Abstract
Abstract. Objectives: Epithelial stem cells of the eye surface, of the cornea and of the conjunctiva, have the ability to give rise to self renewal and progeny production of differentiated cells with no apparent limit. The two epithelia are separated from each other by the transition zone of the limbus. The mechanisms adopted by stem cells of the two epithelia to accomplish their different characteristics, and how their survival, replacement and unequal division that generates differentiated progeny formation are controlled, are complex and still poorly understood. They can be learned only by understanding how stem cells/progenitors are regulated by their neighbouring cells, that may themselves be differently unspecialised, forming particular microenvironments, known as ‘niches’. Stem cells operate by signals and a variety of intercellular interactions and extracellular substrates with adjacent cells in the niche. Technical advances are now making it possible to identify zones in the corneal limbus and conjunctiva that can house stem cells, to isolate and expand them ex vivo and to control their behaviour creating optimal niche conditions. With improvements in biotechnology, regenerative cornea and conjunctiva transplantation using adult epithelial stem cells becomes now a reality. Results and Conclusions: Here we review our current understanding of stem cell niches and illustrate recent significant progress for identification and characterization of adult epithelial stem cells/progenitors at cellular, molecular and mechanistic levels, improvement in cell culture techniques for their selective expansion ex vivo and prospects for a variety of therapeutic applications.
EPITHELIA OF THE EYE SURFACE
The external surfaces, of the cornea and of the conjunctiva, are covered with highly specialized epithelia that are derived from genotypically diverse precursor cells. Both epithelia are of the scaly, non‐keratinized type, formed from adherent layers of cells that continually renew and regenerate themselves. The deeper basal strata contain immature, semiquiescent stem cells capable of autoregeneration and also of creating offspring that mature, migrating towards the external surface, ceasing to divide themselves. They differentiate to death, finally being released into the lachrymal fluid and immediately replaced by subsequent cells migrating from inner layers in continuous turnover that occurs for the whole life of the organism. The two epithelia, of the cornea and of the conjunctiva, are separated from each other by a narrow transition zone known as the limbus.
Mechanisms that regulate contrasting behaviour of the two epithelia are complex and still little understood. Recent basic, preliminary, comparative studies on global expression of the genes of corneal cells, compared to those of conjunctiva, in humans (using oligonucleotide arrays AffimetrixTM) have identified over 11 000 RNA‐transcripts in each of the two tissues, coded by over 8000 different genes, of which there are 256 different transcripts present exclusively in the epithelia of the conjunctiva and 181 in the epithelia of the cornea. Thus, this reveals that homeostatic regulation of the two epithelia is regulated in significantly different ways (Nielsen et al. 2003; Wolosin et al. 2004). The integrity of these epithelia and their capacity to auto‐renew themselves for the whole life of the individuals, depends on the presence of an adequate number of stem cells (SC). Serious damage to the eye surface, for example acid or alkali burns and excessive thermal exposure, and pathological conditions such as Steven–Johnson syndrome, aniridia (which is hereditary), pathologies of the epithelium related to use of contact lenses, cicatricial pemphigoid of the eye, can cause severe SC deficiency (SCD) (Kruse & Tseng 1994; Santos et al. 2005), which may result in accentuated reduction in visual sharpness, and ultimately the possibility of blindness.
Depletion of corneal SC leads to conjunctivalization or migration of the conjunctival elements on the surface of the cornea with consequent opacification, vascularization and progressive marked loss of transparency and of visual capacity (Kruse & Volcker 1997). Various surgical techniques have been proposed for treating SCD (reviewed in Holland & Schwartz 1996; Pellegrini et al. 1997; Tsubota et al. 1999; Schwab et al. 2000; Rama et al. 2001; Grueterich et al. 2002; Ramaesh & Dhillon 2003). It is important to stress that keratoplasty does not correct SCD in the corneal limbus (LSCD). Autologous transplantation of very small monolateral fragments of the corneal limbus for the acquired LSCD is often effective, especially if used for the treatment of unilateral pathology, but it involves the risk of eventual complications that can arise both in the treated donor eye and in the controlateral eye. Allogeneic transplant of fragments of the corneal limbus requires prolonged immunosuppressive treatment with the risk of severe complications for the patient (Lavker et al. 2004; Santos et al. 2005). In the last few years, biotechnological developments have made possible new therapies based on the regenerative capacity of SC/progenies for both autologous and allogeneic use, cultivated ex vivo to successfully obtain adequate numbers for transplantation (reviewed in Limb et al. 2006). The following examples illustrate concepts related to the biology of these SC; recent most significant progress of this new discipline with regard to the regeneration of the epithelia of the eye surface, and hypotheses concerning possibilities of new therapeutic applications.
COMPARATIVE ANALYSIS OF THE EPITHELIA OF THE CORNEA AND OF THE CONJUNCTIVA
The epithelia of the cornea and the conjunctiva have many common characteristics:
-
•
They are both of the scaly, multilayered type in which only the most immature cells of the basal layer have the capability to reproduce themselves.
-
•
They express specific cytokeratins (CK) of epithelial tissues that differ with diverse expressions between the cornea and conjunctiva and in the transition zone of the limbus.
-
•
Their rapid turnover depends on a very small reserve of stem cells (reviewed in Lajtha 1979; Potten & Morris 1988; Pellegrini et al. 1999).
Comparing the phenotypical characteristics of the two epithelia, the corneal epithelium is composed of keratinocytes that extend mosaically in the superimposed layers, while the precursors of the conjunctival epithelium are bipotent, generating both epithelial cells similar to those of the cornea and globose cells that are goblet cells producing mucus. A further significant difference is the diverse angiogenic properties of the two epithelia.
Corneal epithelium is transparent, scaly, multilayered and non‐keratinized; it adheres to the underlying Bowman membrane from its basal layer of cuboidal cell phenotype, devoid of goblet cells. Integrity of the epithelium is essential for guaranteeing protection of the lens and normal visual acuity. The lack of vascularization in the cornea implies either that the cells of its epithelium do not produce any angiogenic factors (Kure et al. 2003; Gabison et al. 2004), or that they release anti‐angiogenic factors. It is remarkable in the cornea that presence of vascular endothelial growth factor (VEGF)‐A (normally a potent stimulator of angiogenesis), and proximity to vascularized tissues yet result in its avascularization. Certain vascular endothelial growth factor receptors (VEGFR) are involved in ‘entraping’ VEGF that inhibits local vascularization (Ambati et al. 2006; Cursiefen et al. 2006). During a re‐epithelialization phase following trauma the epithelial cells secrete, for example, matrilysin (a metalloproteinase) that plays an important anti‐angiogenic role. The contrary occurs in the conjunctival epithelium; in the case of deficit in the number of cells with the capacity to replicate corneal SC (e.g. after a trauma or an extensive caustic destruction of the corneal epithelium), invasion by epithelial cells of the adjoining conjunctiva induces massive neovascularization of the corneal surface (Fini & Stramer 2005).
Conjunctival epithelium extends above well‐vascularized stroma and consists of superimposed layers of cells that are not as tightly organized as those of the cornea. The epithelium is subdivisible into three distinct regions:
-
1
the bulbar epithelium; this adjoins the cornea/limbus zone and covers the eye bulb;
-
2
the fornix, in the region that folds onto the bulb; and
-
3
the eyelid, adjoining the epithelium of the eyelid that covers its tarsal surfaces.
The epithelium here consists of cells that develop as a mosaic; they are generated from stem cells scattered along all conjunctival surfaces in the basal zone, but which are more numerous in the region of the fornix. The stem cells interceded between epithelial cells in three dimensions. Furthermore, here are goblet cells that secrete mucus; these are the principal source of the fluid, an important component of the lachrymal film. Otherwise, known as caliciform cells, they are essential for maintenance of the integrity of the eyeball, and insufficient production of mucus is responsible for serious eye diseases (Dogru et al. 2005).
THE CORNEAL LIMBUS
The epithelium of the limbus consists of a mulitilayer of cells dispersed in the stroma, rich in minute vessels, with the presence of Langherans and melanocyte cells (which produce and secrete pigments) and a gradient of epithelial cells that includes progenitors of the corneal epithelium (Mann 1944; Maumenee & Scholz 1948; Buschke 1949; Kruse & Volcker 1997; Ramaesh & Dhillon 2003). Apparently, the limbus does not contain stem/precursor cells of the conjunctival epithelium, but the basal epithelial cells do generate precursor cells of the corneal epithelium. These include immature transitional cells, which are substantially larger than the basal cells of the corneal epithelium that have a heightened capacity to divide themselves; they are transient amplifying cells (TAC) (Miller et al. 1997). In addition, there is a small fraction of slowly cycling autoreplicating stem cells (Cotsarelis et al. 1989). In this population in human corneal epithelial cells, size correlates with cell differentiation phenotype and proliferative capacity. The slow‐cycling autoreplicating stem cells are small in size with high nucleus: cytoplasmic ratio, whereas TAC and terminally differentiated cells become progressively larger.
Arising from the deepest zone of the limbus, the immature keratinocytes migrate towards the superficial area that adjoins the corneal epithelium, for which they supply the basal constituent elements of the central area (Gipson 1989; Daniels et al. 2001). A transition zone, limbus cells are characterized by phenotypical expression of proteins characteristic of the area; these include at least one sialyltransferase (Wolosin & Wang 1995), α‐enolase (Zieske et al. 1992) and pigments produced and released in the surrounding microenvironment, especially from deep cells with demarcation between the cornea and the limbus. Expression of gap junctions proteins (e.g. Cx43) is rarely detectable in the limbus and corneal domain but tend instead, to be found in cells that form the first layer above the basal row of epithelium (Wolosin et al. 2000; Lavker et al. 2004; Wolosin et al. 2004).
An important marker of the more immature basal and parabasal cells, both of the corneal epithelium and those of the limbus and of conjunctival cells is the protein p63, a nuclear transcription factor partly homologous to p53, whose expression is considered essential for guaranteeing survival, growth and differentiation of epithelial progenitors (Signoretti et al. 2000). An important marker that can be used to evaluate replicative potential of the cells is the nuclear antigen Ki‐67 that is expressed in all dividing cells (Gerdes et al. 1984).
CYTOKERATINS
Cytokeratins are the principal component of a complex network of intermediate filaments of epithelial cells (Green 1980; Watt 1989). They constitute a heterogeneous class of about 30 structurally diverse polypeptides (MW 40–70 kDa) divisible into two subgroups, acidic type and a family of neutral‐basic types (Fuchs et al. 1981; Moll et al. 1982; Kivela & Uusitalo 1998). For formation of intermediate filaments, it is necessary that at least one keratin of each groups is present (Hatzfeld & Franke 1985). In the epithelium of the epidermis, for example, initiation of differentiation is characterized by a switch of expression of the two cytokeratins generally expressed, from the most immature cells of this type of epithelium, CK5 and CK14, to mature cells that express CK1 and CK10 (here, CK1 and CK10 are termed tissue specific cytokeratins, TSCK). In a similiar way, in the epithelium of the eye surface an analogous switch occurs, from expression of CK5 and CK14 to their respective TSCKs, CK3 and CK12 for the cornea and CK13 and CK4 for the conjunctiva (van Muijen et al. 1986; O’Guin et al. 1987; De Saint Jean et al. 2004; Lavker et al. 2004; Paladino et al. 2004).
The principal difference between epidermal epithelium and the two epithelia of the eye's surface occurs in the most advanced phase of their maturity and differentiation. Differerentiated cells of the epidermal epithelium begin to express further proteins that generate the external corneified layer, keratinization. In contrast, differentiation of the external layers of the corneal epithelia and of the conjunctiva is instead accompanied by the expression of proteins delegated for ion transport, metabolites, mucus, proteins responsible for the formation of tight junctions and those of intercellular canals. These are components typically found in secretory epithelium that might be delegated to re‐absorption.
In all cases, keratinization of stratified epithelia is regulated by proteins encoded by a restricted number of critical genes that can operate both to activate specific functions and to block normal discriminative processes. In particular, retinoids and their nuclear receptors are fundamental in this decision. Moreover, degree of keratinization of various epithelial layers reflects their sensitivity to retinoids. Under physiological conditions, keratinocytes of the epithelia of the eye surface, even though potentially capable of producing all the necessary proteins for the synthesis of a cornified epithelium, are inhibited from doing so by the presence of retinoids in the bloodstream and in the lachrymal veil. A diet devoid of, or insufficiently supplied with Vitamin A, in some diseases or in newborns, can result in corneification of the epithelia of the eye surface, with devastating consequences (Kruse & Tseng 1994; Kunimoto et al. 1998).
The pattern of expression of cytokeratins of epithelial cells of the cornea and conjunctiva provides a simple and reliable method for characterization of their grouping and origin, revealing in some cases marked differences during the development. For example in mouse, CK4 is expressed both in corneal and conjunctival epithelium until the adult stage, when it remains confined to conjunctival cells; CK12 is expressed only in the suprabasal layer of the cornea in the foetus, while in the adult it is present in all layers; CK14, on the contrary, is expressed in all layers of the cornea during the foetal stage, but remains restricted to only a few basal cells in the adult (Lavker et al. 2004; Tanifuji‐Terai et al. 2006). In man, a variety of specific markers has been identified in the cornea and in the conjunctiva. CK19 is expressed in the cytoplasm of the most immature cells of the basal limbal layer, while expression of CK3 is selective for the most differentiated cells of the central part of the cornea, in the suprabasal and epibasal layers (Cooper & Sun 1986). In the conjunctiva, CK19 is expressed in the cytoplasm of cells of the suprabasal and the apical layers, CK10 in basal and epibasal layers, yet CK3 is not expressed in this epithelium at all (Dua et al. 2005; Papini et al. 2005).
NICHE, AUTOCRINE AND PARACRINE FACTORS IN THE CORNEA
Survival, proliferation and the differentiation of an epithelium depends on a complex system of interactions between its cells, the external environment and the underlying mesenchyme. Even though little is known of the basic mechanisms of these interactions, it is noted that in response to signals originating from the local microenvironment, cells of epithelial tissues produce and release soluble, biologically active molecules (enzymes, cytokines, growth factors, differentiation factors and more), with wide spheres influence. Such molecules are capable of regulating functions of the epithelial cells that produced them (autocrine signalling) as well as functions of neighbouring cells of the epithelium (paracrine signalling), and of those of the surrounding microenvironment (underlying stroma, capillaries), all contributing to maintenance of homeostasis of the epithelium and to mediation of the repair in the case of damage. The predominant SC model predicts that a SC generates its two daughter cells by asymmetric division, one remaining undifferentiated, thus maintaining the SC pool, and the other cell destined to form a TAC whose progeny ultimately become terminally differentiated cells (TDC).
With regard to inductive methods of terminal differentiation, opinion prevails that a gradient of base to apex and base to lateral factors exist inside the limbal epithelium, which is responsible for the diverse state of maturity between cells of the limbus and those of the cornea, a confirmation of diverse functionality of the compartmentalization that is observed progressing from an SC to TAC to TDC. In the last few years, a significant number of experimental pieces evidence (reviewed in Schlötzer‐Schrehardt & Kruse 2005) have suggested that SCs are retained in their original limbal microenvironment (the so‐called niche) by intrinsic and extrinsic factors that contribute to them maintaining their undifferentiated state. Origins of these factors are the extracellular limbal matrix (in particular the basal membrane) matrix‐to‐cell and cell‐to‐cell interactions. The underlying limbal stroma contains stromal cells and blood vessels, forming the ‘palisade of Vogt’, these facilitate distribution of growth factors and cytokines that can regulate the function and maintenance of limbal SCs. In the cornea, the absence of vascularization determines a progressive lessening of such factors inducing the prevalence of the differentiation. Analogously, basal membranes of the cornea and of the limbus show diverse molecular compositions (Kolega et al. 1989; Ljubimov et al. 1995). In basal membranes of the cornea, α3, α4 and α5 collagen IV chains and α1, α3, β1, β3, γ1, γ2 laminin chains are found, while in the limbus only α1 and α2 of collagen IV chains are present plus the same laminin chains of the cornea plus α2 and β2 (Ljubimov et al. 1995; Tuori et al. 1996; Dietlein et al. 1997; Fukuda et al. 1999). These data together with others, support the idea that differences in composition between basal membranes of the cornea and limbus could, at least in part, explain the differences in phenotype and in behaviour of the basal cells themselves. To support this hypothesis recent experimental evidence demonstrates that lack of expression of CK3 in basal cells of the limbus would have mediated epithelial–mesenchymal interactions of the type between the limbal stroma and basal membrane (Espana et al. 2003). Underlying stroma is also a source of growth factors released by connective tissue cells.
Numerous experiments have demonstrated that embryonic fibroblasts (such as the 3T3 cell line), in coculture with limbal SC/progenitor cells stimulate their division. In such cocultures, the SC/progenitor cells remain, to a large extent, undifferentiated and insensitive to exposure to phorboldiesters that are tumour promoters, a property that is considered typical of less mature progeny of the keratinocytes (Meller et al. 2002), while a further interesting correlation has been noted between epithelial progenitors present in the limbus with high constitutive coexpression of EGF and EGFR, and the capacity of basal epithelial progenitors of the limbus to divide themselves, maintaining a highly undifferentiated phenotype (Zieske & Wasson 1993; Zieske et al. 2000). Although presence of neurotrophins has not been observed in the limbal stroma and limbal epithelium (except a weak expression of NT3 in the epithelium and in the suprabasal layers of the cornea), strong expression of the high affinity receptor for NGF, TrkA, in basal cells of the limbus suggests that NGF is implicated in the control of limbal stem compartments (reviewed in Schlötzer‐Schrehardt & Kruse 2005).
A further important element in regulation of progenitor epithelial cell differentiation is the presence and activity of corneal stromal cells (fibroblasts and keratinocyte precursors). These cells could provide growth factors and cytokines that are the origin of complex autocrine and paracrine interactions that intervene between the fibroblasts, keratinocytes and limbal cells. Three types of interaction, mediated by cytokines, between limbal cells of the cornea and the underlying stroma, have been characterized (Li & Tseng 1995):
-
•
Type I cytokines, in particular, TGFα, IL‐1β, PDGF, are produced by corneal keratinocytes and are active on fibroblasts of the stroma.
-
•
Type II cytokines, such as IGF‐1, TGFβ1, TGFβ2 and b‐FGF, are capable of acting both on keratinocytes and on fibroblasts. The presence of high density of receptors for IGF‐1, TGFβ1, TGFβ2 and b‐FGF, could be responsible for slow progression through the cell cycle of limbal basal cells.
-
•
Type III cytokines are produced by the fibroblasts and are effective in stimulating proliferation and migration of corneal progenitor cells; among these are KGF, produced by fibroblasts of the limbus and HGF produced by corneal fibroblasts.
STEM CELLS
The two principal defining characteristics of stem cells are their capacity to replenish themselves and their capability of providing the origin of differentiated cells. Fine‐tuning of techniques that make isolation and expansion of various adult tissues possible ex vivo has opened enormous applicative potential for regenerative medicine. Diverse tissues, from which it is possible to isolate SCs in the laboratory, have now been identified. These are capable not only of generating cells of the original tissue but also of accomplishing regeneration of different tissues, if cultures are appropriately stimulated in vitro by selective growth factors, or if they should be transplanted in vivo in compatible recipients. These include SC isolated from haematopoietic tissue, from the umbilical cord, from bone marrow in primis and from mesenchymal SC isolated from bone marrow (also called stromal/mesenchymal cells), which can differentiate into haematopoietic precursors, osteoblasts, chondroblasts and adipocytes (Grove et al. 2004; Lakshmipathy & Verfaillie 2005; Suda et al. 2005). Neuronal precursors capable of reproducing and differentiating have also been isolated (Gritti et al. 2002).
MARKERS OF EYE SURFACE STEM CELLS
Considering the countless studies on possible markers that could define stem cell phenotype, conviction prevails that it is not possible to identify expression of a single marker for all stem cells but rather that coexpression of a series of molecules, varying nature, by whose presence or absence of identifies the characteristics of stemness.
With respect to stem cells of the eye surface (those of the cornea and conjunctiva), the limbus is considered to be the centre for corneal stem cell location while conjunctival stem cells are preferentially localized in the epithelial zone of the eyelid, in the fornix and in the muco‐cutaneous epithelia (Wei et al. 1995; Wirtschafter et al. 1999; Chen et al. 2003). A difficulty in study of eye surface stem cells is that often a series of markers may be accurately selected according to the most recent data in the literature, yet these are insufficient to discriminate true stemness of the cells in an unequivocal manner, as there is often a considerable fraction of TAC (even if very primitive), that still retains a phenotype very close to that of total stem cells (Vascotto & Griffith 2006). Reports suggest that expression of specific molecules by cells in regions of eye surface stem cells, can be termed ‘of a stem cell phenotype’ no matter the presence of other primitive cells (early TAC) in that location. Such are; CK19, vimentin, the receptor for hyaluronic acid and the TGFβ type 1 receptor (that are expressed in a specific manner in some basal cells of the limbus and conjunctiva (Vascotto & Griffith 2006)), metallothionein, integrin α9, the carrier ABCG2 that is also found in stem cells of other tissues (Goodell et al. 2005), α‐enolase, p63 (in particular the isoform ΔNp63, which is found confined to a restricted population of basal limbal cells and in the conjunctiva) (Pellegrini et al. 2001; Moore et al. 2002; Di Iorio et al. 2005; Rosellini et al. 2007), the receptor for HGF and the receptor for KGF (reviewed in Schlötzer‐Schrehardt & Kruse 2005). Simultaneously, positivity and/or elevated expression of the markers above, in the same zones, may be accompanied by lack of other markers such as CK3/CK12, connexin 43, P‐cadherin, involucrin, integrin α2, α6, β4 and nestin (reviewed in Schlötzer‐Schrehardt & Kruse 2005).
Among the markers listed, membrane transporter ABCG2 deserves a particular mention. Haematopoietic and many types of organ‐specific stem cells have been recently shown to exhibit the side population (SP) phenotype, based on their ability to efflux the DNA binding dye Hoechst 33342 (Goodell et al. 1996, reviewed in Alison 2003; Challen & Little 2006); SP cells isolated from various adult tissues have stem cell characteristics. ABCG2 is a member of the ATP binding cassette (ABC) transporters; it has been identified as a molecular determinant for SP cells in the bone marrow (Zhou et al. 2001) and in other tissues. Thus, it has been proposed as universal marker for stem cells. Harvested limbal epithelial cell populations contain SP cells expressing ABCG2, and a portion of limbal epithelial basal cells in vivo exclusively express ABCG2 (Watanabe et al. 2004). So far, the ABCG2 transporter has been reported to be the only feasible surface marker suitable to isolate live limbal epithelial stem cells by FACS for transplantation purposes.
Detectable colocalization of stem cell and TAC markers, by means of immunistochemistry on samples from the inside of the same limbal epithelial basal layer, suggests that distribution of the same stem cells and TAC inside the limbal basal layer and the conjunctiva does not follow precise models, but instead is varied and similar to that observed in crypts of the intestinal epithelium (Clatworthy & Subramanian 2001). The discovery of analogous crypts in the corneal limbus supports this hypothesis (Dua et al. 2005). In a recent study, Shanmuganathan et al. (2006) describe the anatomical structure and immunophenotypical characterization of these limbal epithelial crypts (LEC), which appear to be distributed in clusters of different size, cell number and localization within the limbus. They were demonstrated to show the following phenotype: CK3–/CK19+/CD34–/vimentin+/p63+/Connexin43+/Ki67–. Basal cells of the LEC are significantly smaller than basal cells of adjacent rete pegs and are also smaller than suprabasal limbal and central corneal epithelial cells. These characteristics of LEC, together with their ultrastructural phenotype, make this particular subpopulation of cells consistent with it, representing a putative stem cell niche (Shanmuganathan et al. 2006).
An area of novel and promising research is into stem and/or primitive progenitor cells of the stroma underlying the corneal epithelium. In one recent study, a proportion of cells was identified in the substantia propria of the limbus and in the bulbar conjunctiva that express CD44, TNF‐R5, c‐Kit and telomerase – markers associated with stem cells in other tissues (Vascotto & Griffith 2006). Observation of distinct subpopulations of cells on the inside of the substantia propria of the limbus and of the bulbar conjunctiva support the hypothesis of the existence of stromal/mesenchymal‐like stem cells that could give rise to keratinocytes of the eye epithelia. In line with this interesting possibility, there are recent studies in the mouse that have demonstrated haematopoietic origin of a subset of cells of the corneal stroma that express stem cell markers (Nakamura et al. 2004; Sosnova et al. 2005), and multipotent fibroblasts isolated from human cornea/limbal tissue that have been induced to differentiate in vitro into epithelial cells of the cornea (Dravida et al. 2005).
The stromal microenvironment around limbal stem cells is crucial not only to maintain the homeostasis of corneal epithelium but recent findings suggest that the limbal stromal niche is also critical in regulating the fate decision of limbal stem cells towards a corneal or a fibroblast phenotype (Kawakita et al. 2005). This latter is achieved in culture by exposing live limbal epithelium and limbal stroma‐containing cells to air. This finding postulates that limbal stem cells undergo stromal invasion and trans‐differentiate into fibroblast‐like cells through epithelial–mesenchymal transition. Therefore, the reverse process might be also possible and be implicated in the fibrosis observed in patients with SC deficiency.
EPITHELIAL STEM CELL CULTURE
Various effective methods have been fine‐tuned for isolating and expanding epithelial SC of the epidermis with good repeatability (Barrandon & Green 1987; Compton et al. 1989; De Luca et al. 1989; Leighton 1992). Expanding the number of cells on collagen sponges or strips (He & McCulley 1991; Papini et al. 2003), fibrin membranes (Rama et al. 2001), matrices reinforced with hyaluronic acid fibres (Stark et al. 2004) or devitalized membranes (e.g. strips of autologous or allogeneic derma (Matouskova et al. 1997)), the amniotic membrane (Schwab et al. 2000; Meller et al. 2002; Grueterich et al. 2003; Song et al. 2005; reviewed by Gomes et al. 2005) or a layer of oral mucosa (Kinoshita et al. 2004; Inatomi et al. 2005), in the presence or absence of post‐mitotic stromal cells (used like feeder cells), has successfully opened new ways for tissue engineering and regenerative medicine in various fields, and in a particular way in ophthalmology. However, the use of allogeneic biological material is associated with the risk of disease transmission (HIV, hepatitis, bacterial and fungal infections) and allograft rejection. In addition, many of these substrates lack the mechanical properties that provide easy handling for suturing, as well as prolonged endurance after transplantation. Use of biosynthetic materials as stromal substitutes to support epithelial cell growth would overcome some of the problems in the use of allogeneic tissues and biological substrates. Synthetic bioresorbable polymeric materials have been used as matrices for dermal equivalents for skin regeneration (Schmidt et al. 1988; Yoshioka et al. 1994a,b; McAteer et al. 2002; Yoshioka et al. 2003). These include, for example, a polyglactin fibre mesh, poly L‐lactic acid, copolymer of poly(ethylene‐glycolterephthalate) and poly(butylenes‐terephthalate) blocks (Chen et al. 2004; Budak et al. 2005) and more recently temperature‐responsive polymers such as poly‐N‐isopropylacrylamide (Zhang et al. 2002; Liu & Sheardown 2005). Mixtures of 2 thermo‐responsive polymer blocks, poly N‐isopropylacrylamide‐co‐n‐butyl methacrylate and polyethylenglycol (Sudha et al. 2006) have also been used. However, all these matrices have shortcomings, such as poor mechanical strength and the risk of immunological rejection.
Other than being influenced by the type of matrix used, the success of transplantation events depends on the stromal environment encountered in the recipient, as fate of transplanted stem cells is heavily dependent on the stromal environment. In cases of persistent inflammation, viability of transplanted stem cells can be deeply compromised, thus effective control of corneal inflammation is as important as providing viable stem cells in restoring limbal SC deficiency. Recent findings suggest that mesenchymal stem cells produce important soluble factors (growth factors and cytokines) that promote expansion and differentiation of human SCs; they have the ability to modify the response of inflammatory immune cells (reviewed in Le Blank & Ringden 2006). Anti‐proliferative, immunomodulatory and anti‐inflammatory effects of mesenchymal stem cells have focused attention on them as potential therapeutic agents in disorders caused by the immune system, including graft versus host disease, rejection after organ transplantation and autoimmune diseases.
In the course of the last 2–3 years in different laboratories, various methods have been devised for the isolation and expansion of epithelial SC from the cornea and conjunctiva, demonstrating the possibility of culturing epithelial precursor cells of both the cornea and the conjunctiva (Pellegrini et al. 1999; Ramaesh & Dhillon 2003; Papini et al. 2005); these can differentiate, producing cells able to express markers specific to these two tissues (Sun & Lavker 2004; Pajoohesh‐Ganji & Stepp 2005). Refinement of new techniques for organotypic three‐dimensional cultures, in which cells grow and differentiate in the context of their natural environment, in the presence of all cellular components normally present in vivo, has raised the possibility of maintenance, isolation and expansion ex vivo, of stem populations of epithelial tissues. The methodology initially utilized for study of drug toxicity to cells and tissues of a varied nature, cultured for a short period ex vivo (Hanto et al. 1982; Leighton 1992; Margolis et al. 1999), has been recently improved and applied successfully to the study of long‐term cultures ex vivo, of epithelia of a variety of tissues, including the cornea and the conjunctiva (Michelini et al. 2004; Papini et al. 2004; Michelini et al. 2005; Papini et al. 2005). Small undamaged fragments of tissue (1–3 mm2) to be examined are deposited on collagen sponges at the air–liquid interface, in liquid growth media at defined conditions of salts and vitamins with the addition of suitable growth factors specific for the keratinocytes. This is performed in the absence of pituitary extracts previously included, which has been shown to be potential sources of contamination by prions or lentiviruses. In these culture conditions, it is possible to produce selective growth and expansion of undifferentiated epithelial precursors. Then, these cells (isolated from fragments and from the support matrices, by means of enzymatic digestion), are expanded and maintained in culture in the presence of a feeder cell layer with phenotypical characteristics of basal epithelial cells (p63 and CK19 positive, CK3 negative); these undifferentiated cells exhibit high capacity for autoreplication, which can be demonstrated by their expression of the Ki‐67 antigen (Papini et al. 2005). The cells demonstrate their stemness by regenerating in vitro an epithelium‐equivalent that can become phenotypically multilayered similar to that formed in vivo (Fig. 1). The substrate disk, on which the epithelium‐equivalent is regenerated ex vivo, is composed only of basal/epibasal cells that can be transplanted with the newly formed epithelium orientated towards the recipient surface. It is essential that there should be sufficient time for the transplanted stem/basal cells to become incorporated into to the recipient, before the matrix is re‐absorbed, thus reproducing a differentiated, reconstituted new epithelium. Important success factors for transplant are adequate preparation of the stromal surface of the recipient cornea and choice of substrate on which the basal precursor cells will grow in vitro. It has been demonstrated already that initial presence of an equivalent epithelium, formed with very few layers of basal/precursor cells not yet differentiated, offers greater probability for the cells to flourish than a multilayered epithelium containing more layers of differentiated cells. These are probably of minor benefit as differentiated layers are lost immediately after transplant, prolonging the time interval that elapses prior to attachment of de novo basal layers and the creation of the new epithelium. Simultaneously, this delays and reduces involvement of stem cells of the recipient, peripheral to the transplant strip and the process of regeneration of the new epithelium, creating possible eventual deformities in the location or unexpected reactions on the part of components of the stroma, which could require eventual corrective intervention. When planning conjunctival engineering, above all in the eyelid tract, an important factor to consider is choice of the most appropriate substrate‐matrix on which to prepare the equivalent epithelium for transplantation. There is similarity between native conjunctivae. With respect to substrates, autopsy‐derived sclera or a variety of artificial commercial substrates can be used. For example, Medpor (Portex Surgical, Newman, GA, USA), AlloDerm (LifeCell Corp., Branchiburg, NJ, USA) and further comparable matrices, equivalents of human oral mucosa, are at least theoretically more convenient for surgical reconstruction of an extensive eye surface, in particular of the conjunctiva (Nakamura et al. 2004; Yoshizawa et al. 2004; Inatomi et al. 2005). Advantages of this type of procedure include:
Figure 1.
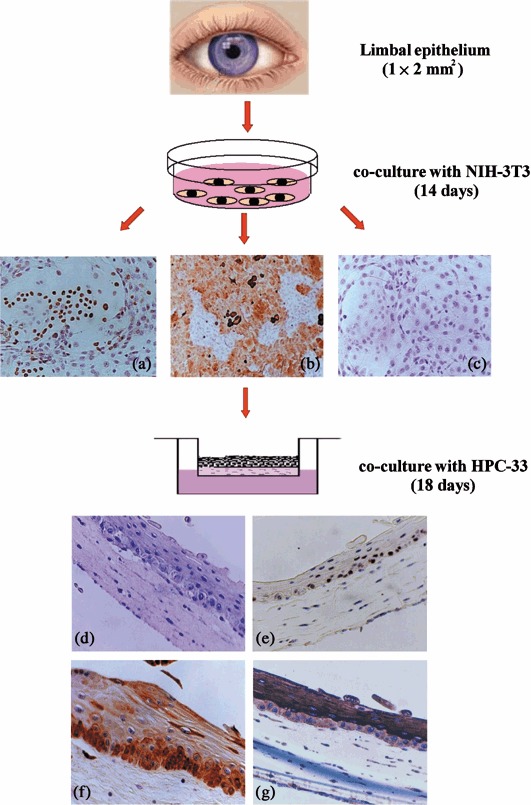
Isolation and expansion of basal corneal cells and demonstration of their stem/progenitor properties. Epithelial cells recovered from fragments of limbal tissue, after enzymatic treatment in the presence of feeder post‐mitotic cells, NIH‐3T3 form clones of epithelial cells p63 (a) and CK‐19 positive (b), negative for CK‐3 (c). These cells, transferred to a collagen matrix containing human corneal stromal fibrobasts (line HPC‐33). Like feeder cells, these form superimposed cohesive layers of non‐keratinized cells capable of differentiation (d), and of maintaining a layer of elements p63 positive (basal) (e), CK‐19 positive (basal and epibasal) (f) and CK‐3 positive (epibasal) (g). Basal corneal cells on collagen matrix without HPC‐33 feeder cells fail to form a multilayered epithelium and remain as a monolayered sheet only (not shown) (enlargement of the original: b, 200×; a, c, d, e, f and g, 200×) (Papini et al. 2005).
-
•
Growth of intact, confluent equivalent‐epithelia on a rigid, human dermal layer is facilitated.
-
•
Transplantation can be carried out with autologous, isologous or heterologous mucosa. In all cases, the initial aim of the procedure is to prepare the recipient substrate onto which keratinocytes adhere, to replicate, to expand in number and to migrate. Initially, very few endogenous stem cells/precursors are present, and these must progressively relocate from the centre of the culture to the periphery. In the case of isologous or heterologous transplantation, it would be necessary for a patient to be prescribed immunosuppressive preventative therapy. Eventually, successive new interventions might be required to transplant tiny strips of autologous tissue that favour development of a sufficient number of stem cells on to a stromal substrate.
-
•
Currently, success of such transplants is improved with reduced morbidity and duration of the surgical intervention. Much is thanks to important innovations in surgical technique and to new instrumental procedures (such as microkeratomy and laser technology) that have improved preparation of the recipient substrate.
PROSPECTS FOR STEM CELL THERAPY
Although regenerative medicine is still in its infancy, important advances have been made in the last few years. The study of epithelial stem cells provides ever‐deepening knowledge concerning their identification. The consequent possibility of isolating and selectively expanding stem cells/precursors of epithelia of the cornea and the conjunctiva in vivo, and of using these cells for engineering equivalent‐epithelia utilisable for transplantation, is here. Today, it is possible to raise the fraction of immature epithelial stem cells/progenitors in vitro, and to separate them from their more differentiated progeny, enriching the fraction of the former in a transplant, and therefore significantly improving the prospect of a successful procedure. Results currently obtained are important for expansion of SC numbers and for fine‐tuning of new optimal culture media. These can be constituted from chemically defined components enriched with soluble recombinant growth factors, obtained using molecular biological techniques and the presence of highly selected isologous and heterologous serum.
With improvements in biotechnology for identification and characterization of SC, and improvement in cell culture techniques for selective SC expansion ex vivo, regenerative medicine becomes a reality. Pathological conditions of optical epithelium, in which three‐dimensional cytoarchitecture of the microenvironment of the corneal and conjunctival epithelia have been seriously compromised, can be repaired. Conditions in which, for genetic reasons, the presence of SC or their precursors is extremely low can be amended. Fundamental is the discovery that SC transplantation does not depend solely on successful routing of sufficient quantities of stem/precursor cells cultured in vitro, then transplanted. Where still present, endogenous stromal/mesenchymal SC that resides in damaged tissues can be augmented. It is important to stress further that new horizons have been opened by the discovery that transplanted adult stem cells can act supportively, creating optimal niche conditions. Preparing a sufficient microenvironment can involve provision of specific growth factors and immunosuppressors, as simultaneously number and function of the endogenous stomal cells contribute directly to synthesis of the new tissue. These results open crucial prospects and stimulate study of new experimental approaches that will be revolutionary in identification of newly discovered auxiliary molecules and growth factors. In regeneration of the epithelia of the cornea and of the conjunctiva in particular, appropriate controls for use prior to or in the course of a transplant will favour successful treatment.
ACKNOWLEDGEMENTS
To those contributors for supporting the development of this work: SOFT Italia SpA, MIUR‐FIRB.PS Quality of Life: ‘Post‐Genome’ and ‘New Organs’; Italian Ministry of Health: C.P. and I.Z.P.S. of Lazio and Tuscany, Ciampino‐Roma; Project Related to Research 11MH1 (Kontakt) between the Ministries of External Affairs of the Czech Republic and of Italy; Foundation‐ONLUS ‘Staminali e Vita’ Pisa, Italy.
REFERENCES
- Alison MR (2003) Tissue‐based stem cells: ABC transporter proteins take centre stage. J. Pathol. 200, 553–560. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J (2006) Corneal avascularity is due to soluble VEGF receptor‐1. Nature 7114, 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H (1987) Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 84, 2302–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM (2005) Ocular surface epithelia contain ABCG2‐dependent side population cells exhibiting features associated with stem cells. J. Cell Sci. 118, 1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke W (1949) Morphologic changes in cells of corneal epithelium in wound healing. Arch. Ophthalmol. 41, 306–316. [DOI] [PubMed] [Google Scholar]
- Challen GA, Little MH (2006) A side order of stem cells: the SP phenotype. Stem Cells 24, 3–12. [DOI] [PubMed] [Google Scholar]
- Chen W, Ishikawa M, Yamaki K, Sakuragi S (2003) Wistar rat palpebral conjunctiva contains more slow‐cycling stem cells that have larger proliferative capacity: implication for conjunctival epithelial homeostasis. Jpn. J. Ophthalmol. 47, 119–128. [DOI] [PubMed] [Google Scholar]
- Chen Z, Depaiva CS, Luo L, Ketzer FL, Pflugfelder SC, Li DQ (2004) Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 22, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy JP, Subramanian V (2001) Stem cells and the regulation of proliferation, differentiation and patterning in the intestinal epithelium: emerging insights from gene expression patterns, transgenic and gene ablation studies. Mech. Dev. 101, 3–9. [DOI] [PubMed] [Google Scholar]
- Compton CC, Gill JM, Bradford DA, Regauer S, Gallico GG, O’Connor NE (1989) Skin regenerated from cultured epithelial autografts on full‐thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab. Invest. 60, 600–612. [PubMed] [Google Scholar]
- Cooper D, Sun TT (1986) Monoclonal antibody analysis of bovine epithelial keratins. Specific pairs as defined by coexpression. J. Biol. Chem. 261, 4646–4654. [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT (1989) Existence of slow‐cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57, 201–209. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Saint‐Geniez M, Hamrah P, Jin Y, Rashid S, Pytowski B, Persaud K, Wu Y, Streilein JW, Dana R (2006) Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 103, 11405–11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JT, Dart JK, Tuft SJ, Khaw PT (2001) Corneal stem cells in press. Wound Repair Regen 9, 483–494. [DOI] [PubMed] [Google Scholar]
- De Luca M, Albanese E, Bondanza S, Megna M, Ugozzoli L, Molina F, Cancedda R, Santi PL, Bormioli M, Stella M, Magliacani G (1989) Multicentre experience in the treatment of burns with autologous and allogenic cultured epithelium, fresh or preserved in a frozen state. Burns 15, 303–309. [DOI] [PubMed] [Google Scholar]
- De Saint Jean M, Baudouin C, Di Nolfo M, Roman S, Lozato P, Warnet JM, Brignole F (2004) Comparison of morphological and functional characteristics of primary‐cultured human conjunctival epithelium and of Wong‐Kilbourne derivative of Chang conjunctival cell line. Exp. Eye Res. 78, 257–274. [DOI] [PubMed] [Google Scholar]
- Dietlein TS, Jacobi PC, Paulsson M, Smyth N, Krieglstein G (1997) Vielfalt der laminin‐isoformen in der limbusregion des auges. Klin. Monatsbl. Augenheilkd. 211, 188–191. [DOI] [PubMed] [Google Scholar]
- Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M (2005) Isoforms of ΔNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. USA 102, 9523–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogru M, Asano‐Kato N, Tanaka M, Igarashi A, Shimmura S, Shimazaki J, Okada N, Takano Y, Fukagawa K, Tsubota K, Fujishima H (2005) Ocular surface and MUC5AC alterations in atopic patients with corneal shield ulcers. Curr. Eye Res. 30, 897–908. [DOI] [PubMed] [Google Scholar]
- Dravida S, Pal R, Khanna A, Tipnis SP, Ravindran G, Khan F (2005) The transdifferentiation potential of limbal fibroblast‐like cells. Brain Res. Dev. Brain Res. 160, 239–251. [DOI] [PubMed] [Google Scholar]
- Dua HS, Shanmuganathan VA, Powell‐Richards AO, Tighe PJ, Joseph A (2005) Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 89, 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana EM, Kawakita T, Romano A, Di Pasquale M, Smiddy R, Liu C, Tseng SCG (2003) Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest. Ophtalmol. Vis Sci. 44, 5130–5135. [DOI] [PubMed] [Google Scholar]
- Fini ME, Stramer BM (2005) How the cornea heals: cornea‐specific repair mechanisms affecting surgical outcomes. Cornea 24 (8 Suppl), S2–S11. [DOI] [PubMed] [Google Scholar]
- Fuchs EV, Coppock SM, Green H, Cleveland DW (1981) Two distinct classes of keratin genes and their evolutionary significance. Cell 27, 75–84. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Chikama T, Nakamura M, Nishida T (1999) Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea 18, 73–79. [PubMed] [Google Scholar]
- Gabison E, Chang JH, Hernandez‐Quintela E, Javier J, Lu PC, Ye H, Kure T, Kato T, Azar DT (2004) Anti‐angiogenic role of angiostatin during corneal wound healing. Exp. Eye Res. 78, 579–589. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J. Immunol. 133, 1710–1715. [PubMed] [Google Scholar]
- Gipson IK (1989) The epithelial basement membrane zone of the limbus. Eye 3, 132–140. [DOI] [PubMed] [Google Scholar]
- Gomes JA, Romano A, Santos MS, Dua HS (2005) Amniotic membrane use in ophthalmology. Curr. Opin. Ophthalmol. 16, 233–240. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo . J. Exp. Med. 183, 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, McKinney‐Freeman S, Camargo FD (2005) Isolation and characterization of side population cells. Methods Mol. Biol. 290, 343–352. [DOI] [PubMed] [Google Scholar]
- Green H (1980) The keratinocyte as differentiated cell type. Harvey Lect. 74, 101–139. [PubMed] [Google Scholar]
- Gritti A, Vescovi AL, Galli R (2002) Adult neural stem cells: plasticity and developmental potential. J. Physiol. (Paris) 96, 81–90. [DOI] [PubMed] [Google Scholar]
- Grove JE, Bruscia E, Krause DS (2004) Plasticity of bone marrow‐derived stem cells. Stem Cells 22, 487–500. [DOI] [PubMed] [Google Scholar]
- Grueterich M, Espana EM, Touhami A, Ti SE, Tseng SC (2002) Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology 109, 1547–1552. [DOI] [PubMed] [Google Scholar]
- Grueterich M, Espana EM, Tseng SC (2003) Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv. Ophthalmol. 48, 631–646. [DOI] [PubMed] [Google Scholar]
- Hanto DW, Hopt UT, Hoffman R, Simmons RL (1982) Lymphocyte recruitment, regional blood flow, and vascular permeability at sites of allogeneic cellular interactions. J. Immunol. 129, 2437–2443. [PubMed] [Google Scholar]
- Hatzfeld M, Franke WW (1985) Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate‐sized filaments by homologous and heterologous recombinations of purified polypeptides. J. Cell Biol. 101, 1826–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YG, McCulley JP (1991) Growing human corneal epithelium on collagen shield and subsequent transfer to denuded cornea in vitro . Curr. Eye Res. 10, 851–863. [DOI] [PubMed] [Google Scholar]
- Holland EJ, Schwartz GS (1996) The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea 15, 549–556. [PubMed] [Google Scholar]
- Inatomi T, Nakamura T, Koizumi N, Sotozono C, Kinoshita S (2005) Current concepts and challenges in ocular surface reconstruction using cultivated mucosal epithelial transplantation. Cornea 24 (8 Suppl), S32–S38. [DOI] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC (2005) Intrastromal invasion by limbal epithelial cells is mediated by epithelial‐mesenchymal transition activated by air exposure. Am. J. Pathol. 167, 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Koizumi N, Nakamura T (2004) Transplantable cultivated mucosal epithelial sheet for ocular surface reconstruction. Exp. Eye Res. 78, 483–491. [DOI] [PubMed] [Google Scholar]
- Kivela T, Uusitalo M (1998) Structure, development and function of cytoskeletal elements in non‐neuronal cells of the human eye. Prog. Retin. Eye Res. 17, 385–428. [DOI] [PubMed] [Google Scholar]
- Kolega J, Manabe M, Sun T‐T (1989) Basement membrane heterogeneity and variation in corneal epithelial differentiation. Differentiation 42, 54–63. [DOI] [PubMed] [Google Scholar]
- Kruse FE, Tseng SC (1994) Retinoic acid regulates clonal growth and differentiation of cultured limbal and peripheral corneal epithelium. Invest. Ophthalmol. Vis. Sci. 35, 2405–2420. [PubMed] [Google Scholar]
- Kruse FE, Volcker HE (1997) Stem cells, wound healing, growth factors, and angiogenesis in the cornea. Curr. Opin. Ophthalmol. 8, 46–54. [DOI] [PubMed] [Google Scholar]
- Kunimoto DY, Sharma S, Reddy MK, Gopinathan U, Jyothi J, Miller D, Rao GN (1998) Microbial keratitis in children. Ophthalmology 105, 252–257. [DOI] [PubMed] [Google Scholar]
- Kure T, Chang JH, Kato T, Hernandez‐Quintela E, Ye H, Lu PC, Matrisian LM, Gatinel D, Shapiro S, Gosheh F, Azar DT (2003) Corneal neovascularization after excimer keratectomy wounds in matrilysin‐deficient mice. Invest. Ophthalmol. Vis. Sci. 44, 137–144. [DOI] [PubMed] [Google Scholar]
- Lajtha LG (1979) Stem cell concepts. Differentiation 14, 23–34. [DOI] [PubMed] [Google Scholar]
- Lakshmipathy U, Verfaillie C (2005) Stem cell plasticity. Blood Rev. 19, 29–38. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Tseng SC, Sun TT (2004) Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp. Eye Res. 78, 433–446. [DOI] [PubMed] [Google Scholar]
- Le Blank K, Ringden O (2006) Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr. Opin. Immunol. 18, 586–591. [DOI] [PubMed] [Google Scholar]
- Leighton J (1992) Structural biology of epithelial tissue in histophysiologic gradient culture. In Vitro Cell. Dev. Biol. 28A, 482–492. [DOI] [PubMed] [Google Scholar]
- Li DQ, Tseng SCG (1995) Three pattern of cytokine expression potentially involved in epithelial fibroblast interactions of human ocular surface. J. Cell. Physiol. 163, 61–79. [DOI] [PubMed] [Google Scholar]
- Limb GA, Daniels JT, Cambrey AD, Secker GA, Shortt AJ, Lawrence JM, Khaw P (2006) Current prospects for adult stem cell‐based therapies in ocular repair and regeneration. Curr. Eye Res. 31, 381–390. [DOI] [PubMed] [Google Scholar]
- Liu L, Sheardown H (2005) Glucose permeable poly (dimethyl siloxane) poly (N‐isopropyl acrylamide) interpenetrating networks as ophthalmic biomaterials. Biomaterials 26, 233–244. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowsky RJ, Michael AF, Sun T (1995) Human corneal basement membrane heterogeneity: topographical differences in the expression of type 4 collagen and laminin isoforms. Lab. Invest. 72, 461–472. [PubMed] [Google Scholar]
- Mann I (1944) Study of epithelial regeneration in living eye. Br. J. Ophthalmol. 28, 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis L, Hatfill S, Chuaqui R, Vocke C, Emmert‐Buck M, Linehan WM, Duray PH (1999) Long term organ culture of human prostate tissue in a NASA‐designed rotating wall bioreactor. J. Urol. 161, 290–297. [PubMed] [Google Scholar]
- Matouskova E, Bucek S, Vogtova D, Vesely P, Chaloupkova A, Broz L, Singernova H, Pavlikova Konigova R (1997) Treatment of burns and donor sites with human allogeneic keratinocytes grown on acellular pig dermis. Br. J. Dermatol. 136, 901–907. [DOI] [PubMed] [Google Scholar]
- Maumenee A, Scholz R (1948) Histopathology of the ocular lesion produced by sulphur and nitrogen mustards. Bull. Johns Hopkins Hosp 82, 121–147. [PubMed] [Google Scholar]
- McAteer JA, Davis JM (2002) Basic cell culture technique and the maintenance of cell lines In: Davis JM, ed. Basic Cell Culture – A Practical Approach, 2nd edn, pp. 93–148. London: Oxford University Press. [Google Scholar]
- Meller D, Dabul V, Tseng SC (2002) Expansion of conjunctival epithelial progenitor cells on amniotic membrane. Exp. Eye Res. 74, 537–545. [DOI] [PubMed] [Google Scholar]
- Michelini M, Rosellini A, Simoncini T, Papini S, Revoltella RP (2004) A three‐dimensional organotypic culture of the human uterine exocervix for studying mucosal epithelial differentiation and migrating leukocytes. Differentiation 72, 138–149. [DOI] [PubMed] [Google Scholar]
- Michelini M, Rosellini A, Mandys V, Simoncini T, Revoltella RP (2005) Cytoarchitecture modifications of the human uterine endocervical mucosa in long‐term three‐dimensional organotypic culture. Pathol. Res. Pract. 201, 679–689. [DOI] [PubMed] [Google Scholar]
- Miller SJ, Lavker RM, Sun TT (1997) Keratinocyte stem cells of cornea, skin, and hair follicle In: Potten C, ed. Stem Cells, p. 331–362. New York: Academic Press. [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24. [DOI] [PubMed] [Google Scholar]
- Moore JE, McMullen CB, Mahon G, Adamis AP (2002) The corneal epithelial stem cell. DNA Cell Biol. 21, 443–451. [DOI] [PubMed] [Google Scholar]
- Van Muijen GN, Ruiter DJ, Franke WW, Achtstatter T, Haasnoot WH, Ponec M, Warnaar SO (1986) Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp. Cell Res. 162, 97–113. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S (2004) Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 88, 1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K, Birkenkamp‐Demtroder K, Ehlers N, Orntoft TF (2003) Identification of differentially expressed genes in keratoconus epithelium analyzed on microarrays. Invest. Ophthalmol. Vis. Sci. 44, 2466–2476. [DOI] [PubMed] [Google Scholar]
- O’Guin WM, Galvin S, Schermer A, Sun TT (1987) Patterns of keratin expression define distinct pathways of epithelial development and differentiation. Curr. Top. Dev. Biol. 22, 97–125. [DOI] [PubMed] [Google Scholar]
- Pajoohesh‐Ganji A, Stepp MA (2005) In search of markers for the stem cells of the corneal epithelium. Biol. Cell 97, 265–276. [DOI] [PubMed] [Google Scholar]
- Paladino G, Marino C, La Terra Mule S, Civiale C, Rusciano D, Enea V (2004) Cytokeratin expression in primary epithelial cell culture from bovine conjunctiva. Tissue Cell 36, 323–332. [DOI] [PubMed] [Google Scholar]
- Papini S, Cecchetti D, Campani D, Fitzgerald W, Grivel JC, Chen S, Margolis L, Revoltella RP (2003) Isolation and clonal analysis of human epidermal keratinocyte stem cells in long‐term culture. Stem Cells 21, 481–494. [DOI] [PubMed] [Google Scholar]
- Papini S, Rosellini A, Campani D, Dematteis A, Selli C, Revoltella RP (2004) Selective growth of epithelial basal cells from human prostate in a three‐dimensional organ culture. Prostate 59, 383–392. [DOI] [PubMed] [Google Scholar]
- Papini S, Rosellini A, Nardi M, Giannarini C, Revoltella RP (2005) Selective growth and expansion of human corneal epithelial basal stem cells in a three‐dimensional‐organ culture. Differentiation 73, 61–68. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M (2001) p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 98, 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M (1997) Long‐term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349, 990–993. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M (1999) Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell. Biol. 145, 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Morris RJ (1988) Epithelial stem cells in vivo . J. Cell Sci. 10 (Suppl), 45–62. [DOI] [PubMed] [Google Scholar]
- Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M, Pellegrini G (2001) Autologous fibrin‐cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 72, 1478–1485. [DOI] [PubMed] [Google Scholar]
- Ramaesh K, Dhillon B (2003) Ex vivo expansion of corneal limbal epithelial/stem cells for corneal surface reconstruction. Eur. J. Ophthalmol. 13, 515–524. [DOI] [PubMed] [Google Scholar]
- Rosellini A, Papini S, Giannarini C, Nardi M, Revoltella RP (2007) Three‐dimensional organotypic culture to investigate human conjunctival epithelial precursor cells and their progeny within their natural microenvironment. Int. J. Dev. Biol. forthcoming. [DOI] [PubMed] [Google Scholar]
- Santos MS, Gomes JA, Hofling‐Lima AL, Rizzo LV, Romano AC, Belfort R Jr (2005) Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am. J. Ophthalmol. 140, 223–230. [DOI] [PubMed] [Google Scholar]
- Schlötzer‐Schrehardt U, Kruse FE (2005) Identification and characterization of limbal stem cells. Exp. Eye Res. 81, 247–264. [DOI] [PubMed] [Google Scholar]
- Schmidt NJ (1988) Cell culture procedures for diagnostic virology In: Schmidt NJ, Emmons RW, eds. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections, 6th edn, pp. 51–100. Washington, DC: American Public Health Association Inc. [Google Scholar]
- Schwab IR, Reyes M, Isseroff RR (2000) Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 19, 421–426. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan VA, Foster T, Kulkarni BB, Hopkinson A, Gray T, Powe DG, Lowe J, Dua HS (2006) Morphological characteristics of the limbal epithelial crypt. Br. J. Ophthalmol. 91, 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M (2000) p63 is a prostate basal cell marker and is required for prostate development. Am. J. Pathol. 157, 1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Yang W, Cui ZH, Dong Y, Sui DM, Guan XK, Ma YL (2005) Transplantation of human limbal cells cultivated on amniotic membrane for reconstruction of rat corneal epithelium after alkaline burn. Chin. Med. J. (Engl.) 118, 927–935. [PubMed] [Google Scholar]
- Sosnova M, Bradl M, Forrester JV (2005) CD 34+ corneal stromal cells are bone‐marrow derived and express hemopoietic stem cell markers. Stem Cells 23, 507–515. [DOI] [PubMed] [Google Scholar]
- Stark HJ, Willhauck MJ, Mirancea N, Boehnke K, Nord I, Breitkreutz D, Pavesio A, Boukamp P, Fusenig NE (2004) Authentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co‐culture. Eur. J. Cell Biol. 83, 631–645. [DOI] [PubMed] [Google Scholar]
- Suda T, Arai F, Shimmura S (2005) Regulation of stem cells in the niche. Cornea 24 (8 Suppl), S12–S17. [DOI] [PubMed] [Google Scholar]
- Sudha B, Madhavan HN, Sitalakshmi G, Malathi J, Krishnakumar S, Mori Y, Yoshioka H, Abraham S (2006) Cultivation of human corneal limbal stem cells in Mebiol gel (R) – A thermo‐reversible gelation polymer. Indian J. Med. Res. 124, 655–664. [PubMed] [Google Scholar]
- Sun TT, Lavker RM (2004) Corneal epithelial stem cells: past, present, and future. J. Investig. Dermatol. Symp. Proc. 9, 202–207. [DOI] [PubMed] [Google Scholar]
- Tanifuji‐Terai N, Terai K, Hayashi Y, Chikama T, Kao WW (2006) Expression of keratin 12 and maturation of corneal epithelium during development and postnatal growth. Invest. Ophthalmol. Vis. Sci. 47, 545–551. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen‐Miyajima H, Shimazaki J (1999) Treatment of severe ocular‐surface disorders with corneal epithelial stem‐cell transplantation. N. Engl. J. Med. 340, 1697–1703. [DOI] [PubMed] [Google Scholar]
- Tuori A, Uusitalo H, Burgeson RE, Terttunen J, Virtanen I (1996) The immunohistochemical composition of the human corneal basement membrane. Cornea 15, 286–294. [DOI] [PubMed] [Google Scholar]
- Vascotto SG, Griffith M (2006) Localization of candidate stem and progenitor cell markers within the human cornea, limbus and bulbar conjunctiva in vivo and in cell culture. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 288, 921–931. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y (2004) Human limbal epithelium contains side population cells expressing the ATP‐binding cassette transporter ABCG2. FEBS Lett. 565, 6–10. [DOI] [PubMed] [Google Scholar]
- Watt FM (1989) Terminal differentiation of epidermal keratinocytes. Curr. Opin. Cell Biol. 1, 1107–1115. [DOI] [PubMed] [Google Scholar]
- Wei ZG, Cotsarelis G, Sun TT, Lavker RM (1995) Label‐retaining cells are preferentially located in fornical epithelium: implication on conjunctival epithelial homeostasis. Invest. Ophthalmol. Vis. Sci. 36, 236–246. [PubMed] [Google Scholar]
- Wirtschafter JD, Ketcham JM, Weinstock RJ, Tabesh T, Mcloon LK (1999) Mucocutaneous junction as the major source of replacement palpebral conjunctival epithelial cells. Invest. Ophthalmol. Vis. Sci. 40, 3138–3146. [PubMed] [Google Scholar]
- Wolosin JM, Wang Y (1995) Alpha‐2,3 sialylation differentiate the limbal and corneal epithelial cell phenotypes. Invest. Ophthalmol. Vis. Sci. 36, 2277–2286. [PubMed] [Google Scholar]
- Wolosin JM, Xiong X, Schutte M, Stegman Z, Tieng A (2000) Stem cells and differentiation stages in the limbo‐corneal epithelium. Prog. Retin. Eye Res. 19, 223–255. [DOI] [PubMed] [Google Scholar]
- Wolosin JM, Budak MT, Akinci MA (2004) Ocular surface epithelial and stem cell development. Int. J. Dev. Biol. 48, 981–991. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Cushman JA, Mori Y (1994a) Synthetic hydrogel with thermoreversible gelation 3: an NMR study of the Sol‐Gel transition. Polym. Adv. Tech. 5, 122–126. [Google Scholar]
- Yoshioka H, Mikami M, Mori Y, Tsuchida E (1994b) A synthetic hydrogel with thermoreversible gelation 1: preparation and rheological properties. J. Macromol. Sci. A31, 113–121. [Google Scholar]
- Yoshioka H, Mori Y, Shimizu M (2003) Separation and recovery of DNA fragments by electrophoresis through a thermoreversible hydrogel composed of poly (ethylene oxide) and poly (propylene oxide). Anal. Biochem. 323, 218–222. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Feinberg SE, Marcelo CL, Elner VM (2004) Ex vivo produced human conjunctiva and oral mucosa equivalents grown in a serum‐free culture system. J. Oral Maxillofac. Surg. 62, 980–988. [DOI] [PubMed] [Google Scholar]
- Zhang XZ, Yang YY, Chung TS (2002) The influence of cold treatment on properties of temperature‐sensitive poly (N‐isopropylacrylamide) hydrogels. J. Colloid Interface Sci. 246, 105–111. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nat. Med. 7, 1028–1034. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Wasson M (1993) Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J. Cell Sci. 106, 145–152. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Bukusoglu G, Yankauckas MA (1992) Characterization of a potential marker of corneal epithelial stem cells. Invest. Ophthalmol. Vis. Sci. 33, 143–152. [PubMed] [Google Scholar]
- Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC (2000) Activation of epidermal growth factor receptor during corneal epithelial migration. Invest. Ophthalmol. Vis. Sci. 41, 1346–1355. [PubMed] [Google Scholar]


