Summary
Aims
The mouse model of allo‐head and body reconstruction (AHBR) has recently been established to further the clinical development of this strategy for patients who are suffering from mortal bodily trauma or disease, yet whose mind remains healthy. Animal model studies are indispensable for developing such novel surgical practices. The goal of this work was to establish head transplant mouse model, then the next step through the feasible biological model to investigate immune rejection and brain function in next step, thereby promoting the goal of translation of AHBR to the clinic in the future.
Methods and Results
Our approach involves retaining adequate blood perfusion in the transplanted head throughout the surgical procedure by establishing donor‐to‐recipient cross‐circulation by cannulating and anastomosing the carotid artery on one side of the body and the jugular vein on the other side. Neurological function was preserved by this strategy as indicated by electroencephalogram and intact cranial nerve reflexes.
Conclusions
The results of this study support the feasibility of this method for avoiding brain ischemia during transplantation, thereby allowing for the possibility of long‐term studies of head transplantation.
Keywords: Allograft, Allo‐head and body reconstruction, composite tissue allotransplantation, Head transplantation, Mouse model
Introduction
Unlike allo‐head and body reconstruction (AHBR), head transplantation is a procedure that places a donor head onto the intact body of the recipient 1, 2. The first successful human hand transplantation was performed at the Christine M. Kleinert Institute for Hand and Microsurgery at the University of Louisville in 1999 3, 4, 5, 6, 7, 8. Tremendous progress in the field of CTA was made throughout the 1990s, and since then, there have been over 100 successful CTA cases, mainly involving the transplantation of hand and facial tissue 9, 10, 11, 12. The practice of CTA has become the standard of care for patients suffering from large tissue defects, and it is revolutionizing the fields of transplant and reconstructive surgery. But for patients suffering from fatal diseases, such as organ failure, muscular dystrophy etc., or bodily trauma, yet whose mind is unaffected, there is an unmet need for a surgical strategy to transplant their head or replace their body. Will AHBR ultimately become as widely accepted as organ transplantation and CTA?
In fact, scientists and physicians began to conceive of the idea of transplanting the human head, the most complex and important organ of the body, in the early 20th century. As early as 1908, Dr. Guthrie reported the transplantation of the head in a dog model 13. Subsequently, Demikhov 14 and Zhao 15 successfully carried out related research in the Soviet Union and China in the 1950s, respectively. Dr. White et al. 16 carried out the first brain and head transplants 17, 18, 19 in a dog and primate model in the late 1960s and early 1970s, which represented important progress in the field. However, these pioneering experiments, limited by technical conditions and requirements, did not achieve long‐term survival and missed the opportunity to study immune rejection and posttransplant CNS functional recovery strategies. Until recently, there had been few advances since that time 1, 2.
Allo‐head and body reconstruction has continued to progress from a theoretical possibility toward becoming a clinical reality 20, 21. An important step in developing AHBR is to explore the process of head transplantation in animal models, which will enable long‐term survival and thus the investigation of drugs to prevent immune rejection, as well as brain function in the transplanted head. Based on experience derived from CTA‐related research, our team proposed a head transplantation procedure that avoids brain ischemia not only through mild hypothermia (31°C) but also by maintaining adequate blood circulation between the grafted head and the recipient body during the procedure. This was accomplished through creating an anastomosis of the carotid arteries and jugular veins. This study describes how to implement and test the head transplant model in mice. Compared with the previous models, we hope this technology will greatly promote the study of AHBR.
Methods
Animals
The experiments were approved by The Animal Care and Use Committee of The Second Affiliated Hospital of Harbin Medical University. Forty Kunming mice and forty C57 wild‐type mice, all male, aged 10–15 weeks, weight 30 ± 5 g, were obtained from the animal center of Harbin Medical University.
Experimental Drugs and Equipment
The following drugs were employed: 3% sodium pentobarbital (90 mg/kg), heparin sodium injection (1.0 mL/100 mg), and norepinephrine (5 mg/kg) to maintain blood pressure. During surgery, respiration was maintained using the Minivent Mouse Ventilator Type 845 (Hugo Sachs Elektronik Harvard Apparatus Gmbh D‐79232 March, Germany). Electroencephalographic (EEG) and electrocardiographic (ECG) recordings were monitored using the data acquisition & analysis system, type BL‐4203, from Chengdu Technology & Market CO., Ltd. (Chengdu, China).
An operating microscope (SXP‐1C) from Shanghai Medical Optical Instruments CO, Ltd., (Shanghai, China), was used. Micro‐ and neurosurgical instruments and surgical supplies were purchased from the Shanghai Instrument Company (Shanghai, China).
Preparation of the Recipient Body
A ventral transverse incision was made at cervical level C4‐C5. The skin and subcutaneous tissue were incised, and tracheal intubation was performed. Next, the carotid artery on one side and the contralateral jugular vein were ligated and cut, and the proximal ends of the vessels were catheterized using a 0.30 mm (I.D.) × 0.64 mm (O.D.) silicone tube to create an anastomosis with the carotid and jugular vessels of the transplanted head. The catheterized ends were flushed with heparin sodium injection (1.0 mL: 100 mg) mixed with sodium chloride injection. The other carotid and jugular vessels were left intact. Thus, the blood perfusion of the recipient body's brain tissue is uninterrupted, which is a key to avoid cerebral hypoxia. According to White's animal model studies, maintaining a blood pressure of at least 40 mmHg within the brain is sufficient to meet its metabolic needs 19.
Next, a transverse incision was made on the dorsal aspect of the recipient mouse at cervical level C3‐C4. The skin and subcutaneous tissue were incised, and the silicone tubes were passed dorsally through the muscles of the neck. The placement of the incision must be such that the vascular anastomosis that is created will remain patent once the donor head is in place, so that blood flow is uninterrupted. All surgical procedures were completed under the microscope and bleeding was stopped by bipolar coagulation.
Preparation of the Donor Head
Ventral and dorsal incisions were made at the cervical level C3‐C4. The skin and subcutaneous tissue were incised, and muscle, nerves, blood vessels, trachea, and esophagus were dissociated. The artery and vein around the spinal cord were ligated at level of C3‐C4. Next, the carotid artery and contralateral jugular vein were ligated and cut. The distal ends were catheterized as before, connecting the corresponding silicone tube to the recipient's carotid artery and jugular vein. During this procedure, the intact contralateral carotid artery and jugular vein provided a continuous blood supply. Then, with the vertebrae exposed, the spinal cord was cut sharply. The muscle, nerves, trachea, and esophagus were ligated and then cut.
Transplantation
The silicone catheter was removed from the recipient mouse's carotid artery and jugular vein, and they were sequentially surgically anastomosed to those of the donor head. The vessels were temporarily clipped to avoid bleeding during this step. Finally, surgical stitching was used to appose the donor and recipient tissues (Figure 1).
Figure 1.
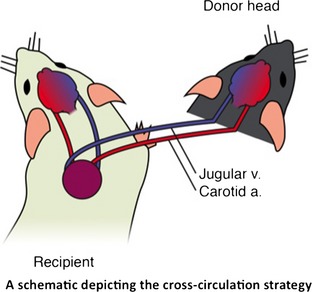
Schematic representation of the head transplantation strategy in mouse model: Ipsilateral carotid artery and jugular vein in donor and recipient mice were connected by the silicone tubes.
Perioperative and Postoperative Monitoring of Vital Signs
Electroencephalography (EEG) and ECG were performed perioperatively and postoperatively to monitor the brain's electrical activity and heart function, respectively. Blood pressure was continuously monitored by tail artery during and after the procedure. Body temperature was monitored to evaluate the perioperative and postoperative body's metabolic condition and maintained at 31°C ± 2°C. Ventilation conditions used were as follows: respiratory rate = 110 ± 5/min and tidal volume = 2.2 mL. When spontaneous breathing resumed, the ventilator was disconnected.
Results
Forty Kunming mice and forty C57 wild‐type mice underwent the head transplant procedure. After allograft, 12 pairs of mice survived over 24 h. Blood pressure was maintained above 100/60 mmHg during surgical procedure, which usually takes 4 h. Within 1.5–2 h after transplantation, the both mice regained consciousness, displaying activity function and responsiveness (Figure 2C,D). With unilateral carotid and jugular cross‐circulation, the cerebral blood supply to the brain during surgery was adequate, and we also observed good peripheral revascularization in the donor head (Figure 2E). EEG recordings made directly from the donor and recipient heads both appeared normal (Figure 3C,D). The finding further supported this that the cranial nerve reflexes were activated, such as blinking and whiskers moving. The ECG also showed normal electrophysiological activity (Figure 3A,B).
Figure 2.
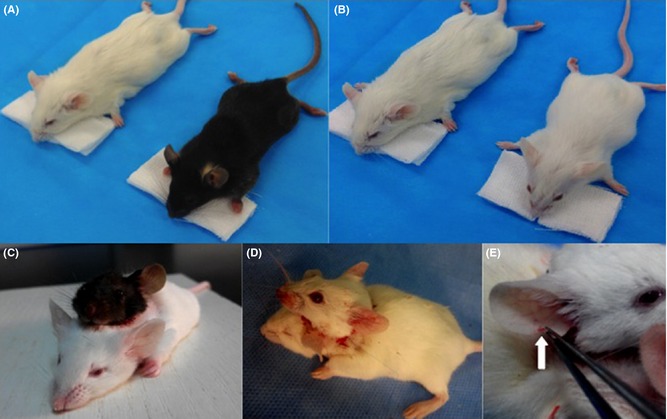
Two pairs of mice pre‐ and postoperation: A and B, black and white mice, C and D, mice after transplantation (black head with white body; white head with white body); E, the white arrow shows good circulation to the donor ear, with induced bleeding postoperation.
Figure 3.
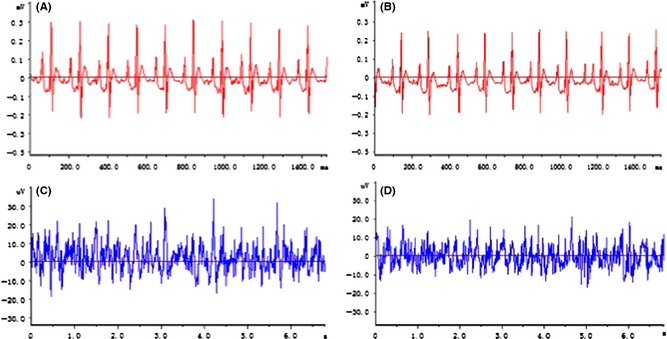
Head transplantation mouse model ECG and EEG recording. A and C, the record of ECG and EEG pre‐operation, and B and D, postoperation. ECG, electrocardiographic; EEG, electroencephalographic.
Discussion
The mouse model of heterotopic head transplantation described herein is an important step in laying the groundwork for human head transplantation. This work was undertaken to provide a model in which immune rejection and brain function can be assessed over time, by prolonging the survival period of the donor and recipient for up to 6 months.
The transplantation procedure was designed to minimize trauma to the recipient, in particular to avoid blood loss and cerebral ischemia, by leaving one carotid artery and one jugular vein intact. Postoperative EEG results from the recipient and the donor heads demonstrate normal and almost identical electrophysiological activity, indicating that neither mouse experienced significant ischemia and that brain function remained intact. After the surgery, the body temperature and activity level of the recipient returned to normal. Our short‐term results so far indicate that the resulting mouse with two heads can recover to a normal physiological state and that the recipient body is capable of supporting the transplanted head.
This study is an extension of AHBR, drawing on its feature of implementing cannulae during the surgical procedure to establish a cross‐circulation and avoid brain ischemia 22. In turn, this procedure will provide the opportunity to determine the optimal immunosuppressive regimen in the scenario of the head as a composite tissue allograft, which will further advance the practice of AHBR 6, 7, 8. Histological methods will be used to evaluate signs of immune rejection over time. Also to be considered is a strategy for neurological recovery. Once this research has been completed, we will be prepared for more in‐depth research using a primate model. These models will be vital to enable the translation of this technique to the clinic 1, 2.
We believe that another important ramification of this research is that the technique of establishing donor‐to‐recipient cross‐circulation during transplantation could be widely applicable to other transplantation scenarios than the head. For instance, during transplantation of other types of composite tissue or even other organs that are intolerant of ischemia, this technique could provide an alternative or adjuvant to the beneficial effects of hypothermia in preserving tissue viability.
In conclusion, our short‐term results with this model are inspiring. The experimental method that we have described can allow for long‐term survival, and thus assessment of transplant rejection and CNS recovery, bringing us one step closer to AHBR in man.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (81470425), the funds for Wu Liande Foundation of HMU (Wld‐qn1414) and the funds for Harbin Science and Technology Bureau (2014RFXYJ023). The authors would like to thank Kristin Luther for help with the manuscript and illustration.
References
- 1. Ren X, Laugel M. The next frontier in composite tissues allotransplantation. CNS Neurosci Ther 2013;19:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ren X, Luther K, Haar L, et al. Concepts, challenges, and opportunities in allo‐head and body reconstruction (AHBR). Neurosci Ther 2014;20:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones JW, Gruber SA, Barker JH, Breidenbach WC. Successful hand transplantation. One‐year follow‐up. Louisville Hand Transplant Team. N Engl J Med 2000;343:468–473. [DOI] [PubMed] [Google Scholar]
- 4. Francois CG, Breidenbach WC, Maldonado C, et al. Hand transplantation: comparisons and observations of the first four clinical cases. Microsurgery 2000;20:360–371. [DOI] [PubMed] [Google Scholar]
- 5. Ren X, Shirbacheh MV, Ustuner ET, et al. Osteomyocutaneous flap as a preclinical composite tissue allograft: swine model. Microsurgery 2000;20:143–149. [DOI] [PubMed] [Google Scholar]
- 6. Jones JW Jr, Ustuner ET, Zdichavsky M, et al. Long‐term survival of an extremity composite tissue allograft with fk506‐mycophenolate mofetil therapy. Surgery 1999;126:384–388. [PubMed] [Google Scholar]
- 7. Ustuner ET, Zdichavsky M, Ren X, et al. Long‐term composite tissue allograft survival in a porcine model with cyclosporine/mycophenolate mofetil therapy. Transplantation 1998;66:1581–1587. [DOI] [PubMed] [Google Scholar]
- 8. Shirbacheh MV, Ren X, Jones JW, et al. Pharmacokinetic advantage of intra‐arterial cyclosporin a delivery to vascularly isolated rabbit forelimb. I. Model development. J Pharmacol Exp Ther 1999;289:1185–1190. [PubMed] [Google Scholar]
- 9. Hautz T, Engelhardt TO, Weissenbacher A, et al. World experience after more than a decade of clinical hand transplantation: update on the innsbruck program. Hand Clin 2011;27:423–431. [DOI] [PubMed] [Google Scholar]
- 10. Foroohar A, Elliott RM, Kim TW, et al. The history and evolution of hand transplantation. Hand Clin 2011;27:405–409. [DOI] [PubMed] [Google Scholar]
- 11. Hautz T, Brandacher G, Engelhardt TO, et al. How reconstructive transplantation is different from organ transplantation–and how it is not. Transplant Proc 2011;43:3504–3511. [DOI] [PubMed] [Google Scholar]
- 12. Gander B, Brown CS, Vasilic D, et al. Composite tissue allotransplantation of the hand and face: a new frontier in transplant and reconstructive surgery. Transpl Int 2006;19:868–880. [DOI] [PubMed] [Google Scholar]
- 13. Guthrie CC. Blood vessel surgery and its applications. London and New York: Edward Arnold; Longmans, Green & Co., 1912. [Google Scholar]
- 14. Demikhov VP. Experimental transplantation of vital organs. New York: Consultants Bureau, 1962. [Google Scholar]
- 15. Dog‐head transplant claimed by Chinese. Washington, DC: The Washington Post, Times Herald 1, 1959. [Google Scholar]
- 16. White RK, Albin MS, Locke GE, et al. Brain transplantation: prolonged survival of brain after carotid‐jugular interposition. Science (New York, NY) 1965;150:779–781. [DOI] [PubMed] [Google Scholar]
- 17. White RJ, Wolin LR, Massopust LC Jr, et al. Cephalic exchange transplantation in the monkey. Surgery 1971;70:135–139. [PubMed] [Google Scholar]
- 18. White RJ, Wolin LR, Massopust LC Jr, et al. Primate cephalic transplantation: neurogenic separation, vascular association. Transplant Proc 1971;3:602–604. [PubMed] [Google Scholar]
- 19. White RJ, Albin MS, Verdura J, et al. The isolation and transplantation of the brain. An historical perspective emphasizing the surgical solutions to the design of these classical models. Neurol Res 1996;18:194–203. [DOI] [PubMed] [Google Scholar]
- 20. Canavero S. The, “Gemini” spinal cord fusion protocol. Surg Neurol Int 2015;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Canavero S. The head anastomosis venture Project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int 2013;4:S335–S342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren X, Song Y, Ye Y. Allogeneic head and body reconstruction: mouse model. CNS Neurosci Ther 2014;20:1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]


