Abstract
Objectives
To determine whether interleukin‐6 (IL‐6) stimulates rat muscle satellite cell proliferation in culture, and if so, to clarify the signalling mechanisms.
Materials and methods
Primary satellite cells were isolated from thirty male F344 rats, 11 weeks of age. IL‐6 at concentrations of 0.01, 0.1, 1, 10 or 100 ng/ml was added to culture media.
Results
IL‐6 at 0.01–1 ng/ml induced dose‐dependent increase in cell proliferation. After treatment with 1 ng/ml IL‐6, cell proliferation increased by 31%, and p‐STAT3+/MyoD+ cells increased in number compared to those in control media (P < 0.05). Inhibitors of JAK2 (AG 490) and STAT3 (STAT3 peptide) blocked the increase in BrdUrd+ cell numbers at 6 h post stimulation with 1 ng/ml IL‐6 (P < 0.05). Furthermore, cyclin D1 mRNA expression and cyclin D1+/MyoD+ cell numbers significantly increased in cultures treated with 1 ng/ml IL‐6 compared to those in control media (P < 0.05). In contrast, treatment with 10 and 100 ng/ml IL‐6 did not stimulate cell proliferation. Treatment with 10 ng/ml IL‐6 induced greater SOCS3 mRNA expression than with 1 ng/ml IL‐6 and control media. Moreover, co‐localization of SOCS3 and myogenin was observed after treatment with 10 ng/ml IL‐6.
Conclusions
IL‐6 induced dose‐dependent increase in satellite cell proliferation by activating the JAK2/STAT3/cyclin D1 pathway.
Introduction
Skeletal muscle satellite cells are located outside the sarcolemma and under the basal lamina of muscle fibres 1. Satellite cells have been shown to play integral roles in skeletal muscle repair, hypertrophy and hyperplasia. In response to various stimuli, satellite cells can increase their activity, proliferate, differentiate and give rise to new myonuclei that are incorporated into existing myofibres, to keep myonuclear domains constant or to repair segmental muscle fibre injury 2, 3, 4. Many growth factors and cytokines have been implicated in regulation of satellite cell responses 2, 3, 5.
Interleukin‐6 (IL‐6) is a pleiotropic cytokine commonly associated with control and coordination of immune responses 6, however, traditionally, IL‐6 is associated with muscle atrophy. Tsujinaka et al. have shown that transgenic mice overexpressing IL‐6 develop skeletal muscle atrophy 7; Haddad et al. also have demonstrated that high concentrations of IL‐6 directly induce skeletal muscle atrophy in healthy rats 8. In addition, high plasma IL‐6 levels are correlated with reduced muscle mass in the elderly 9, and chronically elevated circulating IL‐6 is associated with a variety of muscle wasting diseases 10. Previous research suggests that IL‐6 inhibits cell growth signalling pathways in culture 11, indicating a mechanism by which chronically elevated circulating IL‐6 causes muscle atrophy.
Recently, several studies have provided key evidence that IL‐6 and other cytokines of the interleukin family may be important for muscle regeneration and growth. For example, knockout of leukaemia inhibitory factor (LIF), a member of the IL‐6 family, causes impaired muscle regeneration after muscle damage 12 and impaired muscle growth after muscle overload 13. Conversely, infusion of LIF into LIF knockout mice can stimulate muscle regeneration 12 and muscle growth 13. In addition, IL‐6 knockout mice have been shown to have initial delay in muscle regrowth during recovery from muscle atrophy 14. When IL‐6 is used in combination with LIF, satellite cell proliferation is only slightly increased compared to with IL‐6 treatment alone 15, suggesting that IL‐6 and LIF share common receptors. Thus, IL‐6 and LIF may cause muscle regeneration and growth by activating the same signalling pathway.
Recent studies have elucidated a relationship between IL‐6 and satellite cell responses. Wang et al. showed that IL‐6, LIF or ciliary neurotrophic factor (CNTF) (another member of the IL‐6 family) may be important regulating factors in proliferation and differentiation of myoblasts in vitro 16, but the study did not investigated signalling mechanisms involved. Serrano et al. have demonstrated that IL‐6 knockout mice had blunted hypertrophic responses and less satellite cell‐mediated myonuclear accretion compared to wild type mice, following compensatory hypertrophy, by impairing the proliferative potential of satellite cells 17. In human studies, temporarily elevated IL‐6 expression following muscle contraction has been shown to be important in regulation of satellite cell proliferation and muscle repair 18, 19. Varied effects of IL‐6 on satellite cells may reflect biphasic dose effects of IL‐6. Up to now, no research has been conducted to systematically examine effects of a range of IL‐6 doses on satellite cell functions.
IL‐6 binds to the IL‐6 receptor α chain, which recruits gp130 and induces janus kinases (JAKs; JAK1, JAK2). These proteins then phosphorylate signal transducer and activator of transcription proteins (p‐STATs), STAT1 and STAT3 20. STATs then form homo or heterodimers and translocate to the nucleus where they facilitate transcription of downstream target genes by binding to specific DNA response elements and promoter regions 21. Once in the nucleus, p‐STATs promote transcription of downstream genes, such as cyclin D1 17, p21waf1 22, p27kip1 22 and the suppressor of cytokine signalling 3 (SOCS3) 23, which are associated with regulation of the cell cycle. IL‐6 is a particularly well‐characterized cytokine that activates the JAK2/STAT3 pathway in satellite cells 17, 18, 19. Thus, the IL‐6/JAK2/STAT3 signalling pathway may be a key factor for satellite cell cycle regulation through expression of STAT3‐induced downstream genes. Although IL‐6 may play an important role in regulating satellite cell proliferation, the mechanisms by which it induces proliferative potential of primary satellite cells have not previously been directly elucidated.
The purpose of this investigation was to determine whether IL‐6 induces proliferative potential of satellite cells in culture and to clarify signalling mechanisms involved. We hypothesized that moderate concentrations of IL‐6 may enhance proliferative potential of satellite cells by activating the JAK2/STAT3 signalling pathway, in culture.
Materials and methods
Animals
Male Fisher 344 rats (n = 30) were purchased at the age of 10 weeks (SLC, Tokyo, Japan). All animals were housed at 21 °C in a 12‐h light/12‐h dark cycle environment; rat chow and water were provided ad libitum. Animals were allowed to acclimatize to their new surroundings for 1 week before cell isolation procedures were performed. These experimental procedures were approved by the Tokai University Animal Care and Use Committee and complied with American Physiological Society Animal Care Guidelines.
Materials
The following materials were obtained for primary satellite cell culture experiments: human recombinant IL‐6 (IL‐6; R&D Systems, Minneapolis, MN, USA), Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA), Ham's F‐10 Nutrient Mix (F‐10; Invitrogen), normal horse serum (HS; Invitrogen), foetal bovine serum (FBS; Invitrogen), penicillin/streptomycin (penicillin/streptomycin antibiotic; Invitrogen), Streptomyces griseus (Sigma, St. Louis, MO, USA), and 5‐bromo‐2′‐deoxyuridine‐5′‐monophosphate (BrdUrd; Sigma).
The following materials were obtained from Calbiochem (USA) for pharmacological inhibition: JAK2 inhibitor tyrphostin AG 490 (10 μm) 24, STAT3 inhibitor peptide (STAT3pi, 500 μm) 17, mitogen‐activated protein kinase (MAPK)/extracellular signal‐regulated kinase (ERK) kinase (MEK) inhibitor PD98059 (10 μm) 24, 25, and p38 MAPK inhibitor SB202190 (10 μm) 26. Concentrations of pharmacological inhibitors were determined according to previously described methods 17, 24, 25, 26.
The following materials were used for immunodetection of target proteins in satellite cells: primary antibodies to Pax7 (1:3; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA), myogenin (1:4; DSHB), MyoD (1:100; DAKO, Carpentaria, CA, USA), BrdUrd (Roche Diagnostics, Mannheim, Germany), cyclin D1 (1:100; Epitomics, Burlingame, CA, USA), SOCS3 (1:100; Cell Signaling Technology, Danvers, MA, USA), and p‐STAT3 (1:100; Cell Signaling Technology). Secondary antibodies used were immunoglobulin biotinylated anti‐mouse IgG (1:50; GE Healthcare, Buckinghamshire, UK), Alexa Flour 488‐labelled goat anti‐mouse IgG (1:1000; Invitrogen, Molecular Probes Inc., Carlsbad, CA, USA), and Alexa Flour568‐labelled goat anti‐rabbit IgG (1:1000; Invitrogen, Molecular Probes Inc.).
Isolation of satellite cells
Satellite cells were isolated from the major hind‐limb muscles of rats, according to methods described in Machida et al. 27. Briefly, animals were anesthetized with pentobarbital sodium (50 mg/kg). Major hind limb muscles were removed and trimmed of excess fat and connective tissue, and were digested for 1 h at 37 °C with 1.25 mg/ml pronase. Cells were separated from muscle fibre fragments and tissue debris using differential centrifugation and were plated on an uncoated dish in DMEM containing 10% HS and 1% penicillin/streptomycin for 2 h. Unattached cells in the medium after 2 h incubation were transferred to a fresh uncoated dish. After 24 h, floating cells were collected by centrifugation at 2000 g for 3 min, and the pellet was re‐suspended in F‐10 containing 20% FBS and 1% penicillin/streptomycin, on rat‐tail collagen‐coated culture slides (BD, Franklin Lakes, NJ, USA). All cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2. Immunocytochemical analyses indicated that >90% of these cells were desmin‐ and MyoD‐positive. In subsequent experiments, IL‐6 was added to culture medium at 0.01, 0.1, 1, 10 and 100 ng/ml.
BrdUrd assay
Cultures were pulse‐labelled with 10 μm BrdUrd in medium for the final 1 h, 24 h after stimulation with IL‐6, and numbers of BrdUrd‐positive cells were determined using immunocytochemistry analyses as previously described 27. Activation and proliferation were indicated by percentage of cells BrdUrd+.
Immunocytochemistry
Specimens were rinsed three times in phosphate buffered saline (PBS) at each time point between 3 and 6 h following stimulation with IL‐6. Co‐immunocytochemical staining for MyoD and cyclin D1, MyoD and p‐STAT3, and myogenin and SOCS3, was performed after fixing in 4% paraformaldehyde or methanol, for 20 min at 4 °C, then washing several times. Subsequently, we quantified numbers of satellite cells expressing MyoD (a marker of activated and proliferating satellite cells) with cyclin D1 and p‐STAT3. Specimens were incubated in PBS, then in corresponding antibody blocking solutions for 30 min, and subsequently with appropriately diluted (as indicated above) primary antibodies at 4 °C for 16 h in PBS containing 2% bovine serum albumin. Following thorough rinsing in PBS, cells were incubated with secondary antibodies for 30 min at room temperature. Specimens were then washed in PBS and mounted with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Vectashield; Vector Laboratories, Burlington, ON, Canada). Primary antibodies were detected using Vectastain Elite ABC kit (Vector Laboratories). Immunocytochemistry and nuclear DNA staining were performed using methyl green solution. Stained slides were viewed using a KYENCE BZ‐9000 microscope (KEYENCE, Osaka, Japan) and images were captured and analysed using image software (KEYENCE). For evaluations of nuclei, BrdUrd+ cells, cyclin D1, and p‐STAT3, at least 10 different images were taken per condition, corresponding to 500–1000 myonuclei.
Reverse transcription (RT) – polymerase chain reaction (PCR)
Total RNA was isolated from cultured satellite cells using RNeasy Mini Kit according to the manufacturer's instructions (Qiagen, Valencia, CA, USA) and RNA quantity and purity were assessed by spectrophotometry (Eppendorf, Hamburg, Germany). Synthesis of cDNA was performed using 1 μg total RNA and a reverse transcription kit (TOYOBO, Osaka, Japan) with a random primer (TOYOBO) in 20 μl reaction volumes, using a thermal cycler (Astec, Fukuoka, Japan). PCR was performed using Platium Taq DNA polymerase (Invitrogen); primer sequences are shown in Table 1. PCR products were separated on 2% or 3% agarose gels, visualized using rinse solution containing ethidium bromide, and photographed under UV light. Illuminated bands were quantified using densitometric software (Atto, Tokyo, Japan), and mRNA expression was normalized to that of 18S mRNA (Ambion, Austin, TX, USA) in each sample.
Table 1.
PCR primer pairs
| Product | Forward primer | Reverse primer |
|---|---|---|
| Cyclin D1 | AAGTGCGTGCAGAGGGAGAT | GGGGCGGATAGAGTTGTCAG |
| SOCS3 | ATGGTCACCCACAGCAAGTTCCCG | TTAAAGTGGAGCATCATACTGATCC |
| p21 | GGCAGACCAGCCTAACAGATTT | GGCACTTCAGGGCTTTCTCTT |
| p27 | CGTGAGAGTGTCTAACGGGAG | TCTTCTGTTCTGTTGGCCCT |
Statistical analysis
All values are expressed as mean ± SD. Significant differences were determined using one‐way ANOVA with the Tukey–Kramer test at a probability level of P < 0.05. All statistical analyses were performed using a statistical software package (Prism 4.0; GraphPad Software, La Jolla, CA, USA).
Results
IL‐6‐induced satellite cell proliferation
To analyse satellite cell proliferation, we quantified cell numbers after 24 h treatments with various concentrations of IL‐6 (0.01, 0.1, 1, 10 and 100 ng/ml). Incubation with 0.01–1 ng/ml IL‐6 induced dose‐dependent increase in cell proliferation (Fig. 1a), with 31% increase in cell proliferation at 1 ng/ml compared to that in control media after incubation of 24 h (P < 0.05). In contrast, treatment with 10 and 100 ng/ml IL‐6 did not stimulate cell proliferation compared to control media (Fig. 1a). Thus, we focused on effects of IL‐6 at 1 and 10 ng/ml. To determine timing of IL‐6 induced satellite cell proliferative potential, we stained cells with BrdUrd+ for 24‐h treatments with 1 and 10 ng/ml IL‐6. After 6 h, percentage of BrdUrd+ cells was significantly higher following treatment with 1 ng/ml IL‐6 than with 10 ng/ml IL‐6 or control media only (P < 0.05; Fig. 1b). Additionally, we monitored expression of cyclin D1 protein, which is necessary for cell cycle progression, using immunocytochemistry (Fig. 2b). In comparison to control cultures, treatment with 1 ng/ml IL‐6 caused 25% increase in cyclin D1+/MyoD+ cells after 6 h (P < 0.05; Fig. 2a). However, treatment with 10 ng/ml IL‐6 had no effect on number of cyclin D1+/MyoD+ cells at this time point (Fig. 2a). As these results indicated that the satellite cell response occurred after 6 h stimulation with IL‐6, we monitored protein and mRNA expressions of various cell cycle molecules over this period.
Figure 1.
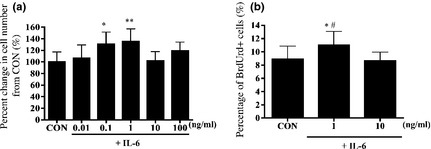
Satellite cell response to various concentrations of interleukin‐6 ( IL ‐6). (a) Satellite cell proliferation after 24 h treatment with various concentrations of interleukin‐6 (IL‐6). (b) Satellite cell proliferation after 6 h treatment with various concentrations of IL‐6. Values are reported as mean ± SD; *P < 0.05 versus CON, **P < 0.01 versus CON, #P < 0.05 versus 10, n = 6–9 per treatment group.
Figure 2.
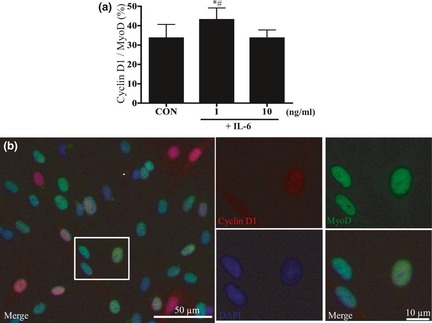
Cyclin D1 protein expression in IL ‐6‐stimulated satellite cells. (a) Comparison of percentage cyclin D1+/MyoD+ cells after 6 h treatment with various concentrations of IL‐6. Values are reported as mean ± SD; *P < 0.05 versus CON, #P < 0.05 versus 10, n = 9 per treatment group. (b) Identification of cyclin D1 and MyoD; immunostaining was performed to visualize localization of cyclin D1 (red), MyoD (green), nuclei (blue) and three images were merged.
IL‐6‐induced proliferation of satellite cells was inhibited by pharmacological inhibition of the JAK2/STAT3 pathway
Among numerous signalling pathways that regulate cell proliferation, we observed changes in the IL‐6‐related JAK2/STAT3 signalling pathway, using the JAK2 inhibitor AG 490 and a STAT3 inhibitory peptide. Both these inhibitors significantly reduced numbers of BrdUrd+ cells after 6 h treatment with 1 ng/ml IL‐6 (P < 0.05; Fig. 3). In contrast, MEK inhibitor PD98059 and p38 MAPK inhibitor SB202190 did not alter numbers of BrdUrd+ cells under these conditions (Fig. 3).
Figure 3.
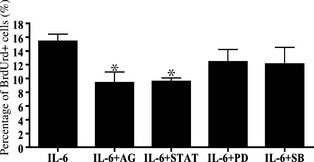
Response of IL ‐6‐related signalling pathways to pharmacological inhibitors. IL‐6 (1 ng/ml) induced satellite cell proliferation after 6 h in the presence of cell signalling pathway inhibitors: AG (10 µm AG490), STAT (500 µm STAT3 peptide), PD (10 µm PD98059) and SB (10 µm SB203580). Values are reported as mean ± SD; *P < 0.05 versus IL‐6, n = 3 per treatment.
IL‐6‐induced phosphorylation of STAT3 protein in satellite cell nuclei
To confirm that IL‐6 induced cell proliferation via the JAK2/STAT3 pathway, we revealed substantial increase in localization of p‐STAT3 protein within nuclei of IL‐6 treated satellite cells, using triple immunofluorescence staining with p‐STAT3, MyoD, and DAPI (Fig. 4b). Treatment with 1 ng/ml IL‐6 for 3 h significantly increased p‐STAT3+/MyoD+ cell numbers by 44% compared to control media only (P < 0.05; Fig. 4a). However, treatment with 10 ng/ml IL‐6 had no effect in these experiments (Fig. 4a).
Figure 4.
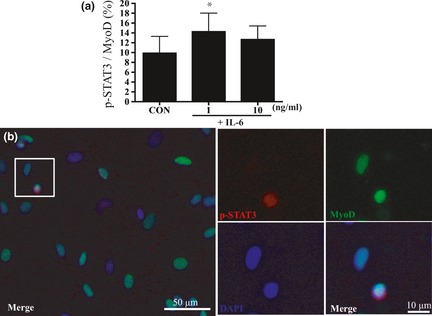
Protein expression of phospho‐ STAT 3 (p‐ STAT 3) in satellite cell nuclei after stimulation with IL ‐6. (a) Phospho‐STAT3 (p‐STAT3) expression as percentage of all MyoD+ cells after 3 h treatment with various concentrations of IL‐6. Values are reported as mean ± SD; *P < 0.05 versus CON, n = 8 per treatment group. (b) Identification of p‐STAT3 and MyoD; immunostaining was performed to visualize localization of p‐STAT3 (red), MyoD (green), nuclei (blue), and three images were merged.
JAK2/STAT3 signalling pathway‐induced downstream genes cylin D1, p21waf1 , p27kip1 and SOCS3 mRNA and localization of SOCS3 protein within myogenin‐positive cells after stimulation with IL‐6
To investigate relevance of the p‐STAT3 response to 1 ng/ml IL‐6, we examined mRNA expression of STAT3‐related downstream genes. Compared to control media, treatment with 1 ng/ml IL‐6 significantly induced cyclin D1 mRNA expression after 6 h (P < 0.05; Fig. 5a). In support of this, mRNA expression of cell cycle inhibitors p21waf1, p27kip1 and SOCS3 (provides a negative feedback loop controling activation of STAT3), was not significantly altered by treatment with 1 ng/ml IL‐6 (Fig. 5b–d). Cyclin D1 mRNA expression was not significantly different following 3 h treatment with 10 ng/ml IL‐6 compared to 1 ng/ml IL‐6 or control media (Fig. 6a). As 10 ng/ml IL‐6 did not induce elevation in cyclin D1 mRNA, we hypothesized that this dose of IL‐6 may increase expression of cell cycle inhibitors p21waf1 and p27kip1 mRNA. However, p21waf1 and p27kip1 mRNA were not significantly changed by treatment with 10 ng/ml IL‐6 after 3 h (Fig. 6b,c) or 6 h compared to control media. In contrast, SOCS3 mRNA expression was increased by 63% after 3 h treatment with 10 ng/ml IL‐6 (Fig. 6d). A previous study has shown that induction of SOCS3 causes cell differentiation 28. Thus, we investigated co‐localization of SOCS3+ and myogenin+ cells after treatment with 10 ng/ml IL‐6. Triple immunofluorescence staining revealed that SOCS3 was present in myogenin+ cells after 3 h treatment with 10 ng/ml IL‐6 (Fig. 7).
Figure 5.
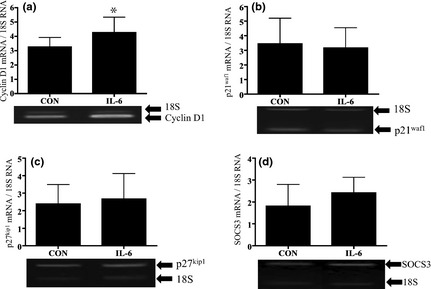
Response of STAT3‐induced downstream genes after stimulation with 1 ng/ml IL‐6. Comparisons of cyclin D1 (a), p21waf1 (b), p27kip1 (c) and SOCS3 (d) mRNA expression after 3 and 6 h treatment with 1 ng/ml IL‐6; All mRNAs were analysed using RT‐PCR and were normalized to expression of 18S mRNA. Values are reported as mean ± SD; *P < 0.05 versus CON, n = 4–9 per treatment.
Figure 6.
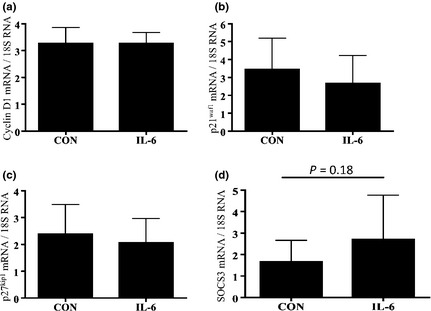
Response of STAT3‐induced downstream genes after stimulation with 10 ng/ml IL‐6. Comparisons of cyclin D1 (a), p21waf1 (b), p27kip1 (c) and SOCS3 (d) mRNA expression after 3 and 6 h treatment with 10 ng/ml IL‐6. All mRNAs were analysed using RT‐PCR and were normalized to expression of 18S mRNA. Values are reported as mean ± SD; *P < 0.05 versus CON, n = 4–9 per treatment.
Figure 7.
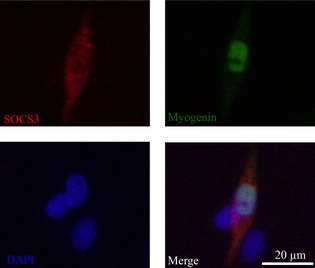
Co‐localization of SOCS 3 protein in myogenin‐positive cells. Immunostaining was performed to visualize localization of SOCS3 (red), myogenin (green), nuclei (blue) and three images were merged.
Discussion
This study demonstrates, for the first time, that IL‐6 can induce dose‐dependent increase in proliferative potential of satellite cells. Using JAK2 inhibitor AG 490, and STAT3 inhibitory peptide, we blocked effects of 1 ng/ml IL‐6 on satellite cell proliferation, indicating that IL‐6 induced a significant increase in p‐STAT3 protein in nuclei of satellite cells at tested concentrations. We also observed upregulation of STAT3‐related downstream genes such as cyclin D1 in response to IL‐6. However, high concentrations of IL‐6, such as 10 ng/ml, prevented proliferative potential of satellite cells by inducing SOCS3.
IL‐6 induced an increase in satellite cell proliferation. One previous study has also shown that LIF can induce proliferation of cultured satellite cells via the JAK2/STAT3 signalling pathway 24. Moreover, attenuated muscle hypertrophy in IL‐6 knockout mice was related to inhibition of STAT3‐mediated satellite cell activity and to a marked reduction in BrdUrd+ cell numbers during compensatory muscle growth 17. Here, numbers of BrdUrd+ cells increased 6 h after stimulation with 1 ng/ml IL‐6. Moreover, JAK2 and STAT3 inhibitors blocked IL‐6‐induced satellite cell proliferation, suggesting that IL‐6 enhanced proliferative potential of satellite cells by activating the JAK2/STAT3 signalling pathway.
Using immunocytochemical analyses, we observed elevated p‐STAT3 protein in nuclei of satellite cells after stimulation with 1 ng/ml IL‐6. On binding of IL‐6 to its receptor IL‐6R, JAK2 is phosphorylated, then STAT3 is phosphorylated by JAK2. Subsequently, p‐STAT3 is translocated to the nucleus where it activates gene transcription 29. The JAK2/STAT3 pathway has been shown to induce genes that regulate cell cycle progression 17, 18, 19. Among these, genes for cyclins such as cyclin D1 are well known effectors of the G1 to S cell cycle phase transition 21. In particular, cyclin D1 is necessary for proliferation of myogenic cells 30. Serrano et al. have demonstrated that forced activation of STAT3 restored cyclin D1 expression in myoblasts from IL‐6 knockout mice and concomitantly restored proliferative potential. These investigators demonstrated significantly reduced p‐STAT3 levels in satellite cells and significantly depressed cyclin D1 expression in IL‐6 knockout mice 17. In the present study, we demonstrated that 1 ng/ml IL‐6 induced cyclin D1 mRNA and led to an increase in numbers of cyclin D1+/MyoD+ cells after 6 h. These findings suggest that activation of cyclin D1 via the IL‐6/JAK2/STAT3 signalling pathway contributed to increased satellite cell proliferation.
We found that 0.01–1 ng/ml IL‐6 induced dose‐dependent increases in cell proliferation, whereas higher concentrations of IL‐6 prevented satellite cell proliferation. Therefore, we hypothesized that high concentrations of IL‐6 may induce downstream cell‐cycle inhibitors p21waf1 and p27kip1, however, our results did not support this hypothesis. The JAK2/STAT3 signalling pathway is moderated by a negative feedback loop involving SOCS3 29. As a downstream target gene of the JAK2/STAT3 signalling pathway 29, SOCS3 may contribute to reduction of nuclear p‐STAT3 that is necessary for differentiation 31, 32. Furthermore, overexpression of SOCS3 has been shown to induce cell differentiation 28. In this study, treatment with 10 ng/ml IL‐6 increased SOCS3 mRNA expression by 63%, but no increase in p‐STAT3 protein expression was observed in nuclei of the satellite cells. Presumably, this increase in SOCS3 expression may prevent IL‐6‐induced satellite cell proliferation by inducing differentiation. This hypothesis is supported by our data demonstrating prevention of increased BrdUrd+ cell numbers after treatment with 10 ng/ml IL‐6. Furthermore, 10 ng/ml IL‐6 increased SOCS3 mRNA expression (by 63%). In addition, we observed localization of SOCS3 protein in myogenin‐positive cells following treatment with 10 ng/ml IL‐6. Although we did not determine whether 10 ng/ml IL‐6 induced satellite cell differentiation, it is possible that the IL‐6/JAK2/STAT3/SOCS3 cascade may contribute to regulation of this process. Further investigations are required to clarify the role of the IL‐6/JAK2/STAT3/SOCS3 pathway in preventing satellite cell proliferation.
In the present study, treatment with 10 ng/ml IL‐6 induced greater SOCS3 mRNA expression than 1 ng/ml IL‐6 and control media. However, details of this bi‐phasic effect remain unclear. Initially, IL‐6 binds receptor IL‐6R, recruits intracellular signal transducer gp130 and initiates various signalling pathways 33. A soluble form of IL‐6R (sIL‐6R) is often secreted, and IL‐6/sIL‐6R complexes have been shown to bind gp130 and activate signal transduction pathways 34. STAT3 regulates a number of its upstream signalling cascade members including IL‐6, gp130 and IL‐6R 19. It is unclear whether sIL‐6R is also regulated by STAT3. However, spontaneous release of sIL‐6R from a human myeloma cell line (U266 cells) has been observed 35. Although we were unable include analyses of IL‐6R, sIL‐6R and gp130 in this study, treatment with 10 ng/ml IL‐6 led to excessive expression of IL‐6/IL‐6R and sIL‐6R/gp130 complexes, and may activate excessive IL‐6 signalling. In support of this, Scheele et al. (36) showed that myocytes from obese patients initiated an IL‐6‐negative control system that protected against elevated levels of circulating IL‐6 36. Thus, high concentrations of IL‐6 may induce negative control systems, such as SOCS3, to protect against excessive IL‐6 signalling.
Various signalling pathways are activated by IL‐6, including JAK/STAT 20, 37, ERK, MEK, MAPK 38 and p38 MAPK pathways 39. Sun et al. have demonstrated that the LIF/JAK1/STAT1/STAT3 pathway promotes proliferation and prevents differentiation of myoblast in vitro 40 and Wang et al. also revealed a novel role for the JAK2/STAT2/STAT3 pathway in muscle cell differentiation in vitro 41. Although we have shown here that IL‐6 activates the JAK2/STAT3 pathway, we were not able to confirm that IL‐6 activated other pathways in satellite cells. The ERK/MAPK signalling pathway induces cell cycle progression by upregulating expression of cyclin D1 42. Spangenburg et al. have revealed that LIF‐induced satellite cell proliferation was not ablated in the presence of a MEK inhibitor 24. Moreover, previous research suggested that IL‐6 induced STAT3 rather than ERK may regulate satellite cell proliferation 17. In accordance with these studies, MEK/ERK inhibitors failed to block cell proliferation in response to IL‐6 here. On the other hand, the p38 MAPK signalling pathway plays an important role in cell differentiation by upregulating MEF2C 43. Accordingly, a previous study with p38 MAPK inhibitor demonstrated reduced expression of differentiation markers in culture 44. Although we did not investigate satellite cell differentiation, we confirmed that p38 MAPK inhibition did not contribute to IL‐6 induced satellite cell proliferation. These findings suggest that JAK2/STAT3 signalling predominates over the MAPK signalling pathway as major mediator of satellite cell responses to IL‐6.
Elevated circulating IL‐6 is associated with muscle atrophy/wasting in many diseases, such as cancer 10. In addition, age‐related increases in serum IL‐6 have been associated with reduced muscle mass 9. Although these studies did not focus on satellite cell responses to IL‐6, elevated IL‐6 may also cause negative effects on satellite cell functions. High concentrations of IL‐6 (10 ng/ml) did not cause satellite cell proliferation by inducing SOCS3 in the present study. In support of this result, IL‐6 infusion into animals caused muscle atrophy that was characterized by preferential loss of myofibrillar protein and increased expression of SOCS3 mRNA 8. The present data provide further evidence that high concentrations of IL‐6 may cause muscle atrophy by reducing satellite cell function.
The results of this investigation demonstrate that IL‐6 plays an important role in both positive and negative regulations of satellite cell proliferation by activating the JAK2/STAT3 signalling pathway. Importantly, contrasting satellite cell responses were observed depending on concentration of IL‐6. These results explain previous observations of IL‐6 mediated muscle atrophy, but indicate the necessity of IL‐6 as a stimulant of muscle growth.
Acknowledgements
We would like to thank Dr. Jonathan Peake at Queensland University of Technology, Australia, for carefully reading this manuscript and for valuable comments. This work was supported by Grant‐in‐Aid for Young Scientists A (#18680048 to SM), Young Scientists B (#22700659 to MK) and Scientific Research A (#18200041 to SM) from the Ministry of Education, Culture, Sports, Science and Technology, and the Japan Society for the Promotion of Science, respectively. Tokai University Supporters Association Research and Study Grant also supported this study.
References
- 1. Mauro A (1961) Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91, 534–551. [DOI] [PubMed] [Google Scholar]
- 3. Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P et al (2005) The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch. 451, 319–327. [DOI] [PubMed] [Google Scholar]
- 4. Ten Broek RW, Grefte S, Von den Hoff JW (2010) Regulatory factors and cell populations involved in skeletal muscle regeneration. J. Cell. Physiol. 224, 7–16. [DOI] [PubMed] [Google Scholar]
- 5. Yablonka‐Reuveni Z (2011) The skeletal muscle satellite cell: still young and fascinating at 50. J. Histochem. Cytochem. 59, 1041–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle‐derived interleukin‐6. Physiol. Rev. 88, 1379–1406. [DOI] [PubMed] [Google Scholar]
- 7. Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A et al (1995) Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin‐6 transgenic mouse. Biochem. Biophys. Res. Commun. 207, 168–174. [DOI] [PubMed] [Google Scholar]
- 8. Haddad F, Zaldivar F, Cooper DM, Adams GR (2005) IL‐6‐induced skeletal muscle atrophy. J. Appl. Physiol. 98, 911–917. [DOI] [PubMed] [Google Scholar]
- 9. Pereira LS, Narciso FM, Oliveira DM, Coelho FM, Souza Dda G, Dias RC (2009) Correlation between manual muscle strength and interleukin‐6 (IL‐6) plasma levels in elderly community‐dwelling women. Arch. Gerontol. Geriatr. 48, 313–316. [DOI] [PubMed] [Google Scholar]
- 10. Baltgalvis KA, Berger FG, Peña MM, Davis JM, White JP, Carson JA (2009) Muscle wasting and interleukin‐6‐induced atrogin‐I expression in the cachectic Apc (Min/+) mouse. Pflugers Arch. 457, 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al‐Shanti N, Stewart CE (2012) Inhibitory effects of IL‐6 on IGF‐1 activity in skeletal myoblasts could be mediated by the activation of SOCS‐3. J. Cell. Biochem. 113, 923–933. [DOI] [PubMed] [Google Scholar]
- 12. Kurek JB, Bower JJ, Romanella M, Koentgen F, Murphy M, Austin L (1997) The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve 20, 815–822. [DOI] [PubMed] [Google Scholar]
- 13. Spangenburg EE, Booth FW (2006) Leukemia inhibitory factor restores the hypertrophic response to increased loading in the LIF(‐/‐) mouse. Cytokine 34, 125–130. [DOI] [PubMed] [Google Scholar]
- 14. Washington TA, White JP, Davis JM, Wilson LB, Lowe LL, Sato S et al (2011) Skeletal muscle mass recovery from atrophy in IL‐6 knockout mice. Acta Physiol. 202, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austin L, Bower J, Kurek J, Vakakis N (1992) Effects of leukaemia inhibitory factor and other cytokines on murine and human myoblast proliferation. J. Neurol. Sci. 112, 185–191. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Wu H, Zhang Z, Liu S, Yang J, Chen X et al (2008) Effects of interleukin‐6, leukemia inhibitory factor, and ciliary neurotrophic factor on the proliferation and differentiation of adult human myoblasts. Cell. Mol. Neurobiol. 28, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serrano AL, Baeza‐Raja B, Perdiguero E, Jardí M, Muñoz‐Cánoves P (2008) Interleukin‐6 is an essential regulator of satellite cell‐mediated skeletal muscle hypertrophy. Cell Metab. 7, 33–44. [DOI] [PubMed] [Google Scholar]
- 18. McKay BR, De Lisio M, Johnston AP, O'Reilly CE, Phillips SM, Tarnopolsky MA et al (2009) Association of interleukin‐6 signalling with the muscle stem cell response following muscle‐lengthening contractions in humans. PLoS ONE 4, e6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toth KG, McKay BR, De Lisio M, Little JP, Tarnopolsky MA, Parise G (2011) IL‐6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS One 6, e17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heinrich PC, Behrmann I, Müller‐Newen G, Schaper F, Graeve L (1998) Interleukin‐6‐type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukada T, Ohtani T, Yoshida Y, Shirogane T, Nishida K, Nakajima K et al (1998) STAT3 orchestrates contradictory signals in cytokine‐induced G1 to S cell‐cycle transition. EMBO J. 17, 6670–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moran DM, Mattocks MA, Cahill PA, Koniaris LG, McKillop IH (2008) Interleukin‐6 mediates G(0)/G(1) growth arrest in hepatocellular carcinoma through a STAT 3‐dependent pathway. J. Surg. Res. 147, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao B, Wang H, Lafdil F, Feng D (2012) STAT proteins ‐ key regulators of anti‐viral responses, inflammation, and tumorigenesis in the liver. J. Hepatol. 57, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spangenburg EE, Booth FW (2002) Multiple signaling pathways mediate LIF‐induced skeletal muscle satellite cell proliferation. Am. J. Physiol. Cell Physiol. 283, C204–211. [DOI] [PubMed] [Google Scholar]
- 25. Plaisance I, Morandi C, Murigande C, Brink M (2008) TNF‐alpha increases protein content in C2C12 and primary myotubes by enhancing protein translation via the TNF‐R1, PI3K, and MEK. Am. J. Physiol. Endocrinol. Metab. 294, E241–250. [DOI] [PubMed] [Google Scholar]
- 26. Kook SH, Choi KC, Son YO, Lee KY, Hwang IH, Lee HJ et al (2008) Involvement of p38 MAPK‐mediated signaling in the calpeptin‐mediated suppression of myogenic differentiation and fusion in C2C12 cells. Mol. Cell. Biochem. 310, 85–92. [DOI] [PubMed] [Google Scholar]
- 27. Machida S, Booth FW (2004) Increased nuclear proteins in muscle satellite cells in aged animals as compared to young growing animals. Exp. Gerontol. 39, 1521–1525. [DOI] [PubMed] [Google Scholar]
- 28. Caldow MK, Steinberg GR, Cameron‐Smith D (2011) Impact of SOCS3 overexpression on human skeletal muscle development in vitro. Cytokine 55, 104–109. [DOI] [PubMed] [Google Scholar]
- 29. Ivashkiv LB, Hu X (2004) Signaling by STATs. Arthritis Res. Ther. 6, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei Q, Paterson BM (2001) Regulation of MyoD function in the dividing myoblast. FEBS Lett. 490, 171–178. [DOI] [PubMed] [Google Scholar]
- 31. Snyder M, Huang XY, Zhang JJ (2008) Identification of novel direct Stat3 target genes for control of growth and differentiation. J. Biol. Chem. 283, 3791–3798. [DOI] [PubMed] [Google Scholar]
- 32. Spangenburg EE (2005) SOCS‐3 induces myoblast differentiation. J. Biol. Chem. 280, 10749–10758. [DOI] [PubMed] [Google Scholar]
- 33. Kalai M, Montero‐Julian FA, Grötzinger J, Fontaine V, Vandenbussche P, Deschuyteneer R et al (1997) Analysis of the human interleukin‐6/human interleukin‐6 receptor binding interface at the amino acid level: proposed mechanism of interaction. Blood 89, 1319–1333. [PubMed] [Google Scholar]
- 34. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller‐Newen G, Schaper F (2003) Principles of interleukin (IL)‐6‐type cytokine signalling and its regulation. Biochem. J. 374, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakajima T, Yamamoto S, Cheng M, Yasukawa K, Hirano T, Kishimoto T et al (1992) Soluble interleukin‐6 receptor is released from receptor‐bearing cell lines in vitro. Jpn. J. Cancer Res. 83, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scheele C, Nielsen S, Kelly M, Broholm C, Nielsen AR, Taudorf S et al (2012) Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL‐6. PLoS One 7, e39657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F et al (1995) A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin‐6. EMBO J. 14, 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ueda T, Bruchovsky N, Sadar MD (2002) Activation of the androgen receptor N‐terminal domain by interleukin‐6 via MAPK and STAT3 signal transduction pathways. J. Biol. Chem. 277, 7076–7085. [DOI] [PubMed] [Google Scholar]
- 39. Baeza‐Raja B, Muñoz‐Cánoves P (2004) p38 MAPK‐induced nuclear factor‐kappaB activity is required for skeletal muscle differentiation: role of interleukin‐6. Mol. Biol. Cell 15, 2013–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun L, Ma K, Wang H, Xiao F, Gao Y, Zhang W et al (2008) JAK1‐STAT1‐STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J. Cell Biol. 179, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang K, Wang C, Xiao F, Wang H, Wu Z (2008) JAK2/STAT2/STAT3 are required for myogenic differentiation. J. Biol. Chem. 283, 34029–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Torii S, Yamamoto T, Tsuchiya Y, Nishida E (2006) ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci. 97, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zetser A, Gredinger E, Bengal E (1999) p38 mitogen‐activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 274, 5193–5200. [DOI] [PubMed] [Google Scholar]
- 44. Cabane C, Englaro W, Yeow K, Ragno M, Dérijard B (2003) Regulation of C2C12 myogenic terminal differentiation by MKK3/p38alpha pathway. Am. J. Physiol. Cell Physiol. 284, C658–666. [DOI] [PubMed] [Google Scholar]


