Abstract
DNA toroids produced by the condensation of λ phage DNA with hexammine cobalt (III) have been investigated by cryoelectron microscopy. Image resolution obtained by this technique has allowed unprecedented views of DNA packing within toroidal condensates. Toroids oriented coplanar with the microscope image plane exhibit circular fringes with a repeat spacing of 2.4 nm. For some toroids these fringes are observed around almost the entire circumference of the toroid. However, for most toroids well-defined fringes are limited to less than one-third of the total toroid circumference. Some toroids oriented perpendicular to the image plane reveal DNA polymers organized in a hexagonal close-packed lattice; however, for other toroids alternative packing arrangements are observed. To aid interpretation of electron micrographs, three-dimensional model toroids were generated with perfect hexagonal DNA packing throughout, as well as more physically realistic models that contain crossover points between DNA loops. Simulated transmission electron microscopy images of these model toroids in different orientations faithfully reproduce most features observed in cryoelectron micrographs of actual toroids.
Keywords: ψ DNA‖gene delivery‖packaging
DNA in living cells is highly condensed and rarely assumes the extended state that it does when free in solution. In both bacteria and eukaryotic cells the active regulation of DNA condensation is known to be an integral part of the cell cycle (1, 2). In sperm cells and viruses, where DNA transcription and replication are inactive, DNA can be packaged at densities that approach the limits of molecular compaction (3, 4). In most vertebrate sperm cells DNA is condensed by arginine-rich proteins into thousands of toroidal structures, each measuring ≈100 nm in outside diameter (5). The DNA of some bacteriophages also is packaged into a single toroid, or spool, with similar dimensions (6–8). Thus, the toroid represents a fundamental morphology selected by nature for the high-density packaging of DNA. In addition to the relevance of DNA toroids to cell biology and virology, DNA condensation is presently of great interest for the development of gene therapies, because controlling DNA condensation is widely appreciated as a key step in the improvement of artificial gene delivery systems (9–12).
Twenty-five years ago it was discovered that the polyamine spermidine
can cause the condensation of DNA into toroidal structures in
vitro with dimensions similar to those expelled from bacteriophage
capsids (13). Numerous subsequent studies demonstrated that toroidal
DNA condensates are produced by a wide range of multivalent cations
[e.g., Co(NH3) , polylysine,
histone H1] and even by monovalent cations in the presence of
dehydrating or crowding agents (5, 14–18). Toroidal DNA condensates
also have attracted the attention of theoreticians who have shown
toroid formation to be a general phenomenon resulting from the
self-association (or collapse) of a semiflexible polymer in solution
(19–23). DNA toroids are most often reported to measure around 100 nm
in outside diameter with a hole of around 30 nm in diameter (24),
although significantly larger toroids also have been observed (25).
Several theories have been presented that attempt to explain why DNA
toroids favor particular dimensions or a particular amount of DNA per
toroid. These theories have used numerous physical factors, including
the percentage of DNA charge neutralized within a toroid, the number of
nearest neighbors for a DNA polymer in a toroid, the size of the DNA
loop that initiates toroid formation, toroid surface tension, and the
density of imperfections in the packing of DNA polymers (22, 24,
26–29). However, no single theory at present is able to faithfully
predict both the size and size distribution observed for DNA toroids.
, polylysine,
histone H1] and even by monovalent cations in the presence of
dehydrating or crowding agents (5, 14–18). Toroidal DNA condensates
also have attracted the attention of theoreticians who have shown
toroid formation to be a general phenomenon resulting from the
self-association (or collapse) of a semiflexible polymer in solution
(19–23). DNA toroids are most often reported to measure around 100 nm
in outside diameter with a hole of around 30 nm in diameter (24),
although significantly larger toroids also have been observed (25).
Several theories have been presented that attempt to explain why DNA
toroids favor particular dimensions or a particular amount of DNA per
toroid. These theories have used numerous physical factors, including
the percentage of DNA charge neutralized within a toroid, the number of
nearest neighbors for a DNA polymer in a toroid, the size of the DNA
loop that initiates toroid formation, toroid surface tension, and the
density of imperfections in the packing of DNA polymers (22, 24,
26–29). However, no single theory at present is able to faithfully
predict both the size and size distribution observed for DNA toroids.
Most investigators have assumed that DNA in toroids is wound as string in a spool, with the loops of DNA polymers packed in a hexagonal lattice (24, 27, 29–31). However, a spool cannot exist with perfect hexagonal packing throughout because of the necessity for crossover points between loops of different radii (26, 28, 32). Additional arguments have been presented by us, with Balhorn, for how an alternative model for DNA organization could explain some experimental observations better than the simple spool model (28). In any case, the complete determination of DNA organization in toroids appears necessary if we are ever to fully understand the factors that govern toroid formation.
Cryo-transmission electron microscopy (TEM) has been used to examine the structure of DNA toroids with nanometer-scale resolution in vitreous ice. The rate of sample freezing is such that the micrograph can be considered as a snapshot of the DNA in solution just before freezing. Using the approach described here we have obtained images with unprecedented detail of DNA organization within toroidal condensates. Our images show directly that some toroids have regular DNA packing around almost the entire circumference of the toroid. However, no images support perfect order for the complete 360° circumference of a toroid. Edge-views of toroids reveal that some of the DNA in toroids is indeed packed in a hexagonal lattice, whereas it is also clear that regions exist where DNA packing is less ordered. Stereo pair micrographs and three-dimensional (3D) models of DNA toroids presented here show that DNA packing within most toroids could be greater than appreciated from any single electron micrograph.
Materials and Methods
DNA Condensate Preparation.
λ phage DNA (λ DNA) was purchased from Life Technologies-GIBCO/BRL (Rockville, MD) and used without further purification. DNA was diluted in 10 mM Tris, 1 mM EDTA, pH 7.0, to a concentration of 20 μg/ml. Co(NH3)6Cl3 was purchased from Sigma and purified by recrystallization as described (33). Stock solutions of Co(NH3)6Cl3 were freshly prepared before usage at a concentration of 200 μM. DNA toroids were prepared by mixing the DNA solution with an equal volume of the Co(NH3)6Cl3 solution at room temperature.
Grid Preparation and Cryo-EM.
Three microliters of condensed λ DNA solution was placed on glow discharged, holey carbon grids. Grids were blotted and plunged into liquid ethane. Frozen-hydrated samples were transferred to a Gatan cryostage and observed in a JEOL 4000 transmission electron microscope operating at 400 kV. Images were recorded on film at ×40,000 magnification and digitized by using a LeafScan 45 scanner at 100 pixels/mm. Grayscale digital images were adjusted for contrast and brightness to produce clearest definitions of DNA condensate features.
Results and Discussion
The plunge-freeze process used to prepare EM grids of solution-state samples traps macromolecular assemblies in vitreous ice with random orientations with respect to the plane of the grid. Maintaining grids at cryogenic temperatures in the electron microscope allows imaging of macromolecular assemblies from different perspectives, thereby providing 3D structural information through a series of two-dimensional (2D) images. In some cases, the 3D structure of an assembly can be digitally reconstructed by combining multiple 2D images. This approach has been particularly successful for obtaining the structure of viral protein capsids that possess a high degree of rotational symmetry (34). Structural investigations of molecular assemblies that lack rotational symmetry, such as the ribosome, also have benefited greatly from the digital synthesis of a large number of 2D cryo-EM images (35). However, this method still requires that the individual molecular assemblies imaged differ from each other only in their orientation with respect to the image plane. As shown below, the images we have acquired for λ DNA toroids reveal a range of DNA curvature (i.e., toroid dimensions), number of λ DNA molecules per toroid, and toroid planarity. These variations make it virtually impossible to reconstruct a high-resolution 3D DNA toroid structure by combining multiple 2D toroid images. Thus, the approach we have taken to determine the fine structure of DNA toroids is to develop a 3D computer model of a toroid that reproduces the principal features of actual toroids imaged in different orientations.
Top-View Cryo-EM Images of DNA Toroids.
A number of toroidal DNA condensates were imaged with the toroid plane approximately parallel to the microscope image plane (Fig. 1). The variation in the outside diameter of these toroids is substantial, at least 95–185 nm. The range of diameters of the toroid holes is also considerable, from around 35 to 85 nm. These dimensions are consistent with those reported previously for λ DNA toroids by EM imaging of freeze-etch replicas (36), a method also capable of determining the size of molecular assemblies in solution just before rapid freezing.
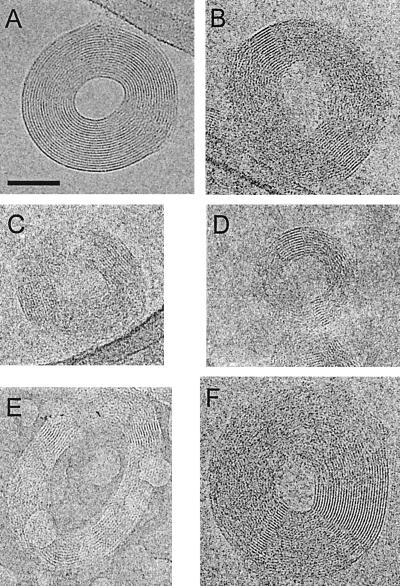
Figure 1.
Cryoelectron micrographs of λ DNA toroids with the plane of the toroid approximately coplanar with the microscope image plane; top-view toroid images. (A) A toroid with DNA fringes visible around almost the entire circumference of the toroid. (B–D) Toroids of various dimensions with small arc angles of well-defined DNA fringes that appear on opposite sides of the toroid center. (E and F) Toroids with well-defined DNA fringes not situated on opposite sides of the toroid center. Many of the toroids are in contact with the carbon film around the edge of the vitreous-ice-filled hole, as in the upper right of A. (Scale bar is 50 nm. All micrographs are shown at the same magnification.)
Most top-view toroids exhibit circumferential fringes with a spacing of around 2.4 nm, suggesting the packing of DNA in a regular lattice (Fig. 1). The cumulative angle around an individual toroid for which fringes are well defined varies from less than 20° to almost 360°. The toroid shown in Fig. 1A has fringes clearly defined around almost its entire circumference. Fringe spacing for this toroid is on average 2.37 nm and varies ± 2% around the circumference of the toroid. The variation in fringe spacing between toroids of a single sample preparation is ≈10%, with a minimum fringe spacing of 2.03 nm.
Many top-view toroids exhibit more than one local region of well-defined fringes (Fig. 1). Of these, over 90% have two of these regions that are usually situated across the toroid center from each other (Fig. 1 B–D). Other toroids have a less regular arrangement of well-defined fringes (Fig. 1 E and F). For some toroids these fringes are curved with the curvature of the toroid, but in others these fringes are relatively straight (Fig. 1). In regions of top-view toroids where well-defined fringes are not observed, the local ordering of DNA polymers is still frequently suggested by intermittent fringes of lower contrast, but with the same spacing as the well-defined fringes.
Edge-View Cryo-EM Images of DNA Toroids.
DNA toroids oriented with the toroid plane perpendicular to the microscope image plane provide complementary information on toroid structure and DNA organization. At the outer regions of an edge-view toroid one is clearly viewing DNA primarily down the polymer axis (i.e., the DNA helical axis is perpendicular to the image plane) (Fig. 2). For some toroids a hexagonal arrangement of discrete spots is visible, indicative of a hexagonal close-packed lattice of DNA polymers (Fig. 2A). For other edge-view toroids this packing is not as clear; however, hexagonal DNA packing is sometimes indicated by the outer regions having a hexagonal cross section with almost perfect 120° angles (Fig. 2B). Still other edge-view toroids have outer regions that lack both a clearly defined hexagonal lattice and a hexagonal cross section, yet alternating lines and spots of high and low electron density indicate the presence of a regular, but nonhexagonal, DNA packing arrangement (Fig. 2C). These nonhexagonal regions can appear on both sides of an edge-view toroid, or on only one side opposite a hexagonally packed region.
Figure 2.
Cryoelectron micrographs of λ DNA toroids with the plane of the toroid oriented ≈90° with respect to the microscope image plane; edge-view toroid images. (A) A toroid where the hexagonal packing of DNA helices is clearly apparent in the outer regions. (Inset) Fourier transform of image region containing the highly ordered DNA lattice. (B) A toroid for which the outer regions are well-defined hexagons. (C) A toroid in which the outer regions appear to have DNA packed in a nonhexagonal lattice. (Scale bar is 50 nm. All micrographs are shown at the same magnification.)
Fourier transforms of edge-view toroid micrographs that contain highly ordered outer regions produce diffraction-like patterns typical of a hexagonal array with a Bragg spacing of (2.4 nm)−1 (Fig. 2A Inset). This finding indicates a radial spacing between helices of 2.8 nm, the Bragg spacing being related to the inverse of the lattice spacing by a factor of 2/√3 for a hexagonal lattice. This measurement is consistent with the 2.4-nm fringe spacing measured for top-view toroids, which therefore can be attributed to the spacing between rows of DNA polymers in a hexagonal array with an interhelix spacing of 2.8 nm. Previous x-ray fiber diffraction and scattering studies of condensed DNA polymers all have been consistent with the notion that DNA within toroidal condensates is packed in a hexagonal lattice (37, 38). However, to the best of our knowledge, there is no prior report of the direct imaging of hexagonally packed DNA within toroidal condensates.
X-ray and electron diffraction studies from the past several decades
have shown the interhelical spacing of DNA polymers in the condensed
state to vary from 1.88 nm to over 3.0 nm, depending on the condensing
agent and level of hydration (37–40). The distance we have measured
for the interhelical spacing of DNA, 2.8 nm, is in excellent agreement
with x-ray diffraction studies of Co(NH3)
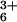 -DNA condensates at relatively high levels of
hydration (40). On the other hand, Böttcher et al.
(32) have reported a fringe spacing in air-dried toroids prepared with
spermine and uranyl acetate of only 1.8 nm. In their EM study the
spacing of well-defined fringes in top-view toroids was interpreted as
the interhelical spacing of DNA. However, the correspondence we report
between fringe spacing in top-view toroids and DNA spacing in edge-view
toroids suggests that the 1.8-nm fringe spacing reported by
Böttcher et al. is actually the spacing between
neighboring rows of DNA polymers in a hexagonal lattice with an
interhelical spacing of 2.1 nm.
-DNA condensates at relatively high levels of
hydration (40). On the other hand, Böttcher et al.
(32) have reported a fringe spacing in air-dried toroids prepared with
spermine and uranyl acetate of only 1.8 nm. In their EM study the
spacing of well-defined fringes in top-view toroids was interpreted as
the interhelical spacing of DNA. However, the correspondence we report
between fringe spacing in top-view toroids and DNA spacing in edge-view
toroids suggests that the 1.8-nm fringe spacing reported by
Böttcher et al. is actually the spacing between
neighboring rows of DNA polymers in a hexagonal lattice with an
interhelical spacing of 2.1 nm.
The volume of the edge-view toroid in Fig. 2B can be determined by directly measuring the cross-sectional area of one of the hexagonal outer regions and the diameter of the toroid. This measurement yields a volume of 2.12 × 105 nm3. If we assume the DNA to be packed in an hexagonal lattice throughout the toroid, with an interhelical spacing of 2.8 nm, this toroid contains ≈92 kb of DNA. The actual amount of DNA in this toroid is therefore likely 96 kb, i.e., two λ DNA molecules, because λ DNA is quantized in units of 48 kb. Other edge-view toroids from the same preparation have considerably larger cross-sectional areas and larger toroid diameters and could contain four or more λ DNA molecules. A circular cross section for toroids has been assumed by most investigators when calculating toroid volumes and support for this assumption has been presented by EM (32, 36). However, the edge-view toroids shown here reveal that this approximation is not always appropriate. For example, if the edge-view toroid in Fig. 2B was viewed from the top and assumed to have a circular cross section its predicted volume would only be 55% of that calculated by using its actual cross-sectional area.
An Idealized Model of a DNA Toroid.
We have constructed a 3D computer model of a DNA toroid in which 179 rings of various radii represent a toroid with DNA packed in a perfect hexagonal lattice. Although this model is highly idealized, projections that simulate TEM images reproduce the dominant features of toroids observed in our cryo-EM micrographs. In Fig. 3 simulated TEM images of the toroid are shown for selected rotation angles between 0° and 90°, with respect to the image plane. For rotation angles between 0° and ≈5° the circular DNA polymers appear as well-defined concentric rings around the entire circumference of the toroid. This finding is very similar to what is observed for the toroid in Fig. 1A. At a rotation angle of only 10° the definition of these rings becomes essentially lost for 85% of the toroid's circumference (Fig. 3). Two regions of fringes do remain upon rotation and are as well defined as the fringes of the toroid at 0° rotation. These pairs of persistent fringes are located across the center of the toroid from each other (i.e., along the tilt axis), similar to what is observed for many toroids in our cryo-EM micrographs (Fig. 1). When the model toroid is rotated further, e.g., to 30° or 45°, these two fringe regions still persist and are even more extensive than those of a toroid rotated only 10° (Fig. 3). The fringes of toroids rotated between ≈15° and 45° are linear and parallel, rather than curved and concentric. This phenomenon illustrates that the well-defined fringes often observed on opposite sides of a toroid may represent sampling of an extensively ordered structure, rather than a higher degree of DNA order at two specific regions of a toroid. As a corollary, given a toroid with perfect hexagonal DNA packing throughout, DNA fringes will be visible around the entire toroid only if the toroid is essentially coplanar with the microscope image plane.
Figure 3.
Simulated TEM images of an idealized model DNA toroid rotated at various angles with respect to the image plane. Model toroid shown was constructed from 179 closed circles packed in an ideal hexagonal lattice.
A simulated image of our idealized toroid rotated 90° with respect to the image plane (i.e., an edge-view toroid) shows the perfect hexagonal lattice of the model DNA polymers in the two outer regions (Fig. 3). However, between the two outer regions DNA order is not apparent. In this central region the distance between rows of DNA polymers, in the edge-view orientation, is only 0.5 times the lattice spacing. This distance is sufficiently close that DNA polymers in this region overlap with each other in projection to an extent that the definition between individual rows of DNA polymers is lost. This finding explains why the central regions of edge-view toroids in our cryo-EM images exhibit the least evidence of DNA order. Our model toroid also illustrates that a slight deviation from a perfect 90° tilt angle does not disrupt the hexagonal shape of the outer regions, but causes the central region to appear slightly wider than the outer region (e.g., 80° tilt angle, Fig. 3). This result is similar to what is seen for the toroid in Fig. 2B, suggesting that this particular toroid is actually tilted slightly less than 90° with respect to the image plane.
Direct Verification of the Tilt-Dependent Apparent Order of DNA in Toroids.
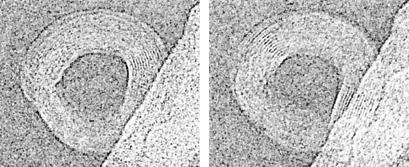
Our idealized model toroid predicts that even toroids with perfect hexagonal DNA packing will exhibit only a pair of well-defined fringes on opposite sides of the toroid when tilted between 10° and ≈50°, with respect to the imaging plane. However, many of the top-view toroids we have imaged contain regions with DNA fringes that are not situated directly across the toroid center from each other. If a 10° tilt can cause DNA in regions away from the toroid tilt axis to lose the appearance of regular packing, it is then possible that the deviation of a toroid from perfect planarity also would eliminate fringes. Additionally, toroid nonplanarity could cause the appearance of DNA fringes off the toroid tilt axis. To test this hypothesis we have acquired cryo-EM stereo pair micrographs of toroids by tilting the microscope stage 10° between the acquisition of two images. These images clearly reveal that our ability to detect local DNA order in a toroid is very sensitive to the orientation of the toroid, as fringe regions in the left stereo image are shifted in position with respect to those of the right image (Fig. 4). Thus, we can conclude that many toroids have more extensively ordered DNA than is apparent in a single image, because tilting and toroid nonplanarity will cause the angle of DNA polymers in some regions of the toroid to be outside the small angle required to produce well-defined fringes. Toroids with circular fringes around almost the entire toroid (e.g., Fig. 1A) are therefore understandably rare, because they must be both perfectly planar and perfectly coplanar with the image plane of the microscope. We also note that the degree of DNA order within toroids may be very sensitive to the exact conditions of sample preparation, as samples prepared by seemingly identical protocols sometimes appear to produce toroids that overall have less well-defined fringes in top-view images.
Figure 4.
Cryoelectron stereo pair micrographs of a λ DNA toroid. Sample stage was rotated 10° between acquisition of left and right micrographs. Note that the exact positions of well-defined DNA fringes shift between images.
Potential Effects of Crossover Regions on DNA Packing.
The idealized model toroid presented above with DNA represented by a collection of closed circles is strictly unrealistic, even though it does faithfully recreate the principal features of actual λ DNA toroids. A more realistic model is one constructed from a single continuous polymer path, because each λ DNA molecule in a toroid is wound into many loops. For example, the average circumference of DNA loops in the toroid of Fig. 2B is around 600 bp, which implies that the λ DNA molecules of this toroid are each wound into a continuous path consisting of ≈80 loops. The winding of a single continuous polymer to form a toroid requires that the polymer cross over itself in space at least as many times as there are loops in the toroid. This necessity for crossovers in DNA toroids has been previously appreciated, as well as the fact that crossovers must result in at least the local disruption of ideal hexagonal packing (26, 28, 32).
To determine whether crossovers are possibly the origin of the nonhexagonal regions of DNA packing reported here we also have created 3D model toroids from a single polymer path consisting of 179 loops. The loops of these model toroids are packed in a perfect hexagonal lattice over a defined arc angle of the toroid, but deviate from a perfect hexagonal lattice over the remaining circumference of the toroid to allow for polymer crossovers. Because there is not a priori one unique or obviously favored set of paths for the loops of a toroid, we present simulated TEM images for a model toroid that uses what is arguably one of the simplest plausible paths for a toroid of 179 loops (see below). With respect to the hexagonal outer region of an edge-view toroid, the path of loops in this model toroid begins at the hexagon apex on one toroid face and proceeds toward the other face in a raster-like fashion (Fig. 5A). An additional consideration in the construction of this model toroid is the arc angle over which the DNA polymers in the toroid deviate from perfect hexagonal packing. This arc angle for crossovers in actual toroids has not yet been determined. For the model toroid shown in Fig. 6 a 359° arc was used for each DNA loop to progress from one position in the perfect hexagonal array to a neighboring position in the same array. In other words, DNA crossovers were distributed around the toroid as much as possible and the polymer loops were packed in a perfect hexagonal lattice over only a 1° arc of the toroid. A hard cylinder potential between polymers was used to adjust the polymer paths over the remaining 359°.
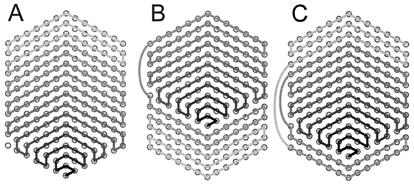
Figure 5.
Schematic representations of three possible paths for the winding of a single DNA polymer into a toroid. (A) A path in which DNA deposition on to the toroid proceeds in a unidirectional manner from one face of the toroid to the other. (B and C) Alternative paths that have places where DNA crosses over the outside of the toroid from one face to the other during toroid growth. Circles represent axial views of DNA polymers. Each hexagonal array represents the cross section of a toroid at an angular position where there is perfect hexagonal packing. Each pair of circles connected by a grayscale line represents the beginning and end of a 360° loop of DNA. The grayscale line of each array follows, from dark to light, the sequential order that loops are added to the growing toroid for the given path. The hole of a toroid would be to the right of each pattern.
Figure 6.
Simulated TEM images of a model toroid constructed from a single continuous path. (Upper) Top-view image; toroid plane is parallel to the image plane. (Lower) Edge-view image; toroid plane is perpendicular to the image plane. At the 9 o'clock position of the top-view image DNA helices are packed in a perfect hexagonal lattice. The progression of each loop from one position in the hexagonal lattice to a neighboring position takes place over a 359° arc. Note that in the top-view image two regions exist where DNA fringe definition is lost. Also note that the DNA fringes at the 9 and 3 o'clock positions of the top-view toroid appear very similar in spacing and clarity. However, the rotation of this toroid by 90° to the edge-view reveals that the packing of DNA polymers at the 3 o'clock position is very different from the perfect hexagonal lattice at the 9 o'clock position.
The top-view image of this model toroid with crossovers (Fig. 6) is very similar to that of the more idealized toroid in Fig. 3. However, a notable difference is the presence of two regions where the definition of DNA rings is lost. These regions occur between the angular position of the toroid where there is perfect hexagonal packing and the position on the opposite side of the toroid where there is the greatest deviation from hexagonal packing. This feature of two regions where DNA fringes are not well defined is similar to what is observed for the toroid in Fig. 1A, which represents the toroid image with the most complete circumferential DNA fringes acquired thus far. An edge-view image of the model toroid with crossovers illustrates the contrast between the perfect hexagonal lattice on one side of the toroid versus the other side where there is maximum deviation from hexagonal packing (Fig. 6). We note that the simple hard-cylinder potential used in the creation of this model has produced a somewhat regular lattice in the nonhexagonal region that is not unlike that observed for some actual edge-view toroids (Fig. 2C). Edge-view images of this model toroid rotated about other axes give the appearance of an intermediate level of DNA organization in both outer regions (not shown).
The arc angle over which crossovers take place in an actual toroid must depend on the energetic cost associated with deviating from a perfect hexagonal lattice versus the cost of bending a DNA polymer the amount necessary to go from one position in the hexagonal lattice to another as a function of crossover arc angle. A DNA toroid where crossovers take place over the entire 360° circumference represents one extreme case. The other extreme is represented by a toroid with perfect hexagonal packing throughout and crossovers occurring over an infinitesimally small toroid arc angle. The former case is represented by the model toroid in Fig. 6, while the latter would be very similar to the toroid shown in Fig. 3. A series of model toroids for which the arc angle of crossovers was varied between these two extremes was also generated (models not shown). Projection images of these models are very similar to that shown for the toroid with 359° crossovers (Fig. 6), except that the angle between the two regions where DNA fringe definition is lost in a top-view decreases in proportion to the crossover angle.
Implications of DNA Toroid Fine Structure on the Mechanism of Toroid Growth.
The hexagons that define the outer regions of some edge-view toroids have their points aligned with the principal axis of the toroid (Fig. 2B). Just as crystal morphology can indicate a preferred direction of crystal growth, the orientation of these hexagons is potentially indicative of how DNA toroids form. Toroids are likely to have more restricted growth patterns than most crystals because of the polymeric nature of DNA, i.e., toroid growth must be compatible with a continuous path for polymer deposition onto the toroid. Furthermore, toroid growth is expected to proceed in a manner that does not require the threading of a DNA polymer through the toroid hole. In Fig. 5 we present three patterns for toroid growth that are consistent with these constraints. The proposed patterns also maximize DNA–DNA contacts in a growing toroid by requiring that each loop of DNA added to a toroid be in contact with two loops that are already part of the growing toroid. The simplest pattern shown (Fig. 5A) was used in the model toroid of Fig. 6. The other two growth patterns illustrate how DNA might cross over the outside of a growing toroid, from one toroid face to the other, without creating a substantial disruption in local DNA packing. There are undoubtedly numerous other possible growth patterns that would also be consistent with the constraints we have considered intrinsic to toroid growth. We note that the toroid growth patterns proposed in Fig. 5 are somewhat similar to growth patterns observed for crystals with screw disorders (41, 42). That is, a screw disorder in a crystal favors monomer addition along an edge that spirals out from a central point as the crystal grows. The patterns for toroid growth we propose adopt a more raster-like appearance, rather than spiral-like, because of our assumption that DNA polymers do not thread through the hole of a growing toroid.
In a previous study (28) we reported a correlation between the average radius of DNA loops in toroids (referred to as the toroid-loop in ref. 27) and the predicted radii of loops that spontaneously form along DNA polymers in solution. This finding served as support for a model in which toroid formation is nucleated by a single DNA loop. More recently we have provided direct support for this model of toroid formation by demonstrating that toroids of a defined size are nucleated by static DNA loops along an otherwise linear DNA polymer (43). However, our previous constant radius of curvature model for DNA organization within toroids (also proposed in ref. 27) is inconsistent with the cryo-EM images presented here. This previous model was proposed, in part, to explain how a toroid nucleated by a loop of a given size could grow to have a hole with a smaller diameter than that of the nucleating loop. The growth patterns proposed here for our cryo-EM-inspired toroid model predict that the hole of a growing toroid will initially decrease in diameter during the early stages of toroid growth.
Concluding Remarks
The results of the cryo-EM study presented here provide direct evidence that DNA within some toroidal condensates is organized in a hexagonal close-packed lattice. This study has also revealed that regions of regular, but nonhexagonal, DNA packing also exist in toroids. Furthermore, both hexagonal and nonhexagonal DNA packing can be present within the same toroid. 3D computer models of DNA toroids have been developed that reproduce virtually all structural features of toroids observed in cryo-EM micrographs and therefore represent reasonable models for the fine structure of DNA toroids. These models illustrate that both the tilting of a toroid, with respect to the EM image plane, and toroid nonplanarity can lead to a substantial underestimation of the degree to which DNA is organized within a toroid. Additionally, these 3D models demonstrate that polymer crossover regions within toroids are a plausible origin of regions where DNA packing is nonhexagonal.
Acknowledgments
N.V.H. gratefully acknowledges support from the National Institutes of Health (Grant GM62873). Work performed at the Lawrence Berkeley National Laboratory was supported by the National Institutes of Health (Grant GM51487) and the Office of Biological and Environmental Research, U.S. Department of Energy under Contract DE-AC03–76SF-00098.
Abbreviations
- TEM
transmission electron microscopy
- 3D
three-dimensional
- λ DNA
λ phage DNA
References
- 1.van Holde K. Chromatin. New York: Springer; 1989. [Google Scholar]
- 2.Holmes V, Cozzarelli N. Proc Natl Acad Sci USA. 2000;97:1322–1324. doi: 10.1073/pnas.040576797. . (First Published February 11, 2000; 10.1073/pnas.040576797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen M, Lee J, Lee C, Balhorn R. Mol Reprod Dev. 1996;45:87–92. doi: 10.1002/(SICI)1098-2795(199609)45:1<87::AID-MRD12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Tikchonenko T. In: Comprehensive Virology. Fraenkel-Conrat H, Wagner R, editors. New York: Plenum; 1975. pp. 1–117. [Google Scholar]
- 5.Hud N V, Allen M J, Downing K H, Lee J, Balhorn R. Biochem Biophys Res Commun. 1993;193:1347–1354. doi: 10.1006/bbrc.1993.1773. [DOI] [PubMed] [Google Scholar]
- 6.Cerritelli M, Cheng N, Rosenberg A, McPherson C, Booy F, Steven A. Cell. 1997;91:271–280. doi: 10.1016/s0092-8674(00)80409-2. [DOI] [PubMed] [Google Scholar]
- 7.Hud N V. Biophys J. 1995;69:1355–1362. doi: 10.1016/S0006-3495(95)80002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klimenko S, Tikchonenko T, Andreev V. J Mol Biol. 1967;23:523–533. doi: 10.1016/s0022-2836(67)80122-0. [DOI] [PubMed] [Google Scholar]
- 9.Luo D, Saltzman W. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 10.Mahato R, Smith L, Rolland A. Adv Genet. 1999;41:95–155. doi: 10.1016/s0065-2660(08)60152-2. [DOI] [PubMed] [Google Scholar]
- 11.Plank C, Tang M X, Wolfe A R, Szoka F C., Jr Hum Gene Ther. 1999;10:319–332. doi: 10.1089/10430349950019101. [DOI] [PubMed] [Google Scholar]
- 12.Rolland A. Crit Rev Ther Drug Carrier Syst. 1998;15:143–198. doi: 10.1615/critrevtherdrugcarriersyst.v15.i2.20. [DOI] [PubMed] [Google Scholar]
- 13.Gosule L C, Schellman J A. Nature (London) 1976;259:333–335. doi: 10.1038/259333a0. [DOI] [PubMed] [Google Scholar]
- 14.Hsiang M W, Cole R D. Proc Natl Acad Sci USA. 1977;74:4852–4856. doi: 10.1073/pnas.74.11.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plum G E, Arscott P G, Bloomfield V A. Biopolymers. 1990;30:631–643. doi: 10.1002/bip.360300515. [DOI] [PubMed] [Google Scholar]
- 16.Widom J, Baldwin R L. J Mol Biol. 1980;144:431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Bloomfield V A. Biophys J. 1999;77:1556–1561. doi: 10.1016/S0006-3495(99)77003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chattoraj D K, Gosule L C, Schellman J A. J Mol Biol. 1978;121:327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- 19.Stevens M J. Biophys J. 2001;80:130–139. doi: 10.1016/S0006-3495(01)76000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuznetsov Y A, Timoshenko E G. J Chem Phys. 1999;111:3744–3752. [Google Scholar]
- 21.Vasilevskaya V, Khokhlov A, Kidoaki S, Yoshikawa K. Biopolymers. 1997;41:51–60. [Google Scholar]
- 22.Bloomfield V. Biopolymers. 1997;44:269–282. doi: 10.1002/(SICI)1097-0282(1997)44:3<269::AID-BIP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov V A, Paul W, Binder K. J Chem Phys. 1998;109:5659–5669. [Google Scholar]
- 24.Bloomfield V A. Biopolymers. 1991;31:1471–1481. doi: 10.1002/bip.360311305. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa Y, Yoshikawa K, Kanbe T. Langmuir. 1999;15:4085–4088. [Google Scholar]
- 26.Park S Y, Harries D, Gelbart W M. Biophys J. 1998;75:714–720. doi: 10.1016/S0006-3495(98)77561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ubbink J, Odijk T. Europhys Lett. 1996;33:353–358. [Google Scholar]
- 28.Hud N V, Downing K H, Balhorn R. Proc Natl Acad Sci USA. 1995;92:3581–3585. doi: 10.1073/pnas.92.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ubbink J, Odijk T. Biophys J. 1995;68:54–61. doi: 10.1016/S0006-3495(95)80158-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earnshaw W, Harrison S. Nature (London) 1977;268:598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- 31.Marx K, Reynolds T. Proc Natl Acad Sci USA. 1982;79:6484–6488. doi: 10.1073/pnas.79.21.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Böttcher C, Endisch C, Fuhrhop J H, Catterall C, Eaton M. J Am Chem Soc. 1998;120:12–17. [Google Scholar]
- 33.Arscott P G, Li A-Z, Bloomfield V A. Biopolymers. 1990;30:619–630. doi: 10.1002/bip.360300514. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z H, Dougherty M, Jakana J, He J, Rixon F J, Chiu W. Science. 2000;288:877–880. doi: 10.1126/science.288.5467.877. [DOI] [PubMed] [Google Scholar]
- 35.Gabashvili I S, Agrawal R K, Spahn C M T, Grassucci R A, Svergun D I, Frank J, Penczek P. Cell. 2000;100:537–549. doi: 10.1016/s0092-8674(00)80690-x. [DOI] [PubMed] [Google Scholar]
- 36.Marx K, Ruben G. J Biomol Struct Dyn. 1986;4:23–39. doi: 10.1080/07391102.1986.10507644. [DOI] [PubMed] [Google Scholar]
- 37.Suwalsky M, Traub W, Shmueli U, Subirana J. J Mol Biol. 1969;42:363–373. doi: 10.1016/0022-2836(69)90049-7. [DOI] [PubMed] [Google Scholar]
- 38.Maniatis T, Venable J J, Lerman L. J Mol Biol. 1974;84:37–64. doi: 10.1016/0022-2836(74)90211-3. [DOI] [PubMed] [Google Scholar]
- 39.Rau D, Parsegian V. Biophys J. 1992;61:246–259. doi: 10.1016/S0006-3495(92)81831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schellman J A, Parthasarathy N. J Mol Biol. 1984;175:313–329. doi: 10.1016/0022-2836(84)90351-6. [DOI] [PubMed] [Google Scholar]
- 41.Read W T J. Dislocations in Crystals. New York: McGraw–Hill; 1953. [Google Scholar]
- 42.Verma A R. Crystal Growth and Dislocations. London: Butterworth; 1953. [Google Scholar]
- 43.Shen M R, Downing K H, Balhorn R, Hud N V. J Am Chem Soc. 2000;122:4833–4834. [Google Scholar]