Abstract
Abstract. Objective: Iron loading has been observed to have a hyperproliferative effect on hepatocytes in vitro and on tumour cells in vivo; removal of this iron being required to induce antitumour activity. Material and Methods: Antiproliferative effects of orally active tridentate iron chelator ICL670 (deferasirox) and bidentate iron chelator CP20 (deferiprone), mediated through the chelation of intracellular iron, were compared in rat hepatoma cell line FAO and human hepatoma cell line HUH7. Results: In FAO cell cultures, we have shown that ICL670 decreased cell viability and DNA replication and induced apoptosis more efficiently than an iron‐binding equivalent concentration of CP20. Moreover, ICL670 decreased significantly the number of the cells in G2‐M phase. In the HUH7 cell cultures, ICL670 and a four‐time higher iron‐binding equivalent concentration of CP20, decreased cell viability and DNA replication in the same range. CP20 increased the number of the cells in G2‐M phase. However, ICL670 inhibited polyamine biosynthesis by decreasing ornithine decarboxylase mRNA level; in contrast, CP20 increased polyamine biosynthesis, particularly putrescine level, by stimulating spermidine‐spermine N1‐acetyl transferase activity that could activate the polyamine retro‐conversion pathway. By mass spectrometry, we observed that ICL670 cellular uptake was six times higher than CP20. Conclusions: These results suggest that ICL670 has a powerful antitumoural effect and blocks cell proliferation in neoplastic cells by a pathway different from that of CP20 and may constitute a potential adjuvant drug for anticancer therapy.
INTRODUCTION
Dysregulation of iron metabolism leads to iron overloading associated with deleterious effects to cells and tissues. Numerous iron chelators have been synthesized in order to treat iron overload diseases, especially thalassaemia. In this context, they could also prevent development of hepatocellular carcinoma or inhibit proliferation of tumour cells in general (Brissot & Loréal 2002). The hyperproliferative effect of iron overload has been observed in vitro in hepatocytes and in vivo in tumour cells (Thompson et al. 1991; Chenoufi et al. 1997). On the other hand, iron depletion by chelators inhibits hepatocyte proliferation by inducing cell cycle arrest that is frequently followed by apoptosis (Chenoufi et al. 1995; Rakba et al. 2000; Gaboriau et al. 2004). Among the various chelators synthesized, hexadentate desferrioxamine (DFO) and the bidentate hydroxypyridinone deferiprone (CP20) are the major molecules used for treatment of iron overload. However, DFO cannot be administered orally and clinical trials have shown that CP20 can induce severe neutropenia (Porter & Davis 2002). Chelators currently used for therapy of iron overload diseases are not optimal compounds. It is, thus, of great interest to develop new, orally efficient and non‐toxic iron chelators. The novel orally active iron chelator ICL670 has appeared to be of special interest because we have previously demonstrated that this compound inhibits tumoural cell proliferation with a higher efficiency than hexadentate iron chelator O‐trensox that itself was more powerful than DFO (Rakba et al. 2000; Chantrel‐Groussard et al. 2006).
Natural polyamines, putrescine, spermidine and spermine, as iron, are definitively required for cell proliferation to occur. Their intracellular levels and uptake are significantly increased for tumour cell proliferation (Wallace et al. 2003). Recently, interaction in metabolism involving cell polyamine transport system as a possible iron cell entry pathway in hamster ovary CHO cells, has been shown (Gaboriau et al. 2004). Moreover, tumour cell proliferation has been shown to be related to key enzymes of polyamine biosynthesis, ornithine decarboxylase (ODC) and polyamine retroconversion pathway spermidine/spermine N1‐acetyltransferase (SSAT) (Yano et al. 1995; Bhasin et al. 2002). ODC, which converts ornithine into putrescine, has increased activity in tumour cells. SSAT, which synthesizes acetylated compounds from spermine and spermidine, has also been shown to be activated in tumour cells.
In the present study, the antiproliferative effect of the two iron chelators, tridentate ICL670 and bidentate CP20, has been compared in rat hepatoma cell line FAO and human hepatoma cell line HUH7 cultures. DNA replication, cytotoxicity and cell cycle analysis have been correlated to intracellular polyamine levels. We have demonstrated that the iron chelator ICL670 inhibits cell proliferation and induces apoptosis with a higher efficiency than CP20. This effect is correlated to modulation of polyamine biosynthesis that results from alteration of SSAT and/or ODC mRNA levels, and of intracellular polyamine content. Comparison of the two molecules indicates that ICL670 has the most powerful antiproliferative potential.
MATERIALS AND METHODS
Cell cultures
The rat hepatoma cell line FAO was used (Deschatrette & Weis 1974) and maintained by subculture in the following medium – 50% HAM F12 and 50% NCTC 135 (Eurobio, Les Ulis, France) – containing per millilitre: glutamine (2 µmol), NaHCO3 (2.2 mg), penicillin (50 IU), streptomycin (50 µg) and foetal calf serum (0.05 mL). The human hepatoma cell line HUH7 was a gift from Dr Ferry (Centre Anticancéreux, Hôpital Ponchaillou, Rennes, France). HUH7 cell cultures were maintained in 75% minimum essential medium (MEM) – 25% medium 199 (Hank's salts) – containing per millilitre, penicillin (50 IU) – streptomycin (50 µg) – glutamine (2 µmol) – hydrocortisone hemisuccinate (0.05 µmol) – insulin (5 µg) – serum albumin bovine (1 mg) and foetal calf serum (0.1 mL). For the experiments, the liver cells were maintained in the same media as above but were deprived of foetal calf serum. Chelators were added 72 h after plating the cells. Viability, proliferation, apoptosis and polyamine metabolism of the cells were analysed 48 h after chelator exposures.
Chelator solutions
Tridentate hydroxyphenyltriazole ICL670 (Rouan et al. 2001) and bidentate hydroxypyridinone CP20 were compared. Solutions of each molecule were used as 1 mm in culture medium – 0.5% dimethyl sulfoxide (DMSO) for ICL670 and culture medium for CP20. These solutions were sterilized by filtration on a 0.2‐µm membrane. Chelators were iron saturated with ferric iron (FeCl3) in stoechiometric conditions of chelation, one mole of iron for two or three moles of ICL670 or CP20, respectively. Two controls were performed for each experiment, one with standard culture medium, and the other with culture medium supplemented with DMSO at the concentration used for ICL670 tests. DMSO‐supplemented controls did not show any difference from standard medium controls; thus, results obtained with controls without DMSO only are reported.
Cell viability and proliferation
The effect of ICL670 and CP20 on cell viability was evaluated by phase contrast microscopy observation and also by measuring mitochondrial succinate dehydrogenase (SDH) activity. SDH activity was evaluated by measuring the formazan formation from 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide (MTT, Sigma, St. Louis, MO, USA) (Mosmann 1983). SDH activity was detected after 2 h incubation in medium, containing 250 µg/mL of MTT. After removing culture medium, formazan salts were solubilized in DMSO and absorbance was read at 535 nm using a FusionTM Packard microplate reader (Packard Bioscience, Meriden, CT, USA).
In order to evaluate DNA synthesis, [3H]methyl‐thymidine (Amersham, Les Ulis, France) was added to the culture medium at a final concentration of 1 µCi/mL, 24 h before cell harvesting. DNA synthesis was evaluated by measuring [3H]methyl‐thymidine incorporation in the absence or in the presence of ICL670 and CP20. Total protein content of the cultures was evaluated by the method of Bradford (1976). Results were expressed as percentage of control values.
Analysis of DNA content was performed by flow cytometry. After trypsinization, DNA content was measured in cells using the Cycle Test Plus kit (Becton Dickinson, San Jose, CA, USA). Cell cycle analysis was performed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA) equipped with an argon laser (488 nm). Data analysis was performed using ModFit software (Verity Software House, Topsham, ME, USA).
Enzyme assay
Lactate dehydrogenase (LDH) activity was measured in both the culture medium and intracellularly as an index of cytotoxicity, employing an LDH kit (Roche Diagnostics, Mannheim, Germany). Extracellular LDH activity was measured by aliquot of cell free medium obtained by centrifugation (100 g/min during 5 min). Intracellular LDH activity was evaluated on liver cells previously lysed in phosphate saline buffer by sonication for 15 s and centrifuged as above. LDH activities were detected by reading absorbance at 485 nm. An LDH standard curve (0–4000 mU/mL) was employed for quantification of enzyme activity (L‐lactate dehydrogenase, Sigma). Experimental results were expressed in terms of LDH release into the medium, as a percentage of total activity per culture.
Apoptosis assays
DNA fragmentation was measured in cells collected by trypsinization and 1 × 106 cells were permeabilized with 0.5% phosphate‐buffered saline‐saponine. After a RNase treatment (100 µg/mL) (Sigma) they were stained with a Tris 10 mm ethylenediaminetetraacetic acid (EDTA), 1 mm solution containing 10 µg/mL of propidium iodide (Sigma). DNA fragmentation was analysed by flow cytometry (FacsCalibur System, BD Biosciences, San Diego, CA, USA). Analysis of the data was performed with ModFit software.
Caspase activity was evaluated on cells lysed as previously described (Stennicke & Salvesen 1997). Aliquots of 50 µL of crude cell lysate were incubated with 25 nm Asp‐Glu‐Val‐Asp 7‐amido‐4‐methyl coumarine (DEVD‐AMC) caspase‐3‐like substrate. At 37 °C for 20 min, caspase‐3 mediates cleavage of DEVD‐AMC peptide (Bachem, Voisin‐le‐Bretonneux, France) generating fluorescent free 7‐amino‐4‐methyl coumarine (AMC) that was measured by spectrofluorometry using a FusionTM Packard microplate reader at excitation/emission wavelengths of 380 and 440 nm, respectively. Caspase activity was measured in arbitrary units per µg total protein. However, DEVD‐AMC caspase activity is not totally specific for caspase‐3 because other caspases can cleave the DEVD‐AMC substrate, however, with much lower efficiency (Stennicke & Salvesen 1997). This activity, usually presented as DEVD‐AMC caspase‐3‐like activity, was expressed in percentage of control cell culture activity.
Polyamine levels
Putrescine, spermidine and spermine levels were measured, as previously described, by mass spectrometry analysis (Gaboriau et al. 2003). Cells were sonicated in 0.2 N perchloric acid. After overnight incubation and centrifugation, protein content was determined in the acid‐insoluble material. 1,10‐Diaminodecane (DAD, internal standard) was added to the supernatant that was treated with dansyl chloride (5 mg/mL) solubilized in acetone. Dansylated polyamines were extracted with cyclohexane, evaporated to dryness and were re‐solubilized in acetonitrile. Mass spectrometry analysis was performed with 50 µL of acetonitrile polyamine solution. Single ion monitoring mode data masses were obtained with an atmospheric pressure chemical ionization sorter to detect the protonated parent ions ([M + H]+; at m/z 555.2, 845.3, 1135.4 and 639.3 for putrescine, spermidine, spermine and 1,10‐diaminodecane, respectively). Polyamine levels were deduced from ionic intensities of the selective ions and were corrected from that of DAD. Polyamine concentrations were deduced from a calibration curve of putrescine, spermidine and spermine (0–50 µm of each polyamine) and were expressed with respect to the protein content.
RNA extraction and amplification
Total RNAs were extracted from 25 cm2 flasks at 80% cell confluency, with SV total RNA isolation system (Promega Corporation, Madison, WI, USA). After reverse transcription of total RNAs, ODC, SSAT and r18S transcripts were amplified by multiplex PCR using three primers sets, respectively: ODC forward 5′‐ACCGGCGTAATCAACCCAGCG‐3′, ODC reverse 5′‐TGCGTGGTCATAGAGTATGC‐3′; SSAT forward 5′‐TTGGGTCATGGTGCCAGCCTG‐3′, SSAT reverse 5′‐CACTTCTGCAACCAGGCAGTG‐3′; r18S forward 5′‐TCGAGGCCTGTAATTGGAA‐3′, r18S reverse 5′‐CCCAAGATCCAACTACGAGCTTT‐3′ (Eurogentec, Angers, France). Amplification fragments were obtained with the following PCR cycles: (94 °C – 2 mn) – 40 × (94 °C – 30 s, 50 °C – 40 s, 72 °C – 30 s) – (72 °C – 5 mn). Length of the co‐amplified fragments of ODC (248 bp), SSAT (300 bp) and r18S (150 bp) were confirmed by gel electrophoresis (2.5% agarose in 0.5% Tris‐acetate‐EDTA buffer). Density of amplified fragments was evaluated by DNA semiquantification with DensiLab software (Microvision Instruments, Evry, France).
Chelator uptake measurement
Chelator uptake was investigated. Three million cells were seeded in 25 cm2 culture flasks. Cell treatments were performed in triplicate in the presence of 40 µm ICL670 and 60 µm CP20. After 48 h of treatment, supernatants were removed and cells were then washed three times with 3 mL of ice‐cold phosphate‐buffered saline solution. Cells were collected by scraping after addition of 1 mL of water and were sonicated for 30 s at 0 °C. Protein content in these cell extracts was measured according to a method adapted from that of Bradford (1976) (Bio‐Rad Protein Assay, Bio‐Rad, Ivry sur Seine, France) and absorbance was read at 595 nm. Ultrafiltration of 200 µL of cell lysates was performed by centrifugation for 20 min at 15 000 g in the NANOSEP™ 3K centrifugal device (Pall Filtron Co., Northborough, MA, USA). Both chelators were quantified in these cell lysates by mass spectrometry analysis using single ion monitoring mode and chemical ionization. No ion was observed at m/z = 374.1 (ICL670) and m/z = 140.1 (CP20), in the mass spectra of the cell lysates obtained from control untreated hepatocytes. Cell lysate samples (50 µL) were directly injected from the HP1100 series autosampler into a stream of acetonitrile/water (3v/1v) at a flow rate of 0.5 mL/min. Areas under the specific ionic signals of 374.1 (ICL670) and 140.1 (CP20) were integrated using the ChemStation 1100 software (Hewlett Packard, Wilmington, DE, USA). Chelator concentrations were then deduced employing the calibration curves obtained from cell lysate enrichments with various concentrations of the two chelators, followed by their sonication and ultrafiltration. Each chelator measurement was determined as triplicate and was corrected for protein content in the cell extracts.
Statistical analysis
Results from at least four replicates were expressed as means ± SD. Statistical analysis was performed using the non‐parametric Mann–Whitney test. Significance level was set at 0.05.
RESULTS
Effect of iron chelators on cell viability and DNA replication in FAO cells
Extracellular LDH activity, SDH activity and DNA replication were measured in FAO cell cultures using comparable iron‐binding equivalent chelator concentrations. A significant increase in extracellular LDH activity was observed in the presence of 20 µm ICL670 (Fig. 1; P < 0.001) while the iron‐binding equivalent concentration 30 µm CP20 was without effect (Fig. 1). Iron saturation of ICL670 inhibited its cytotoxic effect (Fig. 1; P < 0.001). 50% decrease in SDH activity was observed in the presence of 20 µm ICL670 (Fig. 2; P < 0.001), while comparable concentration of CP20 (30 µm) did not modify SDH activity (Fig. 2). Iron saturation of ICL670 inhibited its effect (Fig. 2; P < 0.001). Decrease in DNA replication (–70%) was observed in the presence of 20 µm ICL670 (Fig. 2; P < 0.001), while comparable concentration of CP20 (30 µm) was without effect on DNA replication (Fig. 2). Iron saturation of ICL670 inhibited its antiproliferative effect (Fig. 2; P < 0.001).
Figure 1.
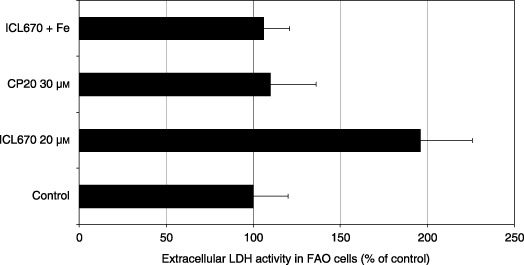
Effect of iron chelators ICL670 and CP20 on extracellular lactate dehydrogenase (LDH) activity in FAO rat cell cultures.
Figure 2.
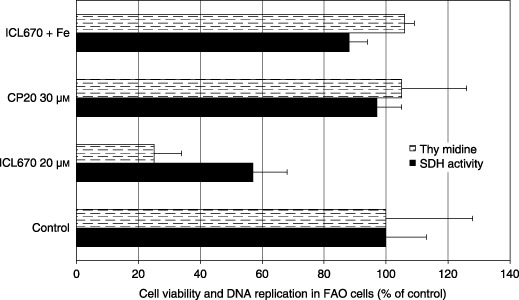
Effect of iron chelators ICL670 and CP20 on cell viability (succinate dehydrogenase activity) and DNA replication in FAO rat cell cultures.
Effect of iron chelators on cell cycle distribution in FAO cells
We observed that 20 µm ICL670 induced accumulation of FAO cells in G0‐G1 phase (65.4% versus 53.6% in the control; Fig. 3, P < 0.01) and in S phase of the cell cycle (24.9% versus 19.1% in the control; Fig. 3, P < 0.05). This increase in the cell number in G0/G1 and S phase cells was correlated to a drop in the number of cells in G2/M (9.6% versus 27.3% in the control; Fig. 3, P < 0.001). Comparable stoiechiometric concentration 30 µm CP20 did not affect cell cycle distribution (Fig. 3).
Figure 3.
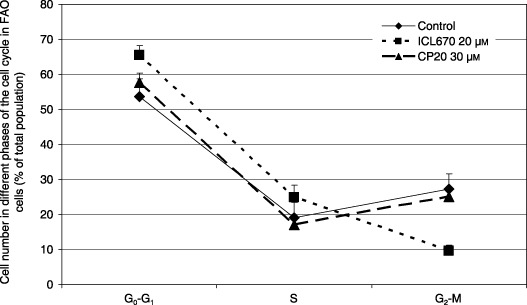
Effect of iron chelators ICL670 and CP20 on cell cycle distribution of FAO rat cell cultures.
Apoptotic effect of the chelators in FAO cells
Phase contrast microscopic observation of FAO cell cultures showed that 30 µm CP20 was without effect on both cell number and cell morphology, while 20 µm of ICL670 enhanced the number of cells floating in the culture medium. These floating cells disappeared when the chelator was iron saturated and thus possibly indicate an apoptotic effect of ICL670 (data not shown). Indeed, compared to control and CP20 treated cultures, ICL670 induced 12 times more DNA fragmentation (Fig. 4; P < 0.001) and 35 times more caspase‐3 activity (Fig. 4; P < 0.001). This apoptotic potential was inhibited by iron saturation of the chelator (Fig. 4; P < 0.001).
Figure 4.
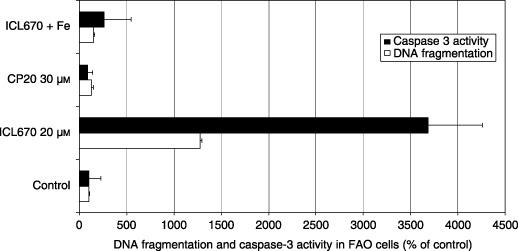
Effect of iron chelators ICL670 and CP20 on nuclear DNA fragmentation and caspase‐3 activity in rat FAO cell cultures.
Effect of iron chelators on cell viability, DNA replication and cell cycle distribution in HUH7 cells
In HUH7 cells, 25 µm ICL670 and a four‐time higher iron‐binding equivalent concentration of CP20 (150 µm) decreased cell viability in a similar manner (–50%) and DNA replication (–90%) (Fig. 5, P < 0.001). In the HUH7 cell cultures, we observed that 25 µm ICL670 and 150 µm CP20, respectively, induced accumulation of cells in S phase (61.2% vsersu 32.5% in the control; Fig. 6, P < 0.01) and G2‐M phase of the cell cycle (98.7% versus 23.7% in the control; Fig. 6, P < 0.001).
Figure 5.
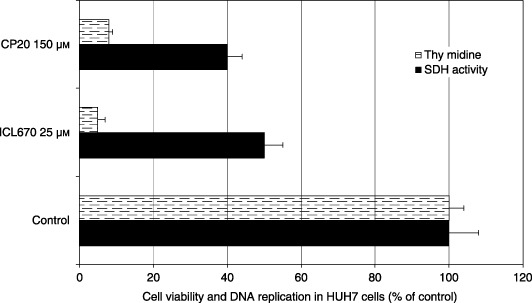
Effect of iron chelators ICL670 and CP20 on cell viability and DNA replication in human hepatoma cell line HUH7 cultures.
Figure 6.
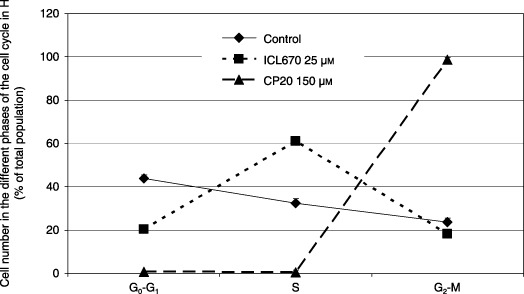
Effect of iron chelators ICL670 and CP20 on cell cycle distribution of HUH7 human cell cultures.
Effect of iron chelators on intracellular polyamine levels
ICL670 (25 µm) decreased levels of the three polyamines (Fig. 7, P < 0.05 or 0.001); a comparable depletion of putrescine and spermidine without significant effect on spermine was obtained using 5 mmα‐difluoromethylornithine (DFMO) treatment during 48 h (Fig. 7). In accordance with these results, a significant decrease of ODC and SSAT mRNA levels was observed in cells treated with ICL670 (Fig. 8, P < 0.001). In contrast, polyamine levels, particularly putrescine, were largely increased by 150 µm CP20 (Fig. 7, P < 0.001). In parallel, HUH7 cells showed high increase in SSAT mRNA (Fig. 8, P < 0.001) and to a lesser extent, ODC mRNA levels (Fig. 8).
Figure 7.
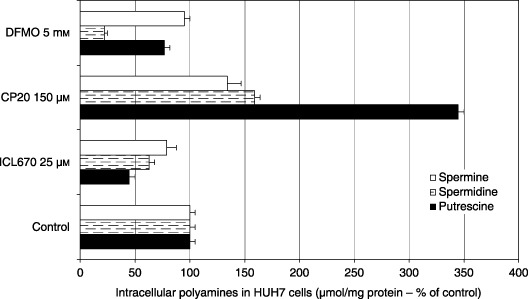
Effect of iron chelators ICL670 and CP20 on intracellular putrescine, spermidine and spermine levels in HUH7 human cell cultures.
Figure 8.
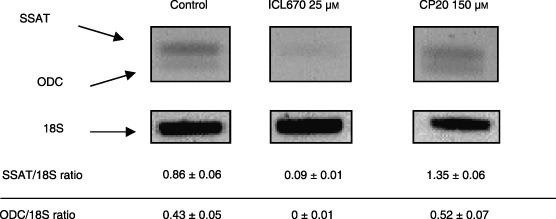
Effect of iron chelators ICL670 and CP20 on ornithine decarboxylase (ODC) and spermidine/spermine N1‐acetyltransferase (SSAT) mRNA levels in HUH7 human cell cultures.
Measurement of chelator uptake by mass spectrometry
FAO cells were incubated for 48 h in the presence of 40 µm of ICL670 and 60 µm of CP20. At the end of the incubation, intracellular contents were: 8.4 ± 4.2 µmol/mg protein and 1.4 ± 0.3 µmol/mg protein, respectively, for ICL670 and CP20. ICL670 in FAO cells attained 6‐fold higher intracellular levels than CP20.
DISCUSSION
CP20 and ICL670 exert cytotoxic and antiproliferative effects on neoplastic cells
CP20 and ICL670 have been shown to mobilize iron of iron‐loaded hepatocytes and consequently to induce cell protection against iron damage (Hershko et al. 2001; Gaboriau et al. 2004). Extracellular LDH increase has been reported in HepG2 cells exposed to CP20 (Chenoufi et al. 1998; Kicic et al. 2001). In FAO cell cultures, ICL670 increases extracellular LDH activity. By analysing the results obtained using comparable iron‐binding equivalent chelator concentrations, we observed that CP20 was devoid of any effect. The ICL670 effect disappeared in conditions of iron saturation, indicating that iron deprivation is a key step in this process.
Antiproliferative ability of iron chelators, like CP20, has been previously shown in different hepatoma cell lines (Chenoufi et al. 1998; Kicic et al. 2001). In FAO cells, the chelator ICL670 induced inhibition of DNA replication; this was closely correlated to decrease of mitochondrial SDH activity that reflects cell viability. However, comparison of the two chelators in similar iron‐binding equivalent conditions indicated high inhibition of DNA replication with ICL670 while CP20 was without effect. Iron saturation of ICL670 completely prevented the decrease in DNA synthesis. Moreover, CP20 has been shown to arrest HepG2 cells in S phase of the cell cycle (Chenoufi et al. 1998). In our study, 30 µm CP20 did not modify the cell cycle of FAO cells. Comparable iron‐binding equivalent concentration of ICL670 (20 µm) induced an increase in cell number in G0‐G1 and S phases of the cell cycle; this correlated to a decrease in cell number in G2‐M.
ICL670 exerts a powerful apoptotic effect in neoplastic cells
Cytotoxic and cytostatic properties of CP20 have already been correlated to DNA fragmentation in human tumour cells, which reflected cell death by apoptosis (Yasumoto et al. 2004). Here, flow cytometry analysis of FAO cells stained with propidium iodide after treatment with iron chelator ICL670, showed a large increase of fragmented DNA. In similar conditions of iron‐binding equivalents, significant DNA alteration was observed using ICL670 while CP20 had no effect. Moreover, data reported with ICL670 indicate that the DNA fragmentation observed reflected cell death by apoptosis, because it correlated to increase of caspase‐3 activity. Thus, in accordance to DNA fragmentation and caspase‐3 activity measurements, cell damage observed after ICL670 could also be related to apoptotic cell death. This indicates a controlled mechanism of cell death by which iron removal may trigger the caspase cascade.
ICL670 and CP20 effects involve deregulation of polyamine metabolism through different pathways
Intracellular polyamine levels and enzymes of their metabolism have been shown to be differentially regulated during cell cycle progression and in induction of apoptotic cell death (Schipper et al. 2000; Oredsson 2003). ODC and SSAT activities have been shown to be high in tumour‐ and other proliferating cells stimulated by growth factors (Matsui & Pegg 1980). On the other hand, inhibition of the first rate‐limiting enzyme of polyamine biosynthesis, ODC, by DL‐α‐difluoromethylornithine (DFMO) induces a cell cycle blockade in G1 or G2 phases of the cycle in numerous cell lines (Marty et al. 2000). In the same way, induction of spermidine/spermine SSAT has been correlated to cell population growth inhibition (Ichimura et al. 1998). In our study, a cytostatic concentration of CP20 (150 µm) considerably increased putrescine level in HUH7 cells. In parallel, we also observed an increase of SSAT mRNA level, as previously reported, in growth arrest of HeLa cells (Ichimura et al. 1998). Moreover, this high putrescine intracellular level could also be correlated to the slight increase of ODC mRNA level. This increase in putrescine, ODC and SSAT mRNA levels induced by CP20 has also been linked to cell cycle arrest in G2‐M phase, because in this phase, elevated levels of these markers was usually observed (Wallace et al. 2003). ICL670, at the concentration 25 µm, had an opposite effect, decreasing levels of the three polyamines. Comparable depletion of putrescine and spermidine was obtained by treating the human hepatoma cells with DFMO. In accordance with these results, a significant decrease of ODC and SSAT mRNA levels was observed in cells treated with ICL670. Furthermore, it appeared possible to correlate decrease in polyamines, ODC and SSAT mRNA levels observed in the presence of ICL670, to cell cycle arrest in S phase, because in this phase, low levels of all three has been described (Wallace et al. 2003). Thus, ICL670 blocks cell proliferation, which is followed by apoptosis; however, it inhibits cell proliferation and exerts its apoptotic effect with higher efficiency than CP20; this possibly is explained by the decreases in putrescine and SSAT mRNA levels. Indeed, previous results have suggested that putrescine is essential for the cell to enter S phase, possibly pushing the cell through the G1 restriction point prior to DNA synthesis (Wallace et al. 2003). Concerning SSAT expression, recent results, obtained from further cancer cell lines, have demonstrated that up‐regulation of SSAT mRNA, in an iron deficient condition using DFO, may exert a protective function in apoptotic cell death (Kim et al. 2005). So, down‐regulation of SSAT mRNA level induced by ICL670 may also contribute to its higher antiproliferative and apoptotic effect compared to CP20. However, we may also consider recent results (Li et al. 1999; Li et al. 2001) that demonstrate polyamine depletion induces or stabilizes p53 gene expression resulting in inhibition of cell population growth. Moreover, mass spectrometry study of cell chelator uptake has shown that ICL670 entered FAO cells six times more than CP20. This could be related to the lipophilicity of ICL670, which presents a partition coefficient higher than that of CP20 (LogP = –0.77 for CP20; LogP = 3.8 for ICL670) (Liu et al. 2002; Nick et al. 2003). This more efficient ICL670 cellular uptake could explain, compared to CP20, its higher cytostatic and apoptotic effect, the effect being correlated to iron depletion, because iron saturation of the chelator inhibited its biological effect.
In conclusion, as summarized in Fig. 9, we have demonstrated that the iron chelator ICL670 inhibits proliferation of FAO rat cell cultures; a comparable result has been obtained in the human HUH7 cell line using a four‐time higher iron‐binding equivalent concentration of CP20 (150 µm). Both chelators can induce cell population growth inhibition associated to deregulation of polyamine metabolism. The cytostatic effect of CP20 is associated to a large increase in putrescine that could result from SSAT activation, the key enzyme involved in the retro‐conversion pathway of polyamine metabolism. Concerning the recent described orally active chelator ICL670, it appears to be more powerful than CP20, and induced a drop in DNA replication associated with decrease of polyamine levels that could result from inhibition of polyamine biosynthesis through ODC inactivation. This study sheds light on a potentially strong relationship between iron depletion and polyamine metabolism. Such interactions appear to depend on the chemical structure of the iron chelator that is probably closely correlated to biological properties independent of iron depletion. Because both iron and polyamines are known to play an essential role in controlling cell proliferation, the present results indicate that ICL670, by inhibiting polyamine metabolism, may constitute a promising adjuvant therapeutic molecule in the strategy for cancer treatment.
Figure 9.
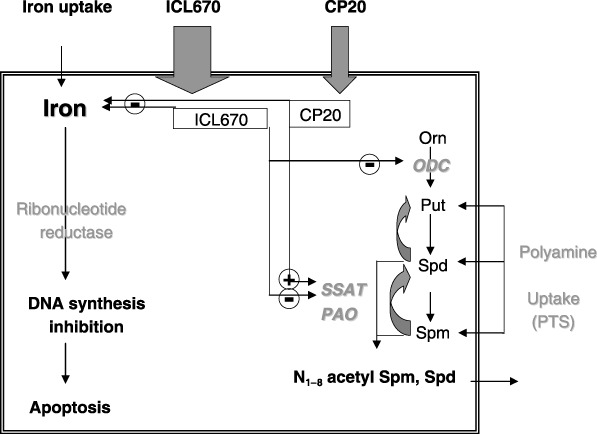
Schematic representation of the effect of iron chelators ICL670 and CP20 on DNA synthesis, apoptosis and polyamine metabolism.
ACKNOWLEDGEMENTS
This work was supported by the ‘5th PCRDT European contract N° QLRT‐2001‐00444: iron in haemochromatosis: deleterious effects of an essential nutrient’ and ACI ‘CAPFER: nouveaux outils chimiques et analytiques pour la détection et le dosage du fer; application à l’étude de son métabolisme; N° 03 2 135’.
REFERENCES
- Bhasin G, Kauser H, Athar M (2002) Low iron state is associated with reduced tumor promotion in a two‐stage mouse skin carcinogenesis model. Food Chem. Toxicol. 40, 1105–1111. [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Brissot P, Loréal O (2002) Role of non‐transferrin‐bound iron in the pathogenesis of iron overload and toxicity. Adv. Exp. Med. Biol. 509, 45–53. [DOI] [PubMed] [Google Scholar]
- Chantrel‐Groussard K, Gaboriau F, Pasdeloup N, Havouis R, Nick HP, Pierre JL, Brissot P, Lescoat G (2006) The new orally active iron chelator ICL670A exhibits a higher antiproliferative effect in human hepatocyte cultures than O‐trensox. Eur. J. Pharmacol. 541, 129–137. [DOI] [PubMed] [Google Scholar]
- Chenoufi N, Drénou B, Loréal O, Pigeon C, Brissot P, Lescoat G (1998) Antiproliferative effect of deferiprone on the HepG2 cell line. Biochem. Pharmacol. 56, 431–437. [DOI] [PubMed] [Google Scholar]
- Chenoufi N, Hubert N, Loréal O, Morel I, Pasdeloup N, Cillard J, Brissot P, Lescoat G (1995) Inhibition of iron toxicity in rat and human hepatocyte cultures by the hydroxypyridin‐4‐ones CP20 and CP94. J. Hepatol. 23, 166–173. [DOI] [PubMed] [Google Scholar]
- Chenoufi N, Loréal O, Drénou B, Cariou S, Hubert N, Leroyer P, Brissot P, Lescoat G (1997) Iron may induce DNA synthesis and repair in rat hepatocytes stimulated by EGF/pyruvate. J. Hepatol. 26, 650–658. [DOI] [PubMed] [Google Scholar]
- Deschatrette J, Weiss MC (1974) Characterization of differentiated clones from a rat hepatoma. Biochimie 56, 1603–1611. [DOI] [PubMed] [Google Scholar]
- Gaboriau F, Chantrel‐Groussard K, Rakba N, Loyer P, Pasdeloup N, Hider RC, Brissot P, Lescoat G (2004) Iron mobilization, cytoprotection, and inhibition of cell proliferation in normal and transformed rat hepatocyte cultures by the hydroxypyridinone CP411, compared to CP20: a biological and physicochemical study. Biochem. Pharmacol. 67, 1479–1487. [DOI] [PubMed] [Google Scholar]
- Gaboriau F, Havouis R, Moulinoux JP, Delcros JG (2003) Atmospheric pressure chemical ionization‐mass spectrometry method to improve the determination of dansylated polyamines. Anal. Biochem. 318, 212–220. [DOI] [PubMed] [Google Scholar]
- Gaboriau F, Kreder A, Clavreul N, Moulinoux J, Delcros J, Lescoat G (2004) Polyamine modulation of iron uptake in CHO cells. Biochem. Pharmacol. 67, 1629–1637. [DOI] [PubMed] [Google Scholar]
- Hershko C, Konijn AM, Nick HP, Breuer W, Cabantchik ZI, Link G (2001) ICL670A: a new synthetic oral chelator: evaluation in hypertransfused rats with selective radioiron probes of hepatocellular and reticuloendothelial iron stores and in iron‐loaded rat heart cells in culture. Blood 97, 1115–1122. [DOI] [PubMed] [Google Scholar]
- Ichimura S, Hamana K, Nenoi M (1998) Significant increases in the steady states of putrescine and spermidine/spermine N1‐acetyltransferase mRNA in HeLa cells accompanied by growth arrest. Biochem. Biophys. Res. Commun. 243, 518–521. [DOI] [PubMed] [Google Scholar]
- Kicic A, Chua AC, Baker E (2001) Effect of iron chelators on proliferation and iron uptake in hepatoma cells. Cancer 92, 3093–3110. [DOI] [PubMed] [Google Scholar]
- Kim K, Ryu JH, Park JW, Kim MS, Chun YS (2005) Induction of a SSAT isoform in response to hypoxia or iron deficiency and its protective effects on cell death. Biochem. Biophys. Res. Commun. 331, 78–85. [DOI] [PubMed] [Google Scholar]
- Li L, Li J, Rao J, Li M, Bass BL, Wang JY (1999) Inhibition of polyamine synthesis induces p53 gene expression but not apoptosis. Am. J. Physiol. Cell Physiol. 276, 946–954. [DOI] [PubMed] [Google Scholar]
- Li L, Rao J, Guo X, Liu L, Santora R, Bass BL, Wang JY (2001) Polyamine depletion stabilizes p53 resulting in inhibition of normal intestinal epithelial cell proliferation. Am. J. Physiol. Cell Physiol. 281, 941–953. [DOI] [PubMed] [Google Scholar]
- Liu YD, Liu ZD, Hider RC (2002) Oral iron chelators – development and application. Best Pract. Res. Clin. Haematol. 15, 369–384. [DOI] [PubMed] [Google Scholar]
- Marty C, Mori G, Sabini L, Rivarola V (2000) Effects of alpha‐difluoromethylornithine on the cyclin A expression in Hep‐2 cells. Biocell 24, 49–52. [PubMed] [Google Scholar]
- Matsui I, Pegg AE (1980) Effect of thioacetamide, growth hormone or partial hepatectomy on spermidine acetylase activity of rat liver cytosol. Biochim. Biophys. Acta 633, 87–94. [DOI] [PubMed] [Google Scholar]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Nick HP, Acklin P, Lattmann R, Buehlmayer P, Hauffe S, Schupp J, Alberti D (2003) Development of tridentate iron chelators: from desferrithiocin to ICL670. Curr. Med. Chem. 10, 1065–1076. [DOI] [PubMed] [Google Scholar]
- Oredsson SM (2003) Polyamine dependence of normal cell‐cycle progression. Biochem. Soc. Trans. 31, 366–370. [DOI] [PubMed] [Google Scholar]
- Porter JB, Davis BA (2002) Monitoring chelation therapy to achieve optimal outcome in the treatment of thalassaemia. Best Pract. Res. Clin. Haematol. 15, 329–368. [PubMed] [Google Scholar]
- Rakba N, Loyer P, Gilot D, Delcros JG, Glaise D, Baret P, Pierre JL, Brissot P, Lescoat G (2000) Antiproliferative and apoptotic effects of O‐Trensox, a new synthetic iron chelator, on differentiated human hepatoma cell lines. Carcinogenesis 21, 943–951. [DOI] [PubMed] [Google Scholar]
- Rouan MC, Marfil F, Mangoni P, Sechaud R, Humbert H, Maurer G (2001) Determination of a new oral iron chelator, ICL670, and its iron complex in plasma by high‐performance liquid chromatography and ultraviolet detection. J. Chromatogr. B Biomed. Sci. Appl. 755, 203–213. [DOI] [PubMed] [Google Scholar]
- Schipper RG, Penning LC, Verhofstad AA (2000) Involvement of polyamines in apoptosis. Facts and controversies: effectors or protectors? Semin. Cancer Biol. 10, 55–68. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS (1997) Biochemical characteristics of caspases‐3‐6‐7 and 8. J. Biol. Chem. 272, 25719–25723. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Kennedy K, Witt M, Juzefyk J (1991) Effect of dietary iron deficiency or excess on the induction of mammary carcinogenenis by 1‐methyl‐1‐nitrosourea. Carcinogenesis 12, 111–114. [DOI] [PubMed] [Google Scholar]
- Wallace HM, Fraser AV, Hughes A (2003) A perspective of polyamine metabolism. Biochem. J. 376, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Obata Y, Yano Y, Otani S, Ichikawa T (1995) Stimulating effect of excess iron feeding on spontaneous lung tumor promotion in mice. Int. J. Vitam. Nutr. Res. 65, 127–131. [PubMed] [Google Scholar]
- Yasumoto E, Nakano K, Nakayachi T, Morshed SR, Hashimoto K, Kikuchi H, Nishikawa H, Kawase M, Sakagami H (2004) Cytotoxic activity of deferiprone, maltol and related hydroxyketones against human tumor cell lines. Anticancer Res. 24, 755–762. [PubMed] [Google Scholar]


