanisms have been reported to be related with IDD and to reverse degenerative trends, abnormal conditions of the living cells and altered cell phenotypes would need to be restored. Promising biological therapeutic strategies still rely on injection of active substances, gene therapy and cell transplantation. With advanced study of tissue engineering protocols based on cell therapy, combined use of seeding cells, bio‐active substances and bio‐compatible materials, are promising for IDD regeneration. Recently reported progenitor cells within discs themselves also hold prospects for future IDD studies. This article describes the background of IDD, current understanding and implications of potential therapeutic strategies.
Introduction
Intervertebral disc degeneration (IDD) is perhaps best defined as a cascade that begins with changes to the local cellular microenvironment and progresses to impairment of their structure and function 1. Prominent changes to IDD are characterized by reduction in active cell numbers, depletion of extracellular matrix (ECM), altered phenotype of normal disc cells, and presence of pro‐inflammatory cytokines and mediators 2, 3. These cell and molecular changes impose a profound influence on progress of IDD and further impair normal function of the intervertebral disc (IVD) and a patient's quality of life. Aetiology of IDD is difficult to precisely characterized, as the degenerative progression can be attributed to multiple factors 4. Ageing, living conditions and biomechanical loading are often related to it; genetic factors can also result in disc degeneration 5. This review article attempts to summarize current understanding of molecular and cellular biology in normal and pathological IVD, as well as to account for potential biological therapeutic strategies for clinical application.
Overview of IDD
According to recent statistics, low back pain (LBP) affects more than half our population during their lives and impacts heavily on the economy and quality of life of the patients 6, 7. With progression in understanding of IDD, many techniques including examination, imaging investigations 8 and trials of intervention 9 have been applied from different directions, to discover causes of the syndrome and to alleviate the pain. IDD can play a pivotal role in LBP and correlates with disc structural breakdown and dysfunction.
Disc degeneration often occurs with advances in ageing, and water content and proteoglycans reduce gradually 10. Lower aggrecan content leads to dehydration which in turn also impairs mechanical functions 11, 12. In addition, genetic, mechanical, environmental and behavioural factors must also be taken into consideration 13.
Over the last two decades, underlying mechanisms of IVD metabolism have benefitted from significant progress. New techniques to examine human tissues, such as immunohistochemistry 14, in situ zymography 15, in situ hybridization 16 and quantitative image analysis 17 have helped to update understanding of mechanisms of IDD.
Current therapeutic strategies to assuage disc degeneration mainly lie in conservative therapies, including physiotherapy and anti‐inflammatory medication. Although these conservative strategies are often able to alleviate symptoms, actual causes of the degeneration are not addressed. When considering spinal surgery, however, adjacent spine segments may experience high risk of accelerated degeneration post‐operatively 18, 19; thus novel therapeutics have emerged and brought new light to address the issue. With the advent of biotherapy, innovative methods may help to restore disc structure and mechanical function.
Structure and function of the IVD
The IVD is a viscoelastic weight‐bearing ‘cushion’ which plays a major role in maintaining flexibility and stability of the spine 20. Anatomically, the outer region [annulus fibrosus (AF)] is composed of lamellae mainly of bundles of type I collagen. The AF is oriented to alternate lamellae to form an angle‐ply structure 21, 22. Central region of the IVD [nucleus pulposus (NP)] consists of type II collagen and aggrecan, but networks of the type II collagen are less organized compared to type I collagen of the AF.
The space‐filling proteoglycan accounts for the most part of the NP and forms large macromolecular aggregates enclosed by the type II collagen network 23. Versican is a further kind of IVD proteoglycan, found in regions between adjacent annular lamellae, which may lubricate collagen bundles 24, 25, 26. These networks effectively mobilize water content of the disc and maintain its internal hydrostatic pressure. There are two thin cartilaginous endplates (EP). EP extend superiorly and inferiorly over the inner AF and NP to separate vertebral bodies and supply nutrients to discs by diffusion 27. The EP provides resilience to prevent collision between vertebral bodies and absorbs load transmitted.
The main function of the IVD is mechanical, it transfers load and provides spinal mobility. Biomechanics play an important role in the process of IDD; unusual complex injury or trauma are often considered to be its major risk factors. Interactions between NP and AF structures contribute to distribution and transmission of the loads between the vertebral bodies 28. When a disc is under high load, hydrostatic pressure is generated within the NP then conducted to the outer AF, generating circumferential stress within the lamellar structure 29. Pressure can also be supported by the inner AF, and proteoglycans may serve as a cushioned pad to slow this process 30. High loads are generated by the human upright posture and load‐bearing activities. Under these, the IVD deforms and its hydrostatic pressure increases. Fluid is slowly squeezed out of the disc which further results in higher osmolarity and lower pH. Loading affects IVD cell metabolism, as changes in physical microenvironment of cells, including fluid content, osmolarity and pH, change the supply of nutrients and bioactive factors within the disc.
Cell and molecular biology of the normal IVD
Cell and molecular biology of IDD are not totally clear, but most experts believe that it is caused by many factors. Increase in pro‐inflammatory cytokines, reduction in disc cell numbers and impaired cell viability are normal changes that occur to ageing discs. These lead to modifications in cell and molecular composition of the IVD. A better understanding of normal cell and molecular development of the IVD will help us correct current errors and discover ideal therapeutic strategies.
Cell components of IVD and their inter‐relationships
The cell population executes crucial machinery for synthesizing and maintaining the IVD matrix. Most cells found in adult NP are small and chondrocyte‐like. However, in both juveniles and adults, a further cell type has been proposed to function in renewal and homoeostasis of the IVD. These are large vacuolated cells of notochordal origin 31. Loss of notochordal cells coincides with the onset of IDD which suggests that this cell population may be involved in maintenance and regeneration of the IVD 32. Human notochord cells have been observed to gradually disappear with ageing, and their depletion correlates with disc degeneration 33, 34. It should also be acknowledged that during the process of disc degeneration, there is remoulding of NP and AF tissues. Based on previous reports, early degenerate adult discs may preserve a population of skeletal progenitor cells. To make this perspective more clear and to assert whether this kind of progenitor/stem cell really exists in human IVDs, innovative studies have confirmed their presence 35, 36. These studies indicated that progenitor cells were present in AF and NPs and express a repertoire of membrane markers common to bone marrow stem cells. Further investigations ensured their presence and defined tissue zones of the skeletal progenitors in the mature rat 37.
More recently, a study undertaken by Sakai et al. 38 identified populations of progenitor cells that were Tie2 positive (Tie2+) and disialoganglioside 2 positive (GD2+) in NP from mice and from humans. This study has far‐reaching impact on our present understanding of cell types of the IVD. They express type II collagen and aggrecan and also they can differentiate into mesenchymal lineages and induce reorganization of NP tissues. Tie2, GD2 and CD24 were proposed to be important markers for recognition of hierarchy of progenitor cells isolated from NP. The sequence originated from Tie2+GD2‐CD24‐ cells, followed in order by Tie2‐GD2+CD24‐ cells and Tie2‐GD2‐CD24‐ cells. Of these, Tie2‐GD2+CD24‐ cells had better self‐renewal capacity and NP tissue reorganization potential. This improved our current information in understanding IVD cell biology.
ECM components of IVD and their role
Structure of the IVD changes with advancing age, specially in composition of disc ECM; these eventually can lead to IDD. Type I and II collagens are the main components of the IVD, accounting for in the region of 80% of IVD collagen between them. Type I collagen makes up major parts of the outer AF and plays an important role in anti‐stretching and repairing damaged tissue 39. Type II collagen is mainly located in inner layers of the AF and NP with the functions of maintaining water content, withstanding and absorbing conductive pressure 40. The major proteoglycan of the disc is aggrecan which occounts for osmotic properties and helps maintain disc height and ability to withstand compression 41, 42.
Molecular biology of the IVD: mechanisms of IVD homoeostasis
The molecular biology of the IVD is complex, with many growth factors, genes and proteinases involved. These molecules coordinate with each other and maintain homoeostasis (Fig. 1). Transforming growth factor‐β (TGF‐β) is comprised of a series of peptides and is considered to have the highest relative relationship with collagen metabolism. TGF‐β not only regulates synthesis of collagen and proteoglycan, but also affects IVD metabolism. PR‐PCR techniques suggest that TGF‐β is a key factor in maintenance and degenerating processes of the IVD 43. Current study has revealed that TGF‐β signalling is essential for endplate cartilage growth over post‐natal life 44. Bone morphogenic protein (BMP) is also a multi‐functional growth factor, belonging to TGF‐β superfamily. BMP receptors are found in the IVD, further indicating that its cells react to growth factors of the BMP superfamily 45. Further investigation indicated that the ligand–receptor model is the way in which BMP plays a pivotal role to regulate events of the IVD, such as increasing synthesis of proteoglycan, upregulating mRNA expression of type II collagen and serving as mitotic agent 46, 47. Under hypoxic conditions, NP cells upregulate expression of vascular endothelial growth factor‐A (VEGFA) and its receptor and membrane‐bound vascular endothelial growth factor receptor‐1 (mbVEGFR‐1), which plays an important role in survival of NP cells 48. Currently, Ang‐1, a ligand of Tie2 was reported to have an innate anti‐apoptotic effect on hNP cells by Sakai et al. 38. According to their study, when added the Tie2‐blocking antibody to the serum‐free culture medium, the number of apoptotic NP cells increased about 2‐fold.
Figure 1.
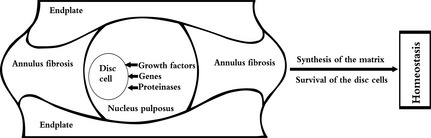
Sagittal structure and homoeostasis of the intervertebral disc.
Molecular biology of the IVD is complex, with many growth factors, genes and proteinases involved. These coordinate with each other to promote synthesis of matrix and survival of disc cells, which help to maintain IVD homoeostasis.
The Sox9 gene is a member of the Sox gene family and is an important transcription factor in the process of type II collagen synthesis. In development of cartilage, Sox9 is an enhancer of type II collagen and remains the most promising gene target for IVD regeneration. When transfected with adenovirus‐mediated Sox9, IVD cells proliferate, and synthesis of proteoglycan and type II collagen follows an upward trend 49, 50.
The IVD is the largest human avascular organ which lacks all immune cells. Destruction of the immune status may lead to degeneration of the IVD. Fas is a type I transmembrane glycoprotein of disc cells. When the Fas ligand of type II transmembrane glycoprotein binds to FasL antibody, death signals are passed into the cell which further results in degeneration of the IVD 51, 52.
Matrix metalloproteinases (MMP) are proteolytic enzymes with metal ions structured within them. They can be divided into five subfamilies, of which MMP‐3 is a matrix enzyme, playing a significant role in degradation of the ECM; imbalance of these enzymes and their inhibitors may lead to IDD 53, 54, 55. Interleukin (IL) is mainly derived from bone marrow stromal cells, monocytes, macrophages, myeloma cells and osteoblasts. IL‐1, a single nuclear factor, is composed of both IL‐1α and IL‐1β. IL‐1 may initiate nerve root pain induced by other inflammatory factors. In addition, IL‐1β can stimulate the IVD to release MMPs which degrade proteoglycan, with concentration and time dependency. In addition, IL‐1β increases the body's sensitivity to pain and plays an important role in metabolism of the cartilage matrix 56. Other ILs are also found in the IVD, such as IL‐6, which may induce sciatica and aggregate inflammatory responses in it.
Cell and molecular hallmarks of IDD
Degenerative changes to the IVD are accompanied by cell and molecular changes. Wide genetic studies have been able to prove the importance of heredity in processes of IDD 57, 58, 59, and at least 14 genes have been shown to be associated with disc degeneration, although their specific functions at the moment remain unknown. Recent investigations have revealed that IL6, SKT and CILP are involved in aetiology of IDD in young adults 60, 61, 62. According to work by Seki et al. 63, CILP protein plays an important role in IVD homoeostasis, based on controlling TGF‐β signals. Several other genes, such as those that code for aggrecan, MMP‐3 and type‐IX collagen, may affect structure and progression of IDD, which still need further investigation.
With harsh conditions of nutrient diffusion through the cartilaginous EP, IVD may undergo continuous increase in levels of cell death 64. However, proliferation is also often seen in degenerating IVD, specially amongst chondrocyte‐like cells 65. Cell death is so commonly observed that it has been taken as an indicator of IDD; as there are no efficient phagocytes in the disc, dead cells are not promptly removed and remain in the matrix for relatively long periods of time. Dead cells, together with proliferating cells eventually increase cell density 66, 67, 68. Meanwhile, numbers of viable cells do not increase with cell density, their number decreases with ageing and degeneration 69. Degenerate disc cells generally have an altered phenotype which includes changes in morphology, metabolism gene expression and more. These features of degenerate cells have been observed as accelerated cell senescence 70. It has been reported that degenerate annular cells become more rounded and chondrocytic, whereas under normal conditions, they are more spindle shaped 71. Annular cells have stellate appearance during degenerative events, with multiple, branching cytoplasmic processes extending into their surrounding matrix 72.
During ageing, numbers of notochordal cells reduce and vary towards more chondrogenic phenotype, observed in the NP region 73. Although the mechanism of disappearance of notochordal cells is unknown, apoptosis induced by antocrine or paracrine Fas‐mediated counterattack are suspected in the process 74. Without sufficient number and activity of cells, the IVD is not able to produce and maintain large matrix molecules which further aggravate loss of proteoglycans and shift in collagen synthesis. Specifically, type I collagen, rarely present in the NP begins to be expressed, while type II collagen, the main NP collagen, is sharply downregulated 75.
Up to now, progenitor cells within discs have been poorly characterized. Tie2+ cells in NP tissues have recently been proven to be progenitor cells, with self‐renewal capacity and multiple differentiation potential 38. Frequency of Tie2+ NP cells reduces with increasing age and is correlated to extent of disc degeneration, which strongly suggests that exhaustion of these cells may cause IVD degeneration itself.
Relevance of cell and molecular biology to potential therapies for IDD
During the process of disc degeneration, reduction in number of viable cells and phenotypic changes to live ones need to be taken into consideration when designing any biological therapeutic applications. Direct injection of active substance therapy, gene therapy and cell transplantation therapy remain to be promising biological strategies.
Active substances therapy
The most direct technique of delivering active substances to disc cells is injection into the IVD; promising results have been reported. Direct injection aims to promote synthesis of proteoglycan, and restoration of disc height. Many active substances, including multiple cytokines and growth factors, are able to move a cell catabolic state to an anabolic one, which is significant in maintaining IVD homoeostasis. Taking clinical applications into consideration, active substance injection into discs is less invasive compared to traumatic surgery, specially when under guidance of fluoroscopy.
A range of studies in vivo has confirmed efficacy of bio‐active substances, when used to repair and regenerate degenerate discs (Table 1). In an early murine IDD model, Walsh et al. 76 compared regenerative effects of a variety of growth factors, including growth and differentiation factor (GDF)‐5, TGF‐β, insulin‐like growth factor (IGF)‐1 and basic fibroblast growth factor (bFGF). IGF‐1 and TGF‐β induced expansion of inner annular fibrochondrocyte populations which actively expressed aggrecan and type II collagen mRNA. The growth factors tested increased cellularity and improved proliferation and a statistically significant increase in disc height was measured after GDF‐5 treatment. In a rabbit IDD model, GDF‐5 was also proven to be an effective bio‐active substance for its regeneration potential, including restoration of disc height, improvement in magnetic resonance imaging scores and histological grading scores 77. Osteogenic protein (OP)‐1 is a promising growth factor for its potential in stimulating production and formation of the ECM. Kawakami et al. 78 have demonstrated that OP‐1 injection into degenerate rat discs increased ECM and inhibited pain‐related behaviour. Subsequently, in the rabbit IDD models, several studies have shown the regenerative efficacy of OP‐1 in degenerate discs 79, 80, 81, 82.
Table 1.
Growth factors confirmed to have regenerative potential in degenerate intervertebral discs: in vivo studies
| Growth factors | Carriers | Species | Animal model of disc degeneration | Outcome | Study | Year |
|---|---|---|---|---|---|---|
| TGF‐β | (−) | Adult, male Swiss Webster mice | Caudal disc compression | Greater percentage of proliferating cells and increased population of anular fibrochondrocytes | Walsh et al. 76 | 2004 |
| IGF‐1 | (−) | Adult, male Swiss Webster mice | Caudal disc compression | Upward trend in cell density | Walsh et al. 76 | 2004 |
| bFGF | (−) | Adult, male Swiss Webster mice | Caudal disc compression | Upward trend in cell density | Walsh et al. 76 | 2004 |
| OP‐1 | (−) | Male Sprague‐Dawley rats | Caudal disc compression | Increased extracellular matrix without mechanical hyperalgesia | Kawakami et al. 78 | 2005 |
| OP‐1 | (−) | New Zealand white rabbits | Age‐related disc degeneration | Increased disc height index and proteoglycan content | An et al. 79 | 2005 |
| OP‐1 | (−) | New Zealand white rabbits | Needle puncture | Restoration of disc height; increase in water content and proteoglycan content | Masuda et al. 80 | 2006 |
| OP‐1 | (−) | New Zealand white rabbits | Needle puncture | Restoration of disc height and biomechanical properties; increase in proteoglycan and collagen content | Yoshiyuki et al. 81 | 2006 |
| OP‐1 | (−) | New Zealand white rabbits | Enzymatic digestion by chondroitinase ABC injection | Reverse of reduction in disc height | Imai Y et al. 82 | 2007 |
| GDF‐5 | (−) | Male Swiss Webster mice | Caudal disc compression | Upward trend in cell density and increase in disc height | Walsh et al. 76 | 2004 |
| GDF‐5 | (−) | New Zealand white rabbits | Needle puncture | Restoration of disc height, improvement of magnetic resonance imaging scores, and histological grading scores | Chujo et al. 77 | 2006 |
| PRP | Gelatin hydrogel microspheres | Male Japanese white rabbits | Nucleus pulposus aspiration (0.005–0.008 ng) | Suppressed progress of IVD degeneration | Nagae et al. 84 | 2007 |
| PRP | Gelatin hydrogel microspheres | Male Japanese white rabbits | Nucleus pulposus aspiration (0.005–0.008 ng) | Preserved disc height and water content; higher mRNA expression levels of PG core protein and type II collagen | Sawamura et al. 85 | 2009 |
| PRP | (−) | Sprague–Dawley rats | Needle puncture | Fewer inflammatory cells and higher fluid content on MRI | Gullung et al. 86 | 2011 |
| PRP | (−) | New Zealand white rabbits | Needle puncture | Restoration of disc height; higher quality of chondrocyte‐like cells | Obata et al. 87 | 2012 |
| PRP | (−) | New Zealand white rabbits | Needle puncture | Increased production of extracellular matrix and maintained MRI signal intensity | Hu et al. 88 | 2012 |
Active substances listed above have exhibited promising potential in regeneration of degenerate discs, indicating potential for active substance therapy.
TGF‐β1, transforming growth factor‐β1; IGF‐1, insulin‐like growth factor‐1; bFGF, basic fibroblastic growth factor; OP‐1, osteogenic protein‐1; GDF‐5, growth and differentiation factor‐5; PRP, platelets‐rich plasma.
However, single growth factor injection may have limitations, as it seems that no single growth factor is potent enough to reverse degenerative trends. As a natural carrier of multiple growth factors, platelet‐rich plasma (PRP) has been introduced into the field of IVD regeneration and enjoys some popularity. PRP is used as a fraction of autologous plasma with high platelet concentration. When activated, platelets from PRP release multiple growth factors, including PDGF, GDF‐5, TGF‐β, vascular endothelial growth factor, bFGF, endothelial growth factor and connective tissue growth factor, among others 83. Some studies have proposed that PRP might be an ideal active substance to serve as a cocktail in a strategy of multiple growth factors 84, 85, 86, 87, 88. Nagae et al. 84 injected PRP carried by gelatin hydrogel microspheres into rabbit degenerate discs, prepared by NP aspiration. Growth factors released successfully suppressed progress of IVD degeneration. Further work by this team has confirmed that PRP injection preserved disc height and water content and expression levels of proteoglycan core protein and type II collagen were also upregulated 85. In a further study, Gullung et al. 86 showed by MRI that PRP reduced inflammatory cells, while increasing fluid content. In a rat IDD model, Obata et al. 87 suggested that PRP was able to restore disc height and increase numbers of chondrocyte‐like cells. A study from our team using PRP in an early rabbit IDD model, also confirmed its regenerative efficacy 88.
Gene therapy
When genes encoding active growth factors are transfected into disc cells, they stably express the corresponding gene products which help to promote cell proliferation and ECM accumulation. Compared to bio‐active substance injection therapy, gene therapy is superior due to its continuous effect of stimulating the sustained expression of ECM. Gene carriers are mainly of two types, viral vectors and non‐viral vectors. In one animal study, Seki et al. 89 evaluated suppressive effects of injections of ADAMTS5 small interference RNA (siRNA) oligonucleotide and results confirmed its efficacy as a potential non‐viral vector for gene therapy of IDD. Non‐viral vectors are safer, but transfection rates end to be rather low. Thus, viral vectors are superior in current gene therapies. Sai et al. 90 constructed an adeno‐associated virus expression system for TGF‐beta3, and confirmed its efficacy in enhancing proteoglycan synthesis of earlier and later dedifferentiated NP cells. For AF gene therapy, lentiviral shRNA silencing of CHOP (C/EBP homologous protein), which is apoptosis regulated, was proven to inhibit stretching‐induced apoptosis in AF cells and to improve MRI and histological scores in a rat model 91. Moon et al. 92 examined biological effects of ‘cocktail’ therapeutic gene transfer into human IVD cells in three‐dimensional cultures. The recombinant adenovirus bore TGF‐beta1 gene (Ad/TGF‐beta1), IGF‐1 gene (Ad/IGF‐1) and BMP‐2 gene (Ad/BMP‐2). Results confirmed that human IVD cultures with triple gene combination transfer demonstrated synergistic amplification effect in proteoglycan synthesis. However, even if production of the respective gene product could be achieved, it should be noted that only if surrounding, starving cells are able to properly respond can an improved matrix be produced. Moreover, with utilization of adenovirus vectors, viruses can transfect other tissues when delivered into the discs 93. As a potential therapy, safety of gene transfer needs to be further investigated, but at the moment, for clinical purposes, non‐virus transfection is safer.
Cell transplantation
Cell transplantation is an ideal approach for IVD regeneration, as this type of therapy can increase both number of viable cells and accumulation of matrix components. Complete structure of the IVD is considered to play an important role in limiting the immune response after cell transplantation. Cells from the NP have been reported to express Fas ligand (FasL) which is immune privileged 94, 95. Thus, some studies considered the ideal cell candidate may be disc cells themselves 96, 97. However, preparation of NP cells for re‐implantation is limited as autologous transplantation requires large numbers of cells, and harvesting them from healthy discs may create unnecessary degeneration of the donor's discs.
As a potential substitute for native disc cells, mesenchymal stem cell (MSC) transplantation holds a better prospect for IVD regeneration 98, 99. MSCs are capable of long‐term self‐renewal and can differentiate into a variety of specialized cells. Also, injection of stem cells into the IVD has confirmed them to migrate to the inner AF for repair and regeneration 100. Synergistic effects of certain active growth factors have been reported to be effective in induction of chondrogenic differentiation of bone marrow mesenchymal stem cells (BMMSCs) in vitro for potential application in IVD repair 41. When co‐cultured with NP cells, MSC exhibited enhanced proliferation and telomerase activity over NP cells cultured alone 101, 102. Thus, NP cells and MSCs may be mixed together as seeding cells for disc regeneration applications. Some studies have illustrated stimulatory effects of notochordal cell conditioned medium on native IVD cells and chondrocytes 103, 104, 105, 106. Results clearly demonstrated the ability of notochord‐conditioned media to direct differentiation of MSCs. Makarand et al. 107 reported evidence for skeletal progenitor cells in degenerate human IVDs. This finding suggested that these endogenous progenitors may be applied to repair degenerate discs. Further investigations revealed locations of these progenitor cells within the IVD 37. A further study identified MSCs from degenerate human NP and indicated that these NP‐derived MSCs were similar to MSCs from bone marrow 108. These findings suggest that stimulating endogenous MSCs of the IVD may be a new target for IDD regeneration strategies.
Recently, tissue engineering strategies based on cell transplantation have enjoyed more popularity 109, 110. Tissue engineering is the combined use of seeding cells, biological scaffolds and bioactive substances. The biological scaffold provides a more suitable micro‐environment to help retain cell morphology and provides primary mechanical stability. In a recent study, a biodegradable AF closure system comprised of a diisocyanate glue, based on polyethylene glycol‐PTMC triblock copolymers, a supporting membrane and an adhesive material, was proven to be a promising potential tissue engineering method to restore function of herniated discs 111. Above, we mentioned PRP as a bio‐active substance being a promising choice for its regenerative properties – when activated, it forms a gel‐like substance. Thus, PRP itself may serve as a good bio‐scaffold for cell implantation, as well as being an activator for cell proliferation and differentiation. In the future, combined use of most suitable seeding cells, best biocompatible materials, and active substances to maintain normal cell phenotype, or directional differentiation, seem to be promising for IDD regeneration strategies.
Summary and perspectives
During the process of degeneration, the IVD undergoes multiple cell and molecular changes, including altered phenotype, cell proliferation, cell density, as well as loss of ECM. To maintain stability and metabolic balance of the IVD, multiple cell and molecular factors function together and compromise changes. Now, the most promising therapeutic strategies lie in three fields active substance injection, gene therapy and cell transplantation. With advanced understanding of cell composition of the IVD, multiple strategies can be jointly applied for better regeneration in the near future. However, pathological mechanisms of IDD still remain unclear. Currently discovered stem/progenitor cells within discs have advanced our knowledge of cell biology of the IVD and how these cells might be related to IDD. In the future, cell mechanisms, and how biological therapies affect endogenous stem/progenitor cells within discs need to be further investigated. Further, current studies are mainly focused on regenerative efficacy of biological strategies, but possibility of adverse effects need to be further addressed when applied in clinical use.
Competing interests
The authors declare that they have no competing interests.
Acknowledgement
This study was supported by National Natural Science Foundation of China (Grant no: 81201422), Jiangsu Province Science Foundation for Youths (Grant no: BK2012334), ‘Summit of the Six Top Talents’ Program of Jiangsu Province (Grant no: 2013‐WSW‐054), China Postdoctoral Science Foundation (Grant no: 2012M520983) and Innovative Foundation of Southeast University (Grant no: 3290002401).
SZ. Wang and YF. Rui have equal contribution to this study.
References
- 1. Freemont AJ (2009) The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 48, 5–10. [DOI] [PubMed] [Google Scholar]
- 2. Singh K, Masuda K, An HS (2005) Animal models for human disc degeneration. Spine J. 5(6 Suppl), 267S–279S. [DOI] [PubMed] [Google Scholar]
- 3. Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM (2011) Degeneration and regeneration of the intervertebral disc: lessons from development. Dis. Model Mech. 4, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schoenfeld AJ, Nelson JH, Burks R, Belmont PJ Jr (2011) Incidence and risk factors for lumbar degenerative disc disease in the United States military 1999–2008. Mil. Med. 176, 1320–1324. [DOI] [PubMed] [Google Scholar]
- 5. Kales SN, Linos A, Chatzis C, Sai Y, Halla M, Nasioulas G et al (2004) The role of collagen IX tryptophan polymorphisms in symptomatic intervertebral disc disease in Southern European patients. Spine (Phila Pa 1976) 29, 1266–1270. [DOI] [PubMed] [Google Scholar]
- 6. McBeth J, Jones K (2007) Epidemiology of chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 21, 403–425. [DOI] [PubMed] [Google Scholar]
- 7. Dagenais S, Caro J, Haldeman S (2008) A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 8, 8–20. [DOI] [PubMed] [Google Scholar]
- 8. Kuslich SD, Ulstrom CL, Michael CJ (1991) The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop. Clin. North Am. 22, 181–187. [PubMed] [Google Scholar]
- 9. Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari‐Juntura E, Lamminen A (2000) Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 25, 487–492. [DOI] [PubMed] [Google Scholar]
- 10. Adams MA, Roughley PJ (2006) What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 31, 2151–2161. [DOI] [PubMed] [Google Scholar]
- 11. Boxberger JI, Sen S, Yerramalli CS, Elliott DM (2006) Nucleus pulposus glycosaminoglycan content is correlated with axial mechanics in rat lumbar motion segments. J. Orthop. Res. 24, 1906–1915. [DOI] [PubMed] [Google Scholar]
- 12. Costi JJ, Stokes IA, Gardner‐Morse MG, Iatridis JC (2008) Frequency‐dependent behavior of the intervertebral disc in response to each of six degree of freedom dynamic loading: solid phase and fluid phase contributions. Spine 33, 1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR (2008) The pathophysiology of disc degeneration: a critical review. J. Bone Joint Surg. Br. 90, 1261–1270. [DOI] [PubMed] [Google Scholar]
- 14. Le Maitre CL, Freemont AJ, Hoyland JA (2004) Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J. Pathol. 204, 47–54. [DOI] [PubMed] [Google Scholar]
- 15. Freemont AJ, Byers RJ, Taiwo YO, Hoyland JA (1999) In situ zymographic localisation of type II collagen degrading activity in osteoarthritic human articular cartilage. Ann. Rheum. Dis. 58, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mee AP, Hoyland JA, Braidman IP, Freemont AJ, Davies M, Mawer EB (1996) Demonstration of vitamin D receptor transcripts in actively resorbing osteoclasts in bone sections. Bone 18, 295–299. [DOI] [PubMed] [Google Scholar]
- 17. Braidman I, Baris C, Wood L, Selby P, Adams J, Freemont A et al (2000) Preliminary evidence for impaired estrogen receptor‐alpha protein expression in osteoblasts and osteocytes from men with idiopathic osteoporosis. Bone 26, 423–427. [DOI] [PubMed] [Google Scholar]
- 18. Mirza SK, Deyo RA (2007) Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine 32, 816–823. [DOI] [PubMed] [Google Scholar]
- 19. Hanley EN Jr, Herkowitz HN, Kirkpatrick JS, Wang JC, Chen MN, Kang JD (2010) Debating the value of spine surgery. J. Bone Joint Surg. Am. 92, 1293–1304. [DOI] [PubMed] [Google Scholar]
- 20. Humzah MD, Soames RW (1988) Human intervertebral disc: structure and function. Anat. Rec. 220, 337–356. [DOI] [PubMed] [Google Scholar]
- 21. Eyre DR, Muir H (1976) Types I and II collagens in interver‐tebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 157, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cassidy JJ, Hiltner A, Baer E (1989) Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 23, 75–88. [DOI] [PubMed] [Google Scholar]
- 23. Roughley PJ (2006) The structure and function of cartilage proteoglycans. Eur. Cell Mater. 12, 92–101. [DOI] [PubMed] [Google Scholar]
- 24. Melrose J, Ghosh P, Taylor TK (2001) A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J. Anat. 198, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melrose J, Smith S, Ghosh P (2000) Differential expression of proteoglycan epitopes by ovine intervertebral disc cells. J. Anat. 197, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melrose J, Smith SM, Appleyard RC, Little CB (2007) Aggrecan, versican and type VI collagen are components of annular translamellar crossbridges in the intervertebral disc. Eur. Spine J. 17, 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Urban JP, Smith S, Fairbank JC (2004) Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 29, 2700–2709. [DOI] [PubMed] [Google Scholar]
- 28. Heuer F, Schmidt H, Wilke HJ (2008) Stepwise reduction of functional spinal structures increase disc bulge and surface strains. J. Biomech. 41, 1953–1960. [DOI] [PubMed] [Google Scholar]
- 29. O'Connell GD, Johannessen W, Vresilovic EJ, Elliott DM (2007) Human internal disc strains in axial compression measured noninvasively using magnetic resonance imaging. Spine (Phila Pa 1976) 32, 2860–2868. [DOI] [PubMed] [Google Scholar]
- 30. Roughley PJ, Melching LI, Heathfield TF, Pearce RH, Mort JS (2006) The structure and degradation of aggrecan in human intervertebral disc. Eur. Spine J. 15(Suppl 3), S326–S332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walmsley R (1953) The development and growth of the intervertebral disc. Edinb. Med. J. 60, 341–364. [PMC free article] [PubMed] [Google Scholar]
- 32. Hunter CJ, Matyas JR, Duncan NA (2003) The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 9, 667–677. [DOI] [PubMed] [Google Scholar]
- 33. Maldonado BA, Oegema TR Jr (1992) Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J. Orthop. Res. 10, 677–690. [DOI] [PubMed] [Google Scholar]
- 34. Weiler C, Nerlich AG, Schaaf R, Bachmeier BE, Wuertz K, Boos N (2010) Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur. Spine J. 19, 1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi KS, Cohn MJ, Harfe BD (2008) Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev. Dyn. 237, 3953–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feng G, Yang X, Shang H, Marks IW, Shen FH, Katz A et al (2010) Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J. Bone Joint Surg. Am. 92, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henriksson H, Thornemo M, Karlsson C, Hägg O, Junevik K, Lindahl A et al (2009) Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 34, 2278–2287. [DOI] [PubMed] [Google Scholar]
- 38. Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S et al (2012) Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 3, 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skaggs DL, Weidenbaum M, Iatridis JC, Ratcliffe A, Mow VC (1994) Regional variation in tensile properties and biochemical composition of the human lumbar anulus fibrosus. Spine (Phila Pa 1976) 19, 1310–1319. [DOI] [PubMed] [Google Scholar]
- 40. Ahsan R, Tajima N, Chosa E, Sugamata M, Sumida M, Hamada M (2001) Biochemical and morphological changes in herniated human intervertebral disc. J. Orthop. Sci. 6, 510–518. [DOI] [PubMed] [Google Scholar]
- 41. Adams MA, Roughley PJ (2006) What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 31, 2151–2161. [DOI] [PubMed] [Google Scholar]
- 42. Setton LA, Chen J (2006) Mechanobiology of the intervertebral disc and relevance to disc degeneration. J. Bone Joint Surg. Am. 88(Suppl 2), 52–57. [DOI] [PubMed] [Google Scholar]
- 43. Konttinen YT, Kemppinen P, Li TF, Waris E, Pihlajamäki H, Sorsa T et al (1999) Transforming and epidermal growth factors in degenerated intervertebral discs. J. Bone Joint Surg. Br. 81, 1058–1063. [DOI] [PubMed] [Google Scholar]
- 44. Jin H, Shen J, Wang B, Wang M, Shu B, Chen D (2011) TGF‐β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 585, 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang H, Kroeber M, Hanke M, Ries R, Schmid C, Poller W et al (2003) Release of active and depot GDF‐5 after adenovirus‐mediated overexpression stimulates rabbit and human intervertebral disc cells. J. Mol. Med. (Berlin) 82, 126–134. [DOI] [PubMed] [Google Scholar]
- 46. Le Maitre CL, Richardson SM, Baird P, Freemont AJ, Hoyland JA (2005) Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc. J. Pathol. 207, 445–452. [DOI] [PubMed] [Google Scholar]
- 47. Wang H, Kroeber M, Hanke M, Ries R, Schmid C, Poller W et al (2004) Release of active and depot GDF‐5 after adenovirus‐overexpression stimulates rabbits and human intervertebral disc cells. J. Mol. Med. (Berlin) 82, 126–134. [DOI] [PubMed] [Google Scholar]
- 48. Fujita N, Imai J, Suzuki T, Yamada M, Ninomiya K, Miyamoto K et al (2008) Vascular endothelial growth factor‐A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem. Biophys. Res. Commun. 372, 367–372. [DOI] [PubMed] [Google Scholar]
- 49. Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W et al (2003) Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976) 28, 755–763. [PMC free article] [PubMed] [Google Scholar]
- 50. Wallach CJ, Gilbertson LG, Kang JD (2003) Gene therapy applications for intervertebral disc degeneration. Spine (Phila Pa 1976) 28(15 Suppl):S93–S98. [DOI] [PubMed] [Google Scholar]
- 51. Kim KW, Kim YS, Ha KY, Woo YK, Park JB, Park WS et al (2005) An autocrine or paracrine Fas‐mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine (Phila Pa 1976) 30, 1247–1251. [DOI] [PubMed] [Google Scholar]
- 52. Heyde CE, Tschoeke SK, Hellmuth M, Hostmann A, Ertel W, Oberholzer A (2006) Trauma induces apoptosis in human thoracolumbar intervertebral discs. BMC Clin. Pathol. 23, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P et al (2003) Gene transfer of the catabolic inhibitor TIMP‐1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine (Phila Pa 1976) 28:2331–2337. [DOI] [PubMed] [Google Scholar]
- 54. Le Maitre CL, Freemont AJ, Hoyland JA (2004) Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J. Pathol. 204, 47–54. [DOI] [PubMed] [Google Scholar]
- 55. Nishida T (1999) Kinetics of tissue and serum matrix metalloproteinase‐3 and tissue inhibitor of metalloproteinases‐1 in intervertebral disc degeneration and disc herniation. Kurume Med. J. 46, 39–50. [DOI] [PubMed] [Google Scholar]
- 56. Patel KP, Sandy JD, Akeda K, Miyamoto K, Chujo T, An HS et al (2007) Aggrecanases and aggrecanase‐generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine (Phila Pa 1976) 32, 2596–2603. [DOI] [PubMed] [Google Scholar]
- 57. Battie MC, Videman T (2006) Lumbar disc degeneration: epidemiology and genetics. J. Bone Joint Surg. Am. 88(Suppl 2), 3–9. [DOI] [PubMed] [Google Scholar]
- 58. Battie MC, Videman T, Kaprio J, Gibbons LE, Gill K, Manninen H et al (2009) The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 9, 47–59. [DOI] [PubMed] [Google Scholar]
- 59. Battie MC, Videman T, Levalahti E, Gill K, Kaprio J (2007) Heritability of low back pain and the role of disc degeneration. Pain 131, 272–280. [DOI] [PubMed] [Google Scholar]
- 60. Kelempisioti A, Eskola PJ, Okuloff A, Karjalainen U, Takatalo J, Daavittila I et al (2011) Genetic susceptibility of intervertebral disc degeneration among young Finnish adults. BMC Med. Genet. 12, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seki S, Kawaguchi Y, Chiba K, Mikami Y, Kizawa H, Oya T et al (2005) A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat. Genet. 37, 607–612. [DOI] [PubMed] [Google Scholar]
- 62. Karasugi T, Semba K, Hirose Y, Kelempisioti A, Nakajima M, Miyake A et al (2009) Association of the tag SNPs in the human SKT gene (KIAA1217) with lumbar disc herniation. J. Bone Miner. Res. 24, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seki S, Tsumaki N, Motomura H, Nogami M, Kawaguchi Y, Hori T et al (2014) Cartilage intermediate layer protein promotes lumbar disc degeneration. Biophys. Res. Commun. pii: S0006‐291X(14)00457‐4. [DOI] [PubMed] [Google Scholar]
- 64. Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG (2002) Classification of age‐related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 27, 2631–2644. [DOI] [PubMed] [Google Scholar]
- 65. Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K (2001) Nucleus pulposus allograft retards intervertebral disc degeneration. Clin. Orthop. Relat. Res. 389, 94–101. [DOI] [PubMed] [Google Scholar]
- 66. Gruber HE, Hanley EN Jr. Analysis of aging and degeneration of the human intervertebral disc (1998) Comparison of surgical specimens with normal controls. Spine (Phila Pa 1976) 23, 751–757. [DOI] [PubMed] [Google Scholar]
- 67. Hastreiter D, Ozuna RM, Spector M (2001) Regional variations in certain cellular characteristics in human lumbar intervertebral discs, including the presence of alpha‐smooth muscle actin. J. Orthop. Res. 19, 597–604. [DOI] [PubMed] [Google Scholar]
- 68. Ishii T, Tsuji H, Sano A, Katoh Y, Matsui H, Terahata N (1991) Histochemical and ultrastructural observations on brown degeneration of human intervertebral disc. J. Orthop. Res. 9, 78–90. [DOI] [PubMed] [Google Scholar]
- 69. Bibby SR, Fairbank JC, Urban MR, Urban JP (2002) Cell viability in scoliotic discs in relation to disc deformity and nutrient levels. Spine (Phila Pa 1976) 27, 2220–2228; discussion 2227–2228. [DOI] [PubMed] [Google Scholar]
- 70. Le Maitre CL, Freemont AJ, Hoyland JA (2007) Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther. 9, R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tolonen J, Grönblad M, Vanharanta H, Virri J, Guyer RD, Rytömaa T et al (2006) Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet‐derived growth factor. Eur. Spine J. 15, 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Johnson WE, Roberts S (2003) Human intervertebral disc cell morphology and cytoskeletal composition: a preliminary study of regional variations in health and disease. J. Anat. 203, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oegema TR Jr (2002) The role of disc cell heterogeneity in determining disc biochemistry: a speculation. Biochem. Soc. Trans. 30, 839–844. [DOI] [PubMed] [Google Scholar]
- 74. Kim KW, Kim YS, Ha KY, Woo YK, Park JB, Park WS et al (2005) An autocrine or paracrine Fas‐mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine (Phila Pa 1976) 30, 1247–1251. [DOI] [PubMed] [Google Scholar]
- 75. Sztrolovics R, Grover J, Cs‐Szabo G, Shi SL, Zhang Y, Mort JS et al (2002) The characterization of versican and its message in human articular cartilage and intervertebral disc. J. Orthop. Res. 20, 257–266. [DOI] [PubMed] [Google Scholar]
- 76. Walsh AJ, Bradford DS, Lotz JC (2004) In vivo growth factor treatment of degenerated intervertebral discs. Spine (Phila Pa 1976) 29, 156–163. [DOI] [PubMed] [Google Scholar]
- 77. Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M et al (2006) Effects of growth differentiation factor‐5 on the intervertebral disc – in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976) 31, 2909–2917. [DOI] [PubMed] [Google Scholar]
- 78. Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Chubinskaya S, Yoshida M (2005) Osteogenic protein‐1 (osteogenic protein‐1/bone morphogenetic protein‐7) inhibits degeneration and pain‐related behavior induced by chronically compressed nucleus pulposus in the rat. Spine (Phila Pa 1976) 30, 1933–1939. [DOI] [PubMed] [Google Scholar]
- 79. An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K et al (2005) Intradiscal administration of osteogenic protein‐1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine (Phila Pa 1976) 30, 25–31. [DOI] [PubMed] [Google Scholar]
- 80. Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K et al (2006) Osteogenic protein‐1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976) 31, 742–754. [DOI] [PubMed] [Google Scholar]
- 81. Miyamoto K, Masuda K, Kim JG, Inoue N, Akeda K, Andersson GB et al (2006) Intradiscal injections of osteogenic protein‐1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 6, 692–703. [DOI] [PubMed] [Google Scholar]
- 82. Imai Y, Okuma M, An HS, Nakagawa K, Yamada M, Muehleman C et al (2007) Restoration of disc height loss by recombinant human osteogenic protein‐1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine (Phila Pa 1976) 32, 1197–1205. [DOI] [PubMed] [Google Scholar]
- 83. Krüger JP, Freymannx U, Vetterlein S, Neumann K, Endres M, Kaps C (2013) Bioactive factors in platelet‐rich plasma obtained by apheresis. Transfus. Med. Hemother. 40, 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nagae M, Ikeda T, Mikami Y, Hase H, Ozawa H, Matsuda K et al (2007) Intervertebral disc regeneration using platelet‐rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 13, 147–158. [DOI] [PubMed] [Google Scholar]
- 85. Sawamura K, Ikeda T, Nagae M, Okamoto S, Mikami Y, Hase H et al (2009) Characterization of in vivo effects of platelet‐rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng. 15, 3719–3727. [DOI] [PubMed] [Google Scholar]
- 86. Gullung GB, Woodall JW, Tucci MA, James J, Black DA, McGuire RA (2011) Platelet‐rich plasma effects on degenerative disc disease: analysis of histology and imaging in an animal model. Evid. Based Spine Care J. 2, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Obata S, Akeda K, Imanishi T, Masuda K, Bae W, Morimoto R et al (2012) Effect of autologous platelet‐rich plasma‐releasate on intervertebral disc degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res. Ther. 14, R241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hu X, Wang C, Rui Y (2012) [An experimental study on effect of autologous platelet‐rich plasma on treatment of early intervertebral disc degeneration.] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 26, 977–983 (in Chinese). [PubMed] [Google Scholar]
- 89. Seki S, Asanuma‐Abe Y, Masuda K, Kawaguchi Y, Asanuma K, Muehleman C, Iwai A et al (2009) Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle‐puncture model. Arthritis Res Ther 11(6), R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sai JM, Hu YG, Wang DC (2007) Constructing adeno‐associated virus‐TGFbeta3 and comparing its biological effect on proteoglycan synthesis in dedifferentiated nucleus pulpous cells with adenovirus‐TGFbeta1. Chin. Med. Sci. J. 22, 113–118. [PubMed] [Google Scholar]
- 91. Zhang YH, Zhao CQ, Jiang LS, Dai LY (2011) Lentiviral shRNA silencing of CHOP inhibits apoptosisinduced by cyclic stretch in rat annular cells and attenuates disc degeneration in the rats. Apoptosis 16, 594–605. [DOI] [PubMed] [Google Scholar]
- 92. Moon SH, Nishida K, Gilbertson LG, Lee HM, Kim H, Hall RA et al (2008) Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine (Phila Pa 1976) 33, 1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Leo BM, Li X, Balian G, Anderson DG (2004) In vivo bioluminescent imaging of virus‐mediated gene transfer and transduced cell transplantation in the intervertebral disc. Spine (Phila Pa 1976) 29, 838–844. [DOI] [PubMed] [Google Scholar]
- 94. Park JB, Chang H, Kim KW (2001) Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine (Phila Pa 1976) 26, 618–621. [DOI] [PubMed] [Google Scholar]
- 95. Takada T, Nishida K, Doita M, Kurosaka M (2002) Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine (Phila Pa 1976) 27, 1526–1530. [DOI] [PubMed] [Google Scholar]
- 96. Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ et al (2003) Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine (Phila Pa 1976) 28, 2609–2620. [DOI] [PubMed] [Google Scholar]
- 97. Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G et al (2002) Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine (Phila Pa 1976) 27, 1626–1633. [DOI] [PubMed] [Google Scholar]
- 98. Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K et al (2005) Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 30, 2379–2387. [DOI] [PubMed] [Google Scholar]
- 99. Zhang YG, Guo X, Xu P, Kang LL, Li J (2005) Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin. Orthop. Relat. Res. 430, 219–226. [DOI] [PubMed] [Google Scholar]
- 100. Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD (2008) Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 8, 888–896. [DOI] [PubMed] [Google Scholar]
- 101. Strassburg S, Richardson SM, Freemont AJ, Hoyland JA (2010) Co‐culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen. Med. 5, 701–711. [DOI] [PubMed] [Google Scholar]
- 102. Kim DH, Kim SH, Heo SJ, Shin JW, Lee SW, Park SA (2009) Enhanced differentiation of mesenchymal stem cells into NP‐like cells via 3D co‐culturing with mechanical stimulation. J. Biosci. Bioeng. 108, 63–67. [DOI] [PubMed] [Google Scholar]
- 103. Erwin WM, Inman RD (2006) Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine (Phila Pa 1976) 31, 1094–1099. [DOI] [PubMed] [Google Scholar]
- 104. Erwin WM, Ashman K, O'Donnel P, Inman RD (2006) Nucleus pulposus notochord cells secrete connective tissue growth factor and up‐regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 54, 3859–3867. [DOI] [PubMed] [Google Scholar]
- 105. Cappello R, Bird JL, Pfeiffer D, Bayliss MT, Dudhia J (2006) Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine (Phila Pa 1976) 31, 873–882; discussion 883. [DOI] [PubMed] [Google Scholar]
- 106. Korecki CL, Taboas JM, Tuan RS, Iatridis JC (2010) Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res. Ther. 1, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR et al (2007) Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 32, 2537–2544. [DOI] [PubMed] [Google Scholar]
- 108. Blanco JF, Graciani IF, Sanchez‐Guijo FM, Muntion S, Hernandez‐Campo P, Santamaria C et al (2010) Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 35, 2259–2265. [DOI] [PubMed] [Google Scholar]
- 109. Lee KI, Moon SH, Kim H, Kwon UH, Kim HJ, Park SN et al (2012) Tissue engineering of the intervertebral disc with cultured nucleus pulposus cells using atelocollagen scaffold and growth factors. Spine (Phila Pa 1976) 37, 452–458. [DOI] [PubMed] [Google Scholar]
- 110. Liang CZ, Li H, Tao YQ, Peng LH, Gao JQ, Wu JJ et al (2013) Dual release of dexamethasone and TGF‐β3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater. 9, 9423–9433. [DOI] [PubMed] [Google Scholar]
- 111. Blanquer SB, Sharifi S, Grijpma DW (2012) Development of poly(trimethylene carbonate) network implants for annulus fibrosus tissue engineering. J. Appl. Biomater. Funct. Mater. 10, 177–184. [DOI] [PubMed] [Google Scholar]


