Abstract
Objectives: Utility of hepatic stem cells could provide a novel solution to the severe shortage of human donor livers, for treatment of liver‐related diseases, due to their ability to proliferate and differentiate into functional hepatocytes. Porcine liver tissues also offer an alternative source from human donor livers. However, morphology, phenotype, successful isolation and culture of porcine hepatic stem cells still require much investigation.
Materials and methods: In the present study, we performed partial hepatectomy to activate hepatic oval cells and developed a procedure utilizing enzymatic digestion and density gradient centrifugation to isolate and purify oval cells derived from porcine livers. We identified ovoid cells by their morphological characteristics and phenotypic properties, thereby providing definitive evidence for the presence of hepatic stem cells in porcine livers. Moreover, we established a culture system, using various growth factors, to provide nourishment for these cells.
Results and conclusions: By transmission electron microscopy, oval‐shaped cells with ovoid nuclei, a high nucleus/cytoplasm ratio and few organelles were demonstrated. Flow cytometry and immunocytochemistry showed that freshly isolated oval cells expressed albumin, cytokeratin 19, alpha fetoprotein (AFP) and OV6 at high levels. Immunofluorescence revealed that porcine hepatic oval cells after culture expressed stem‐cell factor, c‐kit, Thy‐1, CK19, OV6, and AFP. Taken together, this study provides a novel insight into morphological and phenotypic characteristics of porcine hepatic stem cells. Our ability for isolation and culturing porcine hepatic stem cells offers an abundant source of cells for transplantation and tissue engineering to help alleviate liver disease.
Introduction
Wilson and Arber et al. first proposed that there are hepatic stem cells in both foetal and adult liver tissues (1, 2). Oval cells derived from rodent and human livers, and also from bone marrow, have been demonstrated to be hepatic stem cells (3, 4, 5). Hepatic stem cells have recently aroused considerable interest because of their importance in both basic research and clinical applications. On the one hand, hepatic stem cells may participate in liver regeneration and restoration during serious liver injury. Thus, studies on their proliferation, differentiation, and signal transduction pathways that regulate their fate determination, are very helpful for elucidating molecular mechanisms of liver development. On the other hand, hepatic stem cells capable of proliferating can be induced to differentiate into hepatocytes, which could potentially provide a novel approach of cell transplantation, tissue engineering and gene therapy for various kinds of liver disease (6, 7, 8).
It has been widely demonstrated that hepatic stem cells exist in rodent liver tissues (9, 10). We have previously demonstrated that hepatic stem cells derived from adult rat liver could be induced to differentiate into cells with morphological, phenotypic and functional characteristics of mature hepatocytes (11). We have also revealed that rat hepatic stem cells possess high potential for proliferating in vitro, with large increases in cell number while remaining undifferentiated (12). However, morphology, phenotype, isolation and culture of porcine hepatic stem cells require more attention.
Kano et al. have isolated non‐parechymal epithelial cells (NPECs) and hepatic stem‐like cells from normal porcine livers (13, 14, 15). The NPEC phenotypes co‐expressed albumin, cytokeratin (CK) 18, CK7 and α‐fetoprotein during periods of scattering, the cells being strongly positive for α‐1‐antitrypsin and bile duct epithelial cell markers, including γ‐glutamyltransferase and CK‐19, at stages of emergence of duct‐like structures. This group also induced NPECs to differentiate into hepatocytes and bile duct epithelial cells (16), indicating that NPECs were putative hepatic stem cells in porcine livers. However, these NPECs, distinct from hepatic oval cells, were essentially heterogeneous cell populations containing several types of cell.
Shortage of hepatocytes derived from adult human livers is a major handicap for wider application of hepatocyte transplantation and tissue engineering, including development of bio‐artificial livers – use of human foetal and paediatric livers is hotly contested and deeply involved in ethical issues. Thus, more attention is being paid to use of non‐human livers, such as from the pig. As noted, embryonic porcine liver might be able to provide a preferable source of hepatocytes for transplantation (17). There are several advantages for utilization of porcine livers from which to isolate hepatic stem cells. First, sources of porcine liver are extremely abundant. Secondly, the morphology and size of porcine livers are similar to those of human livers. Thirdly, physiological and biochemical characteristics of porcine liver and human liver are alike, and finally, the infectious risks from porcine tissue is lower than from non‐human primates such as the monkey; already, hepatocytes originating from porcine livers have been exploited in bio‐artificial livers to treat patients with acute liver failure (18, 19, 20, 21). Nevertheless, porcine hepatocytes have a disadvantage, that they exhibit limited proliferation and can be cultured in vitro for only short periods. One attractive solution to this shortcoming is to use porcine hepatic stem cells that still proliferate and can be cultured over long time periods.
In this study, we first conducted partial hepatectomy (PH) to activate hepatic oval cells, in porcine livers, and identified porcine oval cells by their morphological and phenotypic characteristics, thereby providing strong evidence for presence of putative hepatic stem cells in porcine liver tissues. We sought to isolate hepatic stem cells with high purity and viability, from porcine liver using enzymatic digestion and density gradient centrifugation, and expanded these cells in in vitro culture. This study offers an unlimited alternative source of hepatic stem cells for potential application in basic research and clinical transplantation.
Materials and methods
Animals
Pigs (3–4 kg body weight) were obtained from the Laboratory Animal Breeding Centre, Chinese Agricultural University, and were fed with standard laboratory chow ad libitum. All animal care and procedures were approved by the Institutional Animal Care Committee.
Establishing the model of porcine oval cell activation
Two‐thirds PH of porcine livers was performed according to the procedure as described previously in rats (12). After a 14‐day recovery period, new liver tissue was used from which to isolate hepatic oval cells.
Isolation and purification of porcine oval cells
Isolation and purification of oval cells were carried out according to a novel, newly patented protocol (patent number: CN1594553). Briefly, newly formed liver tissues were minced in Hank’s balanced salt solution (HBSS) (Gibco, BRL, Grand Island, NY, USA) and digested in Dulbecco’s minimum essential medium (DMEM) (Gibco) containing 0.10% wt/v collagenase IV (Sigma, St. Louis, MO, USA) and 0.025% wt/v EDTA for 15 min at 37 °C in a shaking bath after two‐step collagenase perfusion was accomplished. After centrifugation at 47 g for 5 min, supernatant was centrifuged at 141 g for 5 min. Pellets were suspended in 50 ml of DMEM containing 0.10% wt/v pronase E (Sigma) and 0.005% wt/v DNase I (Sigma), and incubated for 30 min at 37 °C. Suspensions were centrifuged at 141 g for 5 min at 4 °C, and pellets were suspended in 2 ml HBSS. Percoll density gradient was as follows: 1.20 g/ml Percoll, 1.12 g/ml Percoll, cell suspension and HBSS. After centrifugation at 141 g for 20 min at 4 °C, interface between 1.20 and 1.12 g/ml Percoll was decanted and centrifuged at 141 g for 5 min. Viability of oval cells was estimated by staining with 0.25% wt/v trypan blue.
Transmission electron microscopy
Freshly isolated cells were fixed in cold 2.5% wt/v glutaraldehyde in 0.1 m cacodylate buffer (pH 7.4). After extensive washings in phosphate‐buffer saline (PBS) (Sigma), cells were post‐fixed in 1% wt/v OsO4 for 30 min, dehydrated in graded solutions of ethanol and embedded in Epon. Ultrathin sections were cut and examined using a Phillips EM 301 electron microscope after staining with uranyl acetate and lead citrate, according to methods previously described (11, 12).
Flow cytometric analysis
Freshly isolated cells were fixed in cold acetone for 8 min at 4 °C. After centrifugation at 141 g for 5 min at 4 °C, pellets were suspended in 0.1 ml PBS and incubated with the following antibodies, anti‐: albumin, CK19, CK7, alpha fetoprotein (AFP) and OV6 (kind gift from Professor Stewart Sell, Albany Medical College) for 60 min at 37 °C. All other primary antibodies were purchased from Dako Corporation (Kyoto, Japan). Centrifugation was performed at 141 g for 15 min and followed by extensive washes in PBS. Pellets were suspended in 0.1 ml PBS in preparation for cell suspension. Binding of primary antibody was detected by phycoerythrin (PE)‐labelled IgG (Dako, Kyoto, Japan). Cells were assayed by flow cytometry (Becton Dickinson, San Jose, CA, USA) according to methods previously described (11, 12), and data were analysed using Cell‐Quest software (Becton Dickinson). Replacement of primary antibody with PE‐labelled IgG served as a negative control.
Double staining of freshly isolated cells was performed by flow cytometric analysis using primary antibodies against albumin and CK19. Goat anti‐mouse fluorescein isothiocyanate (FITC)‐labelled IgG (Dako) to recognize CK19 and goat anti‐rabbit PE‐labelled IgG (Dako) to label albumin, served as secondary antibodies. The protocol of double staining was similar to the procedure for single staining, except for differences in primary antibodies and secondary antibodies.
Porcine oval cell proliferation in vitro culture
Freshly isolated cells were plated at density of 5 × 105 cells/25 cm2 dish. DMEM was supplemented with 100 U/ml penicillin G and 100 μg/ml streptomycin. Growth factors used in the culture system included 10 ng/ml hepatocyte growth factor (HGF), 20 ng/ml stem‐cell factor (SCF) and 10 ng/ml leukaemia inhibitory factor (LIF). All growth factors mentioned above were purchased from Sigma. Culture was maintained in a humidified incubator in a mixture of 95% air and 5% CO2 at 37 °C. Fresh medium was provided every other day and cell population growth was carefully examined under a phase‐contrast microscope.
Immunofluorescence assay of freshly isolated cells and oval cells, from expanding colonies in culture
Immunofluorescence analysis of oval cells derived from the expanding colonies in culture was performed on day 14. Cells on glass slides were fixed in cold acetone for 5 min, blocked with normal goat serum after extensive washes in PBS (pH 7.4) and incubated with primary antibodies including anti‐: SCF, c‐kit, OV6, CK19, AFP and Thy‐1 at 4 °C overnight. After washing three times in PBS, cells were cultured with PE‐labelled IgG for 30 min at 37 °C, washed three times again in PBS, and observed microscopically for fluorescence, according to methods previously described (22, 23). Replacement of primary antibody with PE‐labelled IgG served as a negative control.
Double staining of freshly isolated cells and oval cells after culture for 14 days, was performed by immunofluorescence using primary antibodies against albumin and OV6. Secondary antibodies were FITC‐labelled IgG (Dako) to recognize OV6 and goat anti‐rabbit PE‐labelled IgG (Dako) to label albumin.
Proliferation assay of porcine oval cells in culture
Porcine oval cells were seeded at a density of 3000 cells in 96‐well microtitre plates and cultured in medium as mentioned above, for 14 days. Proliferation assays were performed using the Non‐Radioactive Cell Proliferation Assay according to the protocol of the manufacturer (Promega, Madison, WI, USA). Optical densities were recorded using Bio‐Rad (Hercules, CA, USA) Ultra Microplate reader at 490 nm wavelength.
Results
Activation of oval cells in porcine liver
We performed two‐thirds hepatectomy on porcine livers, resulting in 100% survival of all pigs after surgery. In this regime for partial hepatectomy, massive proliferation of small cells with oval nuclei and high nucleus/cytoplasm ratio was observed on day 14 after PH (Fig. 1). Activation of oval cells in this model is due to the regenerative stimulus of partial hepatectomy.
Figure 1.
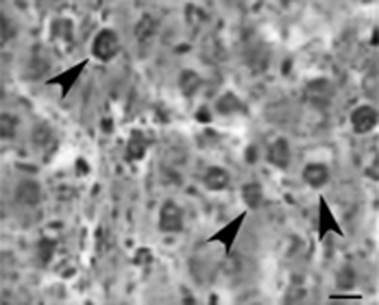
Histological features of porcine liver after partial hepatectomy. Rapid and extensive proliferation of oval cells (arrowheads) with ovoid nuclei was observed. Scale bar = 6 μm.
Morphological features of freshly isolated cells from porcine livers
We first characterized morphological features of the freshly isolated cells from porcine livers. We obtained ∼108 cells/pig with purity of more than 92%, as evaluated by transmission electron microscopy and flow cytometric analysis, as shown below. Viability of freshly isolated cells was 96%, as estimated by their ability to exclude trypan blue (data not shown).
Phase‐contrast microscopy showed that the freshly isolated cells (Fig. 2a) attaching to culture dishes (Fig. 2b) were oval in shape. Transmission electron microscopy revealed that they (Fig. 2c,d) were oval in shape and had ovoid nuclei. These oval cells had high nucleus/cytoplasm ratio (Fig. 2c,d), mitochondria (Mi), endoplasmic reticulum (Er), lysosomes (Ly) and condensed chromatin (Ch) (Fig. 2d). Their median diameter was between 4.5 and 6 μm, around half of that of mature hepatocytes.
Figure 2.
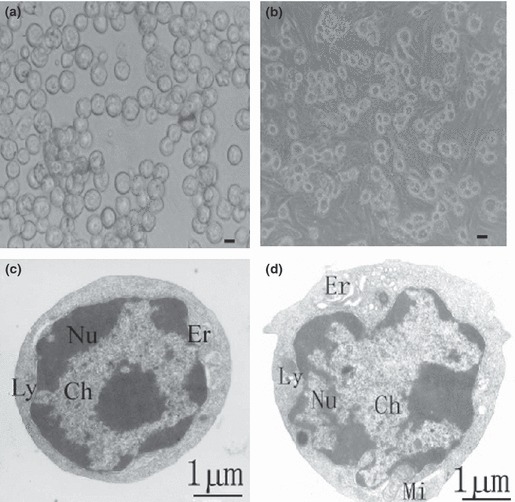
Morphological features of hepatic oval cells derived from porcine liver. (a, b) Phase‐contrast microscopy showing morphology of the freshly isolated porcine hepatic oval cells (a) and cells cultured for one day (b). Scale bar in a = 3 μm, and bar in b = 6 μm. (c, d) Transmission electronic micrograph showing freshly isolated oval cells from porcine liver with their ovoid nuclei (Nu), condensed chromatin (Ch), high nuclei/cytoplasmic ratio, mitochondria (Mi), endoplasmic reticulum (Er) and lysosome (Ly).
Phenotypic characteristics of freshly isolated cells from porcine livers
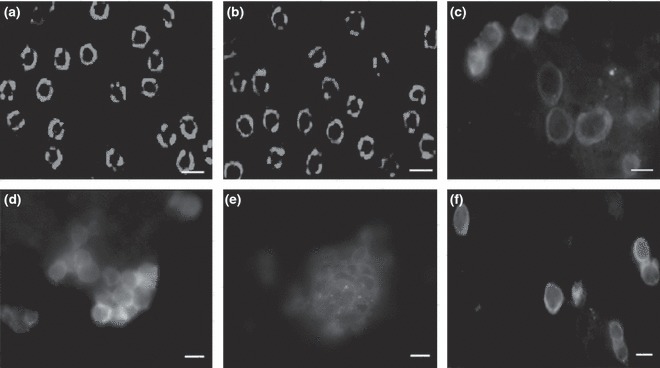
Next, we analysed phenotypic characteristics of freshly isolated oval cells from porcine livers, to confirm their biochemical identity. Flow cytometric analysis indicated that around 93% of the cells expressed albumin (Fig. 3b), and around 96% were positive for CK19 (Fig. 3c). In addition, in the order of 70% of the freshly isolated cells were positive for OV6 (Fig. 3d), a marker for rodent hepatic oval cells (24), and 87% of them expressed AFP (Fig. 3e).
Figure 3.
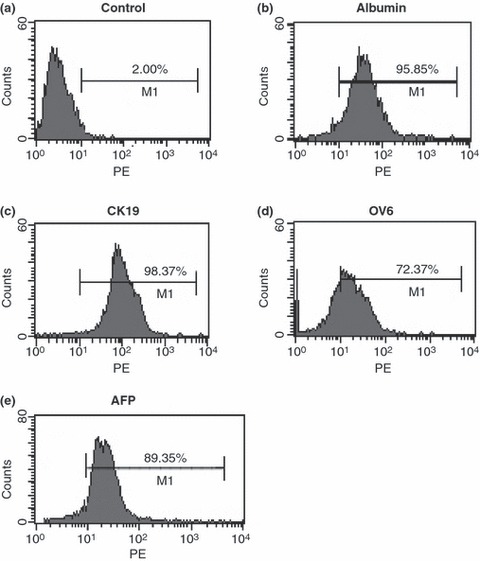
Phenotypic characteristics of oval cells derived from porcine liver. (a) Replacement of primary antibodies with PE‐labelled IgG served as negative control. (b–d) Freshly isolated cells from porcine liver after incubation with primary antibodies including albumin (b), CK19 (c), OV6 (d) and AFP (e), followed by PE‐labelled IgG as secondary antibody. More than 92% of fresh oval cells expressed albumin and CK19.
Double staining analysis using flow cytometry was used to show the hepatobiliary phenotype of such oval cells. We found that 92% of the isolated oval cells from these porcine livers co‐expressed CK19 and albumin (Fig. 4b), suggesting that they had bipotential characteristics of hepatocytes and biliary cells. Double staining using immunofluorescence was used to verify phenotypes of freshly isolated oval cells. We observed that around 90% of the freshly isolated oval cells co‐expressed OV6 and albumin (Fig. 5a).
Figure 4.
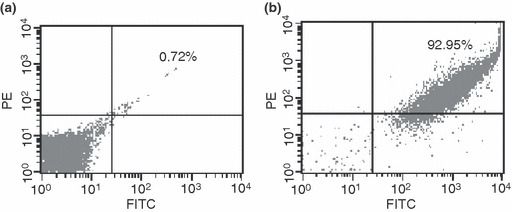
Double staining and flow cytometric analysis to characterize porcine oval cells as expressing hepatobiliary phenotype. (a) Replacement of primary antibodies with PE‐labelled and FITC‐labelled IgG served as negative control. (b) Freshly isolated cells from porcine liver after incubation with primary antibodies including albumin and CK19, followed by PE‐labelled IgG and FITC‐labelled as secondary antibody.
Figure 5.
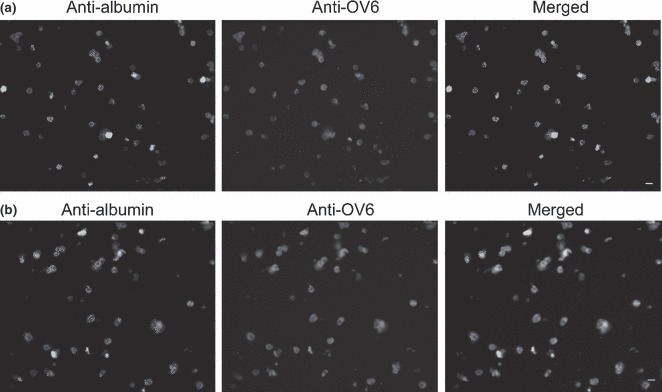
Double staining by immunofluorescence analysis to characterize phenotypes of porcine oval cells. Immunofluorescence analysis showing co‐expression of albumin and OV6 in freshly isolated cells (a) and in oval cells after culture for 14 days (b).
Porcine oval cell proliferation in vitro culture
We further assessed proliferation potential of the porcine oval cells. Freshly isolated cells assumed a cobblestone‐like appearance when seeded in culture dishes. They attached within 24 h after plating and began to proliferate and scatter, while maintaining their oval shape. Numbers of oval cells doubled by 72 h culturing (Fig. 6a), and multiplied quickly with greater increase in number by day 5. On day 6 after plating, 90% of the culture dishes were covered with oval cells (Fig. 6b). The cells could be cultured for second passage, after which they were still able to proliferate, as reflected by increase in cell population. It was noted that after passage the cells aggregated to form discrete colonies with tens of cells, following a period of proliferation (Fig. 6c, denoted by arrows). As described in the Materials and methods section, the cells were cultured in vitro for 14 days, over which time there were eight population doublings, as shown by proliferation (Fig. 6d), confirming that our culture system promoted proliferation of porcine oval cells.
Figure 6.
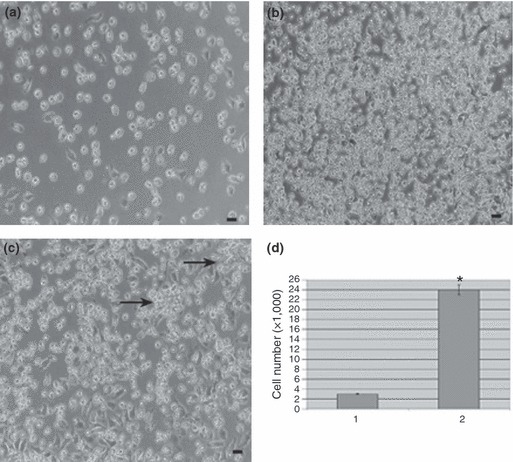
Culture of hepatic oval cells obtained from porcine liver. (a) Porcine hepatic oval cells proliferated with number of population doubling at 72 h after plating. (b) Oval cells proliferated faster with increase in number of cells after culture for 6 days. (c) Porcine oval cells retained the ability to proliferate after two passages. Colonies (indicated by arrows) containing tens of cells were seen following a period of oval cell expansion. Scale bars in a–c = 12 μm. (d) Proliferation assay showed number of oval cells after plating (lane 1) and culture for 14 days (lane 2). Note: *P < 0.05.
Immunofluorescence analysis of proliferative porcine oval cells
The SCF/c‐kit system is believed to play a key role in survival, proliferation and differentiation of stem cells, and expression of SCF is abolished once oval cells differentiate into hepatocytes (25). Hepatic stem cells with high potential for proliferation have been shown to have the ability to form cell colonies (6). To evaluate proliferation capability of our porcine cells derived from colonies, we examined their phenotypic characteristics using immunofluorescence analysis. Here, double immunofluorescence staining revealed that after culture for 14 days, our cells co‐expressed OV6 and albumin (Fig. 5b). As shown in Fig. 7, almost all cells from the expanding colonies expressed SCF (Fig. 7a), c‐kit (Fig. 7b), OV6 (Fig. 7c), CK19 (Fig. 7d), Thy‐1 (Fig. 7e) and AFP (Fig. 7f), indicating that their in vitro culture for 14 days still left them in the undifferentiated state.
Figure 7.
Immunofluorescence analysis of porcine hepatic oval cells after culture for 14 days. Hepatic oval cells from porcine liver after culture for 2 weeks after incubation with antibodies to: SCF (a), c‐kit (b), OV6 (c), CK19 (d), Thy‐1 (e) and AFP (f), and followed by PE‐labelled IgG to detect primary antibodies.
Discussion
Hepatic stem cells remain proliferatively dormant (G0 phase) in normal adult livers. Experimentally, several models using toxins or carcinogens have been utilized to activate hepatic oval cells, but these treatments often cause liver damage. Galactosamine, for example, causes liver injury in pericentral regions, and allyl alcohol engenders periportal hepatocyte death (26, 27). In the present study, PH resulted in liver regeneration with extensive proliferation of hepatic oval cells. It is worth noting that there were no obvious lesions in the livers following these procedures. Thus, the PH model still appears to be an excellent approach for activating oval cells, here in porcine livers.
Due to the lack of specific markers, it proves to be difficult to isolate and purify hepatic stem cells from a heterogeneous liver cell fraction. We and other have isolated hepatic oval cells from adult rodent livers using specific enzymatic digestion and density gradient centrifugation (12, 28). Liver explant culture has been used to separate oval cells derived from mice (29), but the cell isolation technology used led to low purity of oval cells. Kano et al. utilized centrifugation at different velocities to separate NPECs, however, this cell isolation procedure resulted in a mixed cell population containing at least three different kinds of cell (13). Here, we have obtained essentially pure oval cells with a high viability, from adult porcine livers using the separation technique of selective enzymatic digestion and density gradient centrifugation. We also fully characterized morphological and phenotypic features of the isolated and cultured oval cells.
The morphological characteristics of the oval cells we obtained from these porcine livers were similar to those of hepatic oval cells derived from adult rat liver tissues. For example, they both had ovoid nuclei, high nucleus/cytoplasmic ratio, few immature organelles, and median diameter much lower than that of mature hepatocytes. Meanwhile, our porcine hepatic oval cells expressed albumin and CK19 at high levels. Albumin is a specific marker for hepatocytes, whereas CK19 is a special marker of bile duct epithelial cells. Considered together, our hepatic oval cells expressed both hepatocyte and cholangiocyte markers, suggesting that they were phenotypically bipotential stem cells. In addition, our freshly isolated oval cells stained positively for OV6 expression, indicating that there was similarity in phenotypic characteristics between porcine oval cells here and those from rodent livers. All results mentioned above provide definitive evidence that hepatic oval cells exist in porcine livers.
To expand our isolated oval cells from porcine liver tissues, we set up a suitable culture system consisting of certain growth factors including HGF, SCF and LIF. HGF was used as we have found (in common with others) that HGF promotes proliferation of hepatic oval cells derived from adult rat liver (12). We also employed SCF as it is generally shown to be essential for survival of hepatic and other stem cells (30). LIF is essential for maintenance of stem cells in vitro, and thus has been utilized here for our culture of porcine oval cells. Immunofluorescence analysis demonstrated that both SCF ligand and its receptor c‐kit were expressed in expanding cells after 2 weeks, suggesting that SCF signals via an autocrine loop in porcine oval cells. Our results are consistent with findings that both the SCF ligand, and its receptor genes are expressed during early stages of rat oval cell proliferation after partial hepatectomy (30). The c‐kit gene has also been demonstrated to be expressed in human hepatic stem‐like cells (31). We found that our oval cells after culture still expressed CK19, albumin, and OV6, similar to expression patterns of rat and human hepatic stem cells (11, 12, 24, 31). We also observed that after culture our oval cells expressed Thy‐1, which is similar to the expression pattern of adult mouse hepatic stem cells (28). Taken together, porcine oval cells share some important phenotypic features with rodent and human liver stem cells.
In summary, we have for the first time, demonstrated that hepatic oval cells exist in activated porcine liver tissues. We believe that these are putative hepatic stem cells, based on morphological features, phenotypic characteristics and their proliferation ability. We have shown that our porcine hepatic oval cells share certain phenotypes with rodent and human liver stem cells and were able to proliferate in in vitro culture. This could provide an important source of hepatic stem cells for basic research and potentially in the future for clinical application.
Disclosure
The authors declare that no competing financial interests exist.
Acknowledgements
We are thankful to Professor Stewart Sell, New York State Department of Health, Wadsworth Center, for his kind provision of an antibody to OV6. The work was supported by grants from the High–tech Project of the Chinese Ministry of Science and Technology (National ‘863’ Project) (No. 2001AA216051), the Natural Science Foundation of Beijing (No. 7022023), the National Science Foundation of China (No. 31171422), and Shanghai Pujiang Program (No. 11PJ1406400).
References
- 1. Wilson JW, Leduc EH (1958) Role of cholangioles in restoration of the liver of the mouse after dietary injury. J. Pathol. Bacteriol. 76, 441–449. [DOI] [PubMed] [Google Scholar]
- 2. Arber N, Zajicek G, Ariel I (1988) The streaming liver. II. Hepatocyte life history. Liver 8, 80–87. [DOI] [PubMed] [Google Scholar]
- 3. Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA (1996) Identification of bipotential progenitor cells in human liver development. Hepatology 23, 476–481. [DOI] [PubMed] [Google Scholar]
- 4. Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N et al. (1999) Bone marrow as a potential source of hepatic oval cells. Science 284, 1168–1170. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Foster M, Al‐Dhalimy M, Lagasse E, Finegold M, Grompe M (2003) The origin and liver repopulating capacity of murine oval cells. Proc. Natl. Acad. Sci. USA 100(Suppl. 1), 11881–11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suzuki A, Zheng YW, Fukao K, Nakauchi H, Taniguchi H (2001) Hepatic stem/progenitor cells with high proliferative potential in liver organ formation. Transplant. Proc. 33, 585–586. [DOI] [PubMed] [Google Scholar]
- 7. Strain AJ, Neuberger JM (2002) A bioartificial liver – state of the art. Science 295, 1005–1009. [DOI] [PubMed] [Google Scholar]
- 8. Faris RA, Konkin T, Halpert G (2001) Liver stem cells: a potential source of hepatocytes for the treatment of human liver disease. Artif. Organs 25, 513–521. [DOI] [PubMed] [Google Scholar]
- 9. Smith PG, Tee LB, Yeoh GC (1996) Appearance of oval cells in the liver of rats after long‐term exposure to ethanol. Hepatology 23, 145–154. [DOI] [PubMed] [Google Scholar]
- 10. Sakamoto T, Ezure T, Lunz J, Murase N, Tsuji H, Fung JJ et al. (2000) Concanavalin A simultaneously primes liver hematopoietic and epithelial progenitor cells for parallel expansion during liver regeneration after partial hepatectomy in mice. Hepatology 32, 256–267. [DOI] [PubMed] [Google Scholar]
- 11. He ZP, Tan WQ, Tang YF, Feng MF (2003) Differentiation of putative hepatic stem cells derived from adult rats into mature hepatocytes in the presence of epidermal growth factor and hepatocyte growth factor. Differentiation 71, 281–290. [DOI] [PubMed] [Google Scholar]
- 12. He ZP, Tan WQ, Tang YF, Zhang HJ, Feng MF (2004) Activation, isolation, identification and in vitro proliferation of oval cells from adult rat livers. Cell Prolif. 37, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kano J, Noguchi M, Kodama M, Tokiwa T (2000) The in vitro differentiating capacity of nonparenchymal epithelial cells derived from adult porcine livers. Am. J. Pathol. 156, 2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kano J, Ishiyama T, Iijima T, Morishita Y, Murata S, Hisakura K et al. (2008) Differentially expressed genes in a porcine adult hepatic stem‐like cell line and their expression in developing and regenerating liver. Lab. Invest. 88, 132–143. [DOI] [PubMed] [Google Scholar]
- 15. Kano J, Ishiyama T, Nakamura N, Iijima T, Morishita Y, Noguchi M (2003) Establishment of hepatic stem‐like cell lines from normal adult porcine liver in a poly‐D‐lysine‐coated dish with NAIR‐1 medium. In Vitro Cell. Dev. Biol. Anim. 39, 440–448. [DOI] [PubMed] [Google Scholar]
- 16. Kano J, Tokiwa T, Zhou X, Kodama M (1998) Colonial growth and differentiation of epithelial cells derived from abattoir adult porcine livers. J. Gastroenterol. Hepatol. 13(Suppl.), S62–S69. [PubMed] [Google Scholar]
- 17. Katchman H, Tal O, Eventov‐Friedman S, Shezen E, Aronovich A, Tchorsh D et al. (2008) Embryonic porcine liver as a source for transplantation: advantage of intact liver implants over isolated hepatoblasts in overcoming homeostatic inhibition by the quiescent host liver. Stem Cells 26, 1347–1355. [DOI] [PubMed] [Google Scholar]
- 18. Chung YJ, Lee HJ, Koh YT, Kim SB, Kim SH, Choi SH et al. (2002) Isolation and culture of pig hepatocyte in large scale for the application of bioartificial liver system. Taehan Kan Hakhoe Chi 8, 249–255. [PubMed] [Google Scholar]
- 19. Linti C, Zipfel A, Schenk M, Dauner M, Doser M, Viebahn R et al. (2002) Cultivation of porcine hepatocytes in polyurethane nonwovens as part of a biohybrid liver support system. Int. J. Artif. Organs 25, 994–1000. [DOI] [PubMed] [Google Scholar]
- 20. Abrahamse SL, van de Kerkhove MP, Sosef MN, Hartman R, Chamuleau RA, van Gulik TM (2002) Treatment of acute liver failure in pigs reduces hepatocyte function in a bioartificial liver support system. Int. J. Artif. Organs 25, 966–974. [DOI] [PubMed] [Google Scholar]
- 21. Jasmund I, Langsch A, Simmoteit R, Bader A (2002) Cultivation of primary porcine hepatocytes in an OXY‐HFB for use as a bioartificial liver device. Biotechnol. Prog. 18, 839–846. [DOI] [PubMed] [Google Scholar]
- 22. He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M (2008) Gdnf upregulates c‐Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells 26, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He Z, Jiang J, Kokkinaki M, Dym M (2009) Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct‐4 activation. Stem Cells 27, 2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crosby HA, Hubscher SG, Joplin RE, Kelly DA, Strain AJ (1998) Immunolocalization of OV‐6, a putative progenitor cell marker in human fetal and diseased pediatric liver. Hepatology 28, 980–985. [DOI] [PubMed] [Google Scholar]
- 25. Blakolmer K, Jaskiewicz K, Dunsford HA, Robson SC (1995) Hematopoietic stem cell markers are expressed by ductal plate and bile duct cells in developing human liver. Hepatology 21, 1510–1516. [PubMed] [Google Scholar]
- 26. Dabeva MD, Alpini G, Hurston E, Shafritz DA (1993) Models for hepatic progenitor cell activation. Proc. Soc. Exp. Biol. Med. 204, 242–252. [DOI] [PubMed] [Google Scholar]
- 27. Dabeva MD, Shafritz DA (1993) Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D‐galactosamine model of liver regeneration. Am. J. Pathol. 143, 1606–1620. [PMC free article] [PubMed] [Google Scholar]
- 28. Li WL, Su J, Yao YC, Tao XR, Yan YB, Yu HY et al. (2006) Isolation and characterization of bipotent liver progenitor cells from adult mouse. Stem Cells 24, 322–332. [DOI] [PubMed] [Google Scholar]
- 29. Monga SP, Tang Y, Candotti F, Rashid A, Wildner O, Mishra B et al. (2001) Expansion of hepatic and hematopoietic stem cells utilizing mouse embryonic liver explants. Cell Transplant. 10, 81–89. [PubMed] [Google Scholar]
- 30. Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS (1994) Expression of stem cell factor and its receptor, c‐kit, during liver regeneration from putative stem cells in adult rat. Lab. Invest. 70, 511–516. [PubMed] [Google Scholar]
- 31. Crosby HA, Kelly DA, Strain AJ (2001) Human hepatic stem‐like cells isolated using c‐kit or CD34 can differentiate into biliary epithelium. Gastroenterology 120, 534–544. [DOI] [PubMed] [Google Scholar]


