Abstract
Objectives
Pulmonary arterial hypertension (PAH) is a fast progressing vascular disease characterized by uncontrolled cell proliferation of pulmonary artery smooth muscle cells (PASMCs). Some studies have suggested that PAH and cancers share an apoptosis‐resistant state, featuring excessive cell proliferation. The miR‐34 family consists of tumour‐suppressive miRNAs, and its reduced expression has been reported in numerous cancers; however, its role in hypoxia‐induced PAH has not been previously studied.
Materials and methods
miR‐34 family expression was evaluated in a rat model with hypoxia and in cultured hypoxic PASMCs, using real‐time quantitative PCR (RT‐qPCR). Function of miR‐34 family was assessed by transfecting miR‐34 mimics and inhibitors. Dual luciferase reporter gene assays, RT‐qPCR and Western blotting were performed to validate target genes of miR‐34.
Results
Significant down‐regulation of miR‐34a in hypoxic lung tissue, pulmonary artery and PASMCs was identified and then effects of miR‐34a in modulating cell proliferation in human pulmonary artery smooth muscle cells (hPASMCs) was investigated in vitro. Reduction of miR‐34a levels in hPASMCs caused increased proliferation and these effects were reversed by overexpression of miR‐34a. miR‐34a overexpression down‐regulated platelet‐derived growth factor receptor alpha (PDGFRA) expression, which is a key factor in PAH development. These results suggest that miR‐34a is a potential regulator of proliferation in PASMCs, and that it could be used as a novel treatment strategy in PAH.
1. Introduction
Pulmonary arterial hypertension (PAH) is a fast progressing vascular disease characterized by uncontrolled cell proliferation and reduced apoptosis of pulmonary artery smooth muscle cells (PASMCs),1, 2 leading to right ventricular failure and ultimately death. Although therapeutic progress has been achieved in PAH patients, survival rates still remain exceedingly low. The pathogenesis of PAH is not clear, hypoxia is the important cause.
MicroRNAs (miRNAs) have been identified as a novel class of short single‐stranded small RNA molecules (~22 nucleotides). miRNAs regulate gene expression by binding and targeting mRNAs for degradation or suppressing translation,3 and notably, miRNAs may control 30% of all genes in human genomes.4 A number of studies reflect that miRNAs have essential functions in various kinds of biological processes, including cellular proliferation,5 differentiation6, 7 and apoptosis.8 It has been elucidated that miRNAs play critical roles in hypoxia‐induced PAH.9, 10, 11, 12, 13, 14 Zhu et al. and other lab have found several miRNA target genes, including L‐type calcium channel‐α1, Mst1, BMPR2 and NFAT to be predicted to be highly enriched in PAH‐associated pathways,10, 15, 16, 17, 18 suggesting extensive miRNA‐regulated control of this disease. Nevertheless, their molecular roles in pathogenesis remain unclear.
Voelkel et al.19 and Archer et al.20 proposed that both PAH and cancer shared characteristics by excessive cell proliferation and resistance to apoptosis. miR‐34a is down‐regulated in many forms of cancers.21, 22, 23, 24, 25 In our study, in vivo experiments showed that miR‐34a was down‐regulated in the rat lung tissue and distal pulmonary arteries (PAs). Based on this evidence, the effect of miR‐34a in modulating cell proliferation was investigated. We report for the first time that overexpression of miR‐34a under hypoxic conditions decreases proliferation and inhibits pulmonary vascular remodelling. miR‐34a also reduced PDGFRA protein expression levels in hPASMCs. The findings suggested that miR‐34a could act as novel therapeutic target for anti‐remodelling drugs.
2. Material and methods
For detailed Material and methods, please see Data S1 (Tables S1–S3).
3. Results
3.1. miR‐34a levels are down‐regulated in hypoxia‐induced PAH and PASMCs
Uncontrolled cell proliferation and reduced apoptosis of PASMCs were a major component leading to the development of pulmonary remodelling and previous studies have showed that miR‐34a expression was altered by hypoxia.26 To investigate whether the expression of miR‐34a was the underlying factor in hypoxia‐induced PAH. We detected miR‐34 family expression in normoxia and hypoxia‐induced PAH using qRT‐PCR analysis. As shown in Fig. 1, miR‐34a level was significantly down‐regulated in the rat lung tissue and distal PAs (Fig. 1a,b), but was not changed in miR‐34b and miR‐34c. To characterize whether down‐regulated miR‐34a levels were specific to the lung in rats with pulmonary hypertension, we compared organ‐specific levels of miR‐34a between normal and pulmonary hypertensive rats. The results showed that miR‐34a levels were only down‐regulated in the lung and PAs but not in the heart, kidney, liver and aorta of hypoxic rats compared to normal rats (Fig. S1). Next, we cultured rat PASMCs and human PASMCs to examine the miR‐34 family expression in vitro experiment. The PASMCs were identified by smooth muscle cell α‐actin antibody.27 Immunofluorescence revealed that the purity of PASMCs was more than 98% (Fig. S2). Real‐time PCR results showed hypoxia only inhibited the miR‐34a expression (Fig. 1c,d).
Figure 1.
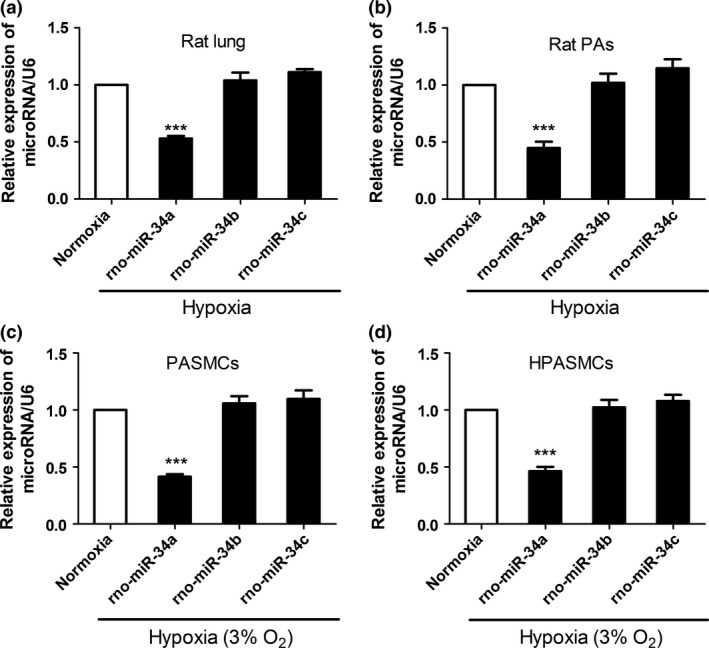
Hypoxia down‐regulated the expression of miR‐34 family members in pulmonary arterial hypertension (PAH). (a) miR‐34a is down‐regulated in hypoxia‐induced PAH rat. The expression of miR‐34 family members were estimated in rat lung (n=6). A significant down‐regulation in the expression of miR‐34a in hypoxic PAH rat compared to normoxia was observed, whereas the expression of miR‐34b and miR‐34c is not obvious change. (b) The expression of miR‐34a was down‐regulated in distal pulmonary arteries. (c, d) Hypoxia down‐regulated the expression of miR‐34a in rat pulmonary artery smooth muscle cells (PASMCs) and human PASMCs (***P<0.0001)
3.2. Nebulization of miR‐34a mimics reverses hypoxia‐induced PAH
To test whether restoration of miR‐34a level can reverse symptoms of PAH in the rat model, synthetic miR‐34a RNA molecules were selectively delivered to the lung of hypoxia‐induced PAH rats by intratracheal nebulization. As shown in Fig. 2a, miR‐34a restoration decreases mean PA pressure. To determine whether synthetic miR‐34a delivery can reduce PA remodelling in hypoxia‐induced PAH animals, we measured medial wall thickness. We observed that hypoxia for 3 weeks caused significant increases in thickness of the pulmonary vascular walls in the smooth muscle layer of pulmonary arterioles of hypoxia group. Hypoxia failed to increase medial thickness of the pulmonary vascular walls in the smooth muscle layer of pulmonary arterioles in the synthetic miR‐34a treatment (Fig. 2b,c). These results indicated that miR‐34a mimic treatment prevents hypoxia‐induced pulmonary hypertension and pulmonary vascular remodelling.
Figure 2.
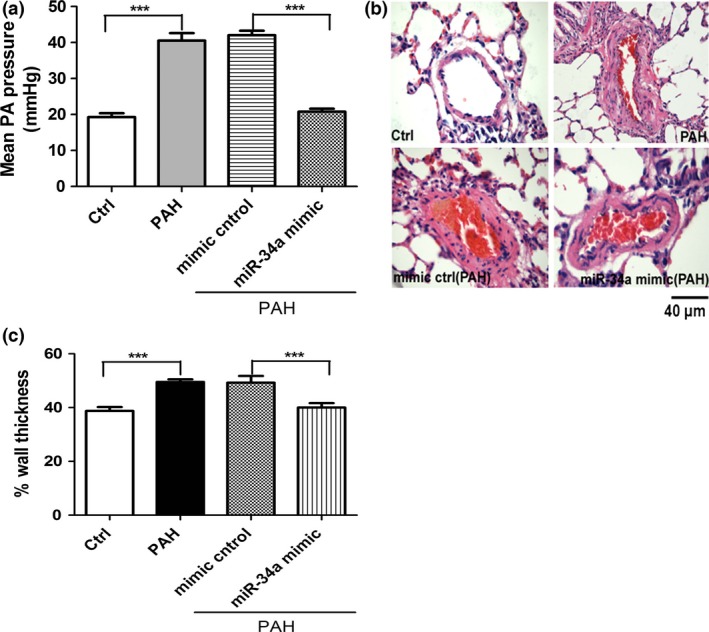
Restoring miR‐34a level reverses hypoxia‐induced pulmonary arterial hypertension. (a) miR‐34a mimic decreases mean pulmonary arterie (PA) pressure. Mean PA pressure measured by right catheterization in closed chest rats (n=4 rats per group) is shown. (b) miR‐34a mimic decreases PA wall thickness. PA remodelling was measured by the percentage of media wall thickness on lung sections stained by haematoxylin and eosin (n=5). (c) Wall thickness of pulmonary vascular. Hypoxia significantly increased wall thickness of pulmonary vascular compared with normoxic rats, which was reversible by administration of miR‐34a mimic to rats (***P<0.0001, n=6)
3.3. The overexpression of miR‐34a decreases proliferation in hPASMCs
miR‐34a expression levels were found to be significantly lower in rat with pulmonary hypertension induced by hypoxia as compared to normoxic rat and suggest that miR‐34a may play a key role in regulating the proliferative phenotype of the pulmonary vasculature. To demonstrate the functional role of miR‐34a, hPASMCs were transfected with miR‐34a inhibitor or miR‐34a mimics. Notably, miR‐34a inhibitor enhanced the growth of hPASMCs significantly compared to miRNA inhibitor control. In contrast, miR‐34a mimic reduced cell number of hPASMCs compared with miRNA mimic control (Fig. 3a,b). PCNA (proliferating cell nuclear antigen) has been generally regarded as a hallmark for cellular proliferation. Western blots revealed that the expression of PCNA was obviously increased by hypoxia or miR‐34a inhibitor compared to normoxia or miRNA inhibitor control in hPASMCs, whereas miR‐34a mimic inhibited the up‐regulation of PCNA induced by hypoxia when compared with miRNA mimic control in hPASMCs (Fig. 3c,d). Collectively, these results implicate that miR‐34a inhibits the proliferation of hPASMCs.
Figure 3.
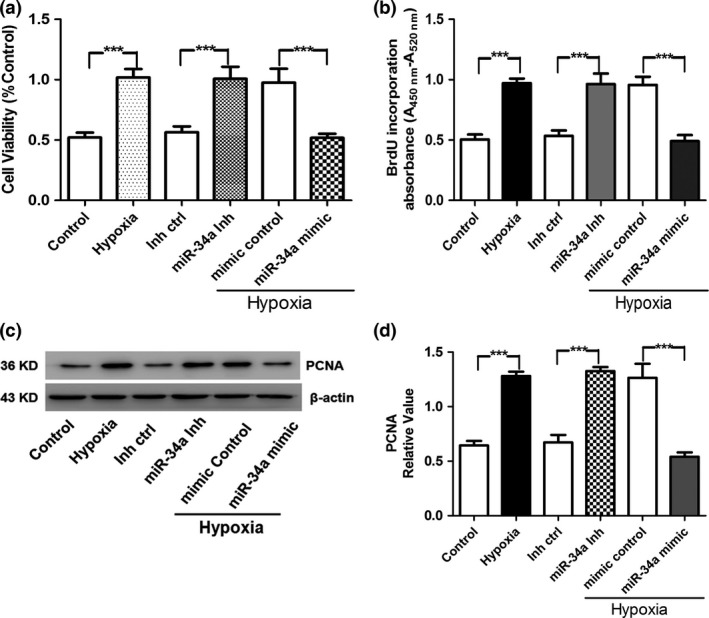
Role of miR‐34a in hypoxia‐induced pulmonary artery smooth muscle cell (PASMC) proliferation. (a) hPASMC viability was determined by measuring MTT after hypoxia and miR‐34a treatment. (b) BrdU incorporation experiment was performed to detect the proliferation of hPASMCs. (c, d) Proliferating cell nuclear antigen (PCNA) is a critical eukaryotic replication accessory factor that supports DNA binding in DNA processing. It participates in the progress of DNA replication, repair, and recombination. PCNA consists of three toroidal‐shaped monomers that encircle doublestranded DNA. PCNA protein was detected by Western blot in cultured hPASMCs after hypoxia and miR‐34a mimic treatment (***P<0.0005)
3.4. miR‐34a resisted the proliferation of hPASMCs via the inhibition of Cyclin A and Cyclin E
We further examined whether miR‐34a changed the expression of cell cycle regulators, including Cyclin A and Cyclin E in hPASMCs after transfection of miR‐34a mimics and inhibitor. Western blots displayed that expression of Cyclin A and Cyclin E was elevated by hypoxia and miR‐34a inhibitor, whereas the expression of these proteins was decreased by miR‐34a mimic induced by hypoxia (Fig. 4a,b), indicating that down‐regulation of miR‐34a contributed to the cell cycle activity alternation in hypoxia condition.
Figure 4.
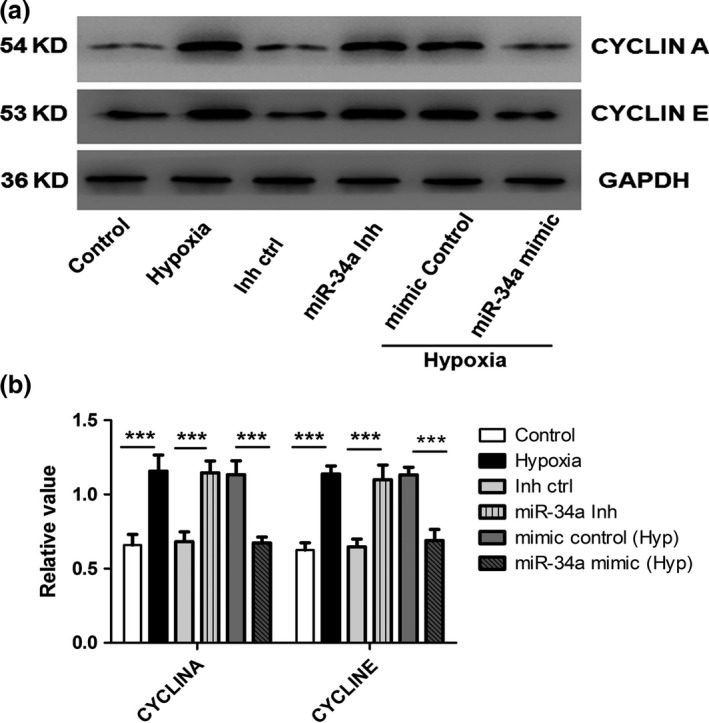
Role of miR‐34a on hypoxia‐induced human pulmonary artery smooth muscle cells (hPASMCs') cell cycle progress. (a, b) Protein expressions of cyclin A and cyclin D were detected by Western blot in cultured hPASMCs. Both the hypoxia and miR‐34a inhibitor increased the cyclin A and cyclin E expression, whereas miR‐34a mimic attenuated the hypoxia‐increased cyclin A and cyclin E expression in PASMCs (***P<0.0001)
3.5. PDGFA was a direct target of miR‐34a in hPASMCs
To gain novel insights into molecular mechanisms underlying the function of miR‐34a in regulating hPASMCs, we identified the targets of miR‐34a. Using miRNA predict software, namely TargetScan, we predicted that PDGFRA was a binding target of miR‐34a and there are ≥7 nucleotides that match the 5′ end of miR‐34a in rats and human (Fig. 5a). To verify whether PDGFRA was direct targets of miR‐34a, PDGFRA 3′UTR, containing two miR‐34a binding sites (Fig. 5b), was cloned downstream of the luciferase open reading frame. Then, luciferase reporter assays were performed in HEK293 cells that did not express miR‐34a in order to test whether miR‐34a regulates PDGFRA expression. The luciferase reporter gene linked to the wild‐type 3′UTR of PDGFRA was transfected into HEK293 cells, and co‐transfection of miR‐34a mimic resulted in a significant decrease of luciferase activity, but was not changed in co‐transfection of miR‐34a inhibitor. Reporter plasmid with mutant sequence of PDGFRA produced no change in luciferase activity, indicating a direct interaction between the miRNAs and PDGFRA 3′UTRs (Fig. 5c). Next, we tested whether the expression of PDGFRA was altered by miR‐34a in cultured hPASMCs. Real‐time PCR and Western blotting showed that PDGFRA was expressed at a higher level in hypoxia and miR‐34a inhibitor treatment than in control and inhibitor control. In contrast, application of miR‐34a mimic repressed the up‐regulation of PDGFRA induced by hypoxia (Fig. 5d,e). Taken together, these results indicate that miR‐34a is the direct target of PDGFRA.
Figure 5.
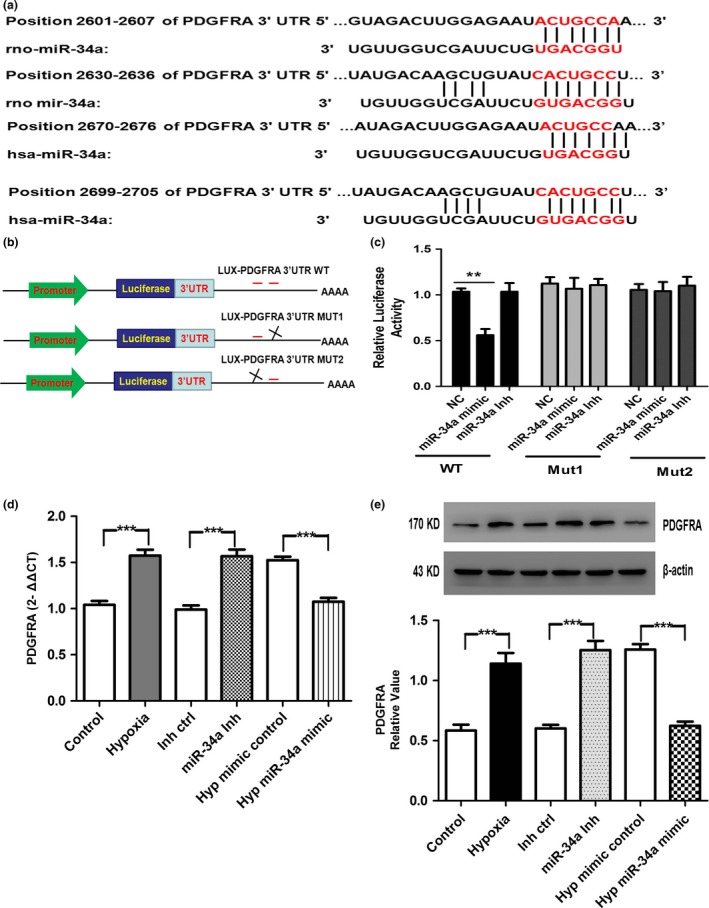
miR‐34a targeted PDGFRA 3′UTRs. (a) The imprecisely complementary sequences of miR‐34a with PDGFRA 3′UTRs is shown. (b) PGL3 control‐PDGFRA constructs containing two PDGFRA‐binding sites (in red). Deletion of one of the two PDGFRA sites was used to generate the mutant luciferase plasmids. (c) PDGFRA 3′UTRs is direct targets of miR‐34a. pGL3‐PDGFRA luciferase constructs, containing a wild‐type (left side of the histograms) or mutated (right side of the histograms) PDGFRA a3′UTRs, were transfected into HEK293 T cells. miR‐34a mimic significantly decreased the luciferase activity compared with negative control (NC), whereas miR‐34a inhibitor did not affect the luciferase activity. The reporter assays were performed three times with essentially identical results. (d, e) Hypoxia and miR‐34a inhibitor significantly increased the mRNA and protein expression of PDGFRA. However, miR‐34a mimic significantly blocked the hypoxia‐induced up‐regulation (n=3, respectively) (**P<0.01; ***P<0.0001)
3.6. miR‐34a mimic decreases cellular migration in hPASMCs
It has been reported that proliferation and migration of human vascular SMC contribute to vascular remodeling in pulmonary hypertension and atherosclerosis.28 We studied the effect of miR‐34a overexpression on hypoxia‐induced hPASMCs migration using the wound healing (migration) assay. Firstly, hPASMCs were transfected with the respective miRs. Then, wound field was created and photographs were taken at 0 and 24 hours via microscopy to analyse the degree of migration in treatment group. As shown in Fig. 6, hypoxia and miR‐34a inhibitor increased hPASMCs migration, but miR‐34a mimic reversed the increasing migration induced by hypoxia. These results suggest that overexpression of miR‐34a resulted in reduced cell migration in hypoxia‐induced increase in cell migration.
Figure 6.
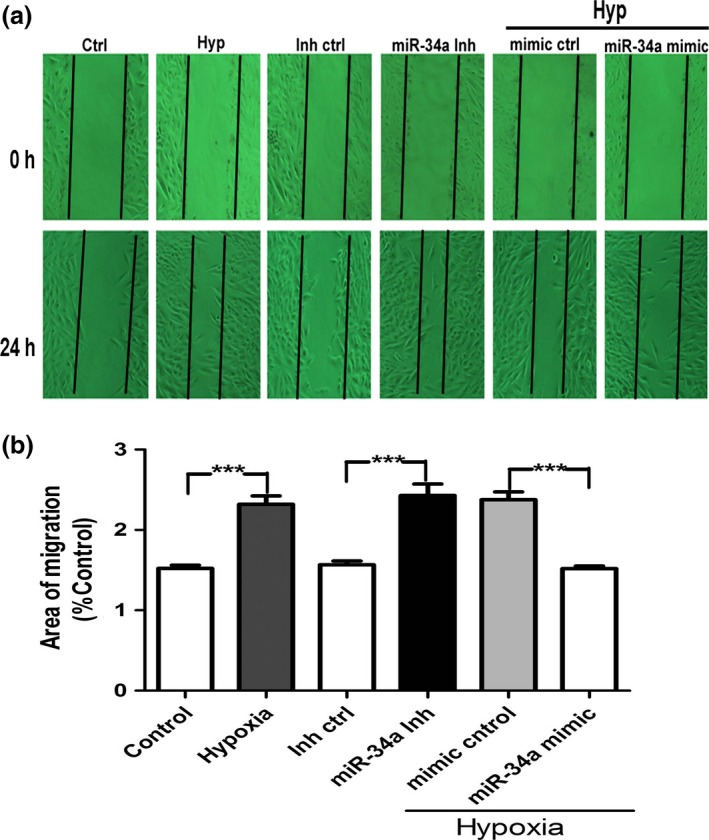
miR‐34a decreases migration of human pulmonary artery smooth muscle cells (hPASMCs). Migration of hPASMCs was transfected with miR‐34a mimic, miR‐34a inhibitor, and control miR was assessed by wound healing assay. Hypoxia or miR‐34a inhibitor increased the hPASMCs migration, but miR‐34a mimetic inhibited the increased migration induced by hypoxia. The data are presented as the mean±SD. n=6 experiments. Hyp indicates hypoxia; Inh ctrl indicates inhibitor negative control; miR‐34a inh indicates miR‐34a inhibitor (***P<0.0001)
3.7. miR‐34a mimic decreases DNA damage and promotes apoptosis in hypoxia hPASMCs
DNA damage signalling pathway is important for PAH development. We measured DNA damage in distal PAs from hypoxic rats and normal rats, using the expression of the γ‐H2AX (a DNA damage marker) by Western blotting. As shown in Fig. 7a, DNA damage is significantly increased in PAH in comparison with normal tissue controls, and miR‐34a mimic decreases DNA damage in PAH. DNA damage is sustained in cultured hPASMCs in hypoxia, miR‐34a inhibitor and miRNA mimics control treatment in comparison with normal control. The expression of γ‐H2AX in hPASMCs is reduced by the addition of the miR‐34a mimics in hypoxia condition, suggesting that miR‐34a is important for the repair of these damages (Fig. 7b,c). We next studied the implication of miR‐34a in hypoxic hPASMCs survival. We found that hPASMCs have a significant apoptosis‐resistant in hypoxia in comparison with control hPASMCs. However, the number of TUNEL‐positive cells was increased after addition of the miR‐34a mimics in hypoxia condition (Fig. 7d,e).
Figure 7.
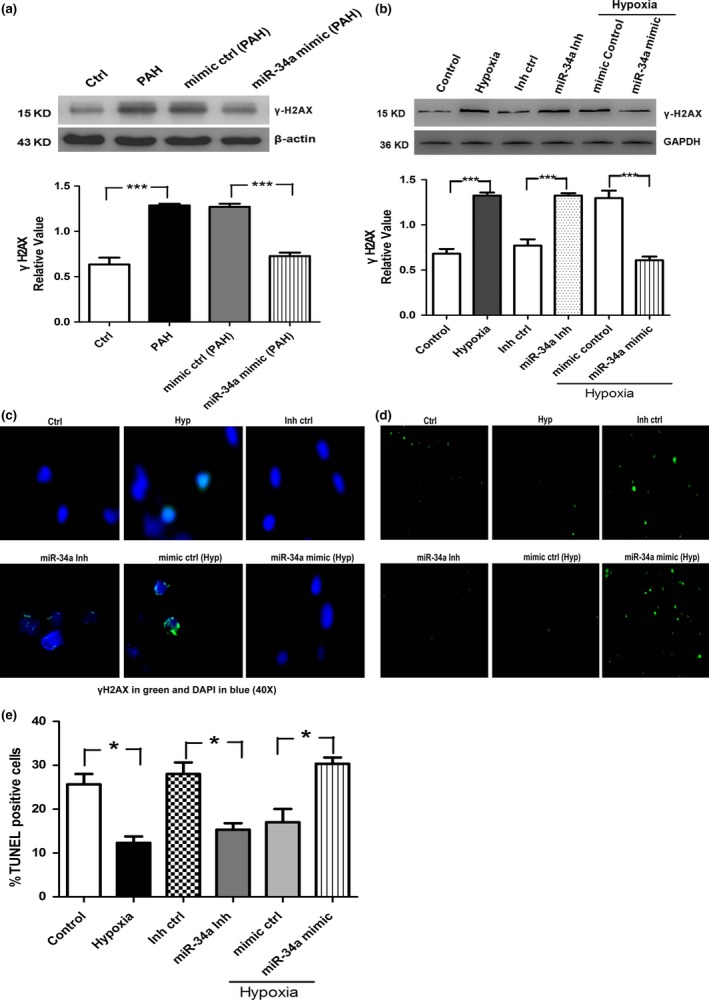
DNA damage is increased in pulmonary arterial hypertension distal pulmonary arteries and hypoxic hPASMCs. (a) DNA damage (γH2AX) was quantified in distal pulmonary arteries by Western blotting. (b, c) Hypoxic hPASMCs are also associated with increased DNA damage measured by γH2AX protein expression and nuclear staining. (d) Representative photographs of TUNEL staining in different groups. (e) Quantitative analysis of TUNEL‐positive cells content among groups (*P<0.05; ***P<0.0001)
3.8. miR‐34a mimic strongly increased the expression of KCNK3 and reduced NFATc2 expression and activation in hPASMCs
Recently, KCNK3 has been identified as a new predisposing gene for PAH by whole‐exome sequencing,29 and loss of KCNK3 is a key event in PAH pathogenesis. By Western blot, we demonstrated that hypoxia reduces the expression of KCNK3, and miR‐34a mimic increases KCNK3 expression in hypoxia (Fig. 8a,b). The KCNK3 channel participates in the regulation of plasma membrane resting potential and the decreased KCNK3 expression could lead to vasoconstriction. Next, we provide evidence that the cytosolic calcium concentration is investigated in hPASMCs. Results showed that [Ca2+]i FLuo‐3 intensity is increased by hypoxia and miR‐34a mimic weakened the [Ca2+]i FLuo‐3 intensity by hypoxia in hPASMCs (Fig. 8c,d).
Figure 8.
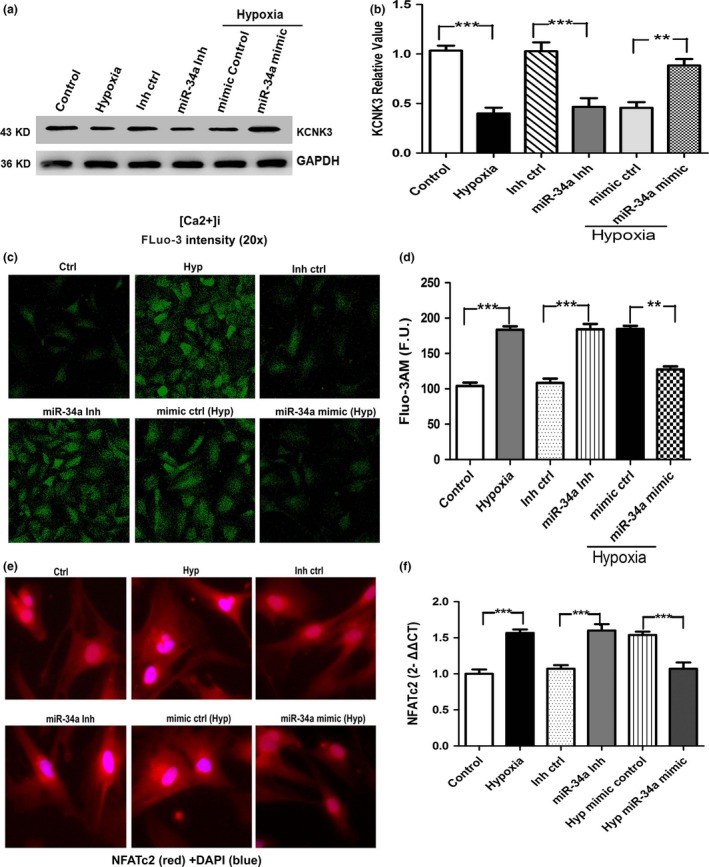
(a, b) KCNK3 protein was detected by Western blot in cultured hPASMCs after hypoxia and miR‐34a mimic treatment. (c, d) miR‐34a mimic in hypoxic hPASMCs decreases [Ca2+]i FLuo‐3. (e, f) miR‐34a mimic decreases NFATc2 activation and expression measured by immunofluorescence and real‐time PCR (n=3, respectively) (**P<0.01; ***P<0.0001)
4. Discussion
miR‐34a has been well‐studied in various cancer cells21, 22, 23, 24, 25; however, little is known about its expression and function in hPASMCs in hypoxia. Our study is the first to investigate that miR‐34a targets PDGFRA expression, resulting in hypoxia‐induced PASMC proliferation and vascular remodelling. In our experiment, we found that miR‐34a is significantly down‐regulated in hypoxic rat PAH compared to normoxia. Consistent with the in vivo experiment, our in vitro studies using PASMCs show that hypoxia down‐regulates the expression of miR‐34a. miR‐34a directly binds to the 3′‐UTR of PDGFRA mRNA, leading to the decreased expression of PDGFRA. These findings provide new insight into miR‐34a's function as a negative regulator of PDGFRA expression, which may underline the aetiology of hypoxia pulmonary hypertension.
miRNAs may contribute to the pathogenesis of PAH and represent a possible therapeutic target for altering the remodelling phenotype in the pulmonary vasculature. Caruso et al. has found miR‐22, miR‐30, and let‐7f were down‐regulated while miR‐322 and miR‐451 were up‐regulated in two commonly used rodent models of PAH [exposure to chronic hypoxia and monocrotaline (MCT) insult in the rat].9 Other study showed that in human PAs from PAH patients, miR‐204 was down‐regulated in PAs and PASMCs from idiopathic PAH patients. It has been reported that miR‐21 promotes the proliferation and migration of PASMCs by regulating multiple gene targets in hypoxia condition.30 Previous studies have examined that miR‐34a expression was altered by hypoxia.26 miR‐34a is located on human chromosome 1 and thought to be involved in various cancer pathways.21, 22, 23, 24, 25 Overexpression of miR‐34a leads to a reduction in proliferation and increased apoptosis.31, 32 In this study, we found that miR‐34a mimic inhibited the proliferation of hPASMCs induced by hypoxia, which is consistent with previous studies in cancer cells. PDGF signalling plays a key role in several diseases including diabetes,33 cardiomyopathy34 and cancer,35 and meanwhile several studies have demonstrated its involvement in the progression of pulmonary hypertension.36 PDGFR‐alpha mRNA expression was increased in small PAs from patients displaying idiopathic PAH as compared with control subjects. PDGFR‐alpha mainly stained PASMCs and to a lesser extent endothelial cells.36 Although a miRNA could involve many potential target genes, the imprecise complementary sequences of miR‐34a with PDGFRA gene shows new significant role of miR‐34a on hypoxia‐induced cell proliferation. It has been reported that PDGFRα plays a critical role in cell proliferation and survival.37 As expected, our results showed that overexpression of miR‐34a repressed the PDGFRA mRNA and protein expression, and this effect was reversed by inhibitor of miR‐34a (Fig. 5). Taken together, these studies have shown specific miRNA species that could be targeted as potential therapeutic applications in PAH.
Previous study reported that migration of proliferating vascular SMCs is important histopathology finding displayed in vascular remodelling of PAH.38 Literature showed that hypoxia increases cell migration in ovine foetal pulmonary artery SMCs via cGMP‐dependent protein kinase PKG.39 PASMCs were treated with different growth factors, which can modulate expression of various miRNAs and affect phenotypic transition of PASMCs, and subsequently cell growth, migration and differentiation were altered. For example, the TGF‐β‐superfamily of growth factors (TGF‐β and BMP4) increases the expression of miR‐21.40 In this study, we have proved that miR‐34a plays a role in migration of hPASMCs in hypoxia. miR‐34a regulates cell migration in some cancer cells has been demonstrated in vitro. Thus, it can be concluded that the overexpression of miR‐34a in hypoxia is important for both hPASMC proliferation and migration.
Recent study found that DNA damage/PARP‐1 signalling pathway is important for PAH development by Meloche et al.41 We measured DNA damage in rat distal PAs and hPASMCs using γ‐H2AX as DNA damage marker by Western blotting and immunofluorescence. We found that DNA damage is significantly increased in PAH and hypoxic hPASMCs and the effect is decreased by the addition of the miR‐34a mimic, suggesting that miR‐34a is important for the repair of these damages. In addition, our studies provide the first evidence that miR‐34a promotes apoptosis and increases TUNEL‐positive cells in hypoxia. Some miRNAs have been implicated in vascular diseases. For example, Courboulin et al. have demonstrated that re‐establishing miR‐204 expression should be explored as a potential new therapeutic approach for human PAH.42 The role of miR‐34a in vascular tissues remained unknown. Our study performed in rat distal PAs and hPASMCs has demonstrated that the down‐regulation of miR‐34a is associated with enhanced PDGFRA expression, cell proliferation and the down‐regulation of KCNK3 channels, which in turn depolarizes hPASMCs membrane potential. These findings reinforced the importance of miR‐34a in the aetiology of PAH. In PAH, NFATc2 activation leads to the down‐regulation of Kv1.5, causing PASMC depolarization, increasing [Ca2+]i and promoting PASMCs proliferation.43, 44 Pim1 is a NFAT activator and identified as an attractive therapeutic target for PAH.45 NFATc2 expression (qRT‐PCR) and activation (immunofluorescence) are increased in hypoxic hPASMCs compared with control PASMCs. hPASMCs treated with miR‐34a mimic showed a significant decrease in both NFATc2 mRNA and activation levels. Finally, we provide further evidence that miR‐34a mimic in hypoxic hPASMCs decreases [Ca2+]i (FLuo‐3). These findings was further confirmed miR‐34a mimic was a novel, specific and attractive therapeutic strategy to reverse PAH.
In conclusion, we have performed detailed experimental characterization of hypoxia inducible miRNA‐34a in vitro and in vivo and its regulation by the cyclin A/E pathway in hPASMC. We have shown that PDGFRA is the direct target of miR‐34a in hPASMCs. Although many of these signalling pathways have previously been implicated in the development of PAH, in which the expression of miR‐34a is shown to be reduced, suggesting that miR‐34a may act as a crucial signalling mediator during remodelling of the pulmonary vasculature. Thus, our study illustrates a novel role for miR‐34a as a therapeutic target in PAH.
Author contributions
H.Z. and J.H. conceived and designed the experiments; P.W. performed the experiments; P.W. and X.J. analysed the data; Z.H. and F.W. contributed reagents/materials/analysis tools; P.W. and Y.S. wrote the paper; J.W. conducted the Western blotting and quantitative real‐time PCR experiments.
Conflicts of interest
The authors declare no conflict of interest.
Supporting information
Acknowledgements
This study was supported by grants from Natural Science Foundation of Heilongjiang Province of China (H2015110), the subject of Daqing Science and Technology Bureau (Szd‐2011‐41) and Seed Fund of Harbin Medical University (Daqing) (DQ2014‐04).
References
- 1. Stenmark KR, Fagan KA, Frid MG. Hypoxia‐induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. [DOI] [PubMed] [Google Scholar]
- 2. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. [DOI] [PubMed] [Google Scholar]
- 3. Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science (New York, NY). 2004;303:95–98. [DOI] [PubMed] [Google Scholar]
- 4. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- 5. Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. [DOI] [PubMed] [Google Scholar]
- 6. Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. [DOI] [PubMed] [Google Scholar]
- 7. Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. [DOI] [PubMed] [Google Scholar]
- 9. Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. [DOI] [PubMed] [Google Scholar]
- 10. Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, et al. miR‐21 regulates chronic hypoxia‐induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302:L521–L529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, et al. A role for miR‐145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111:290–300. [DOI] [PubMed] [Google Scholar]
- 12. Meloche J, Le Guen M, Potus F, Vinck J, Ranchoux B, Johnson I, et al. miR‐223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2015;309:C363–C372. [DOI] [PubMed] [Google Scholar]
- 13. Smith KA, Yuan JX, Schumacker PT. MicroRNAs and PARP: co‐conspirators with ROS in pulmonary hypertension. Focus on “miR‐223 reverses experimental pulmonary arterial hypertension”. Am J Physiol Cell Physiol. 2015;309:C361–C362. [DOI] [PubMed] [Google Scholar]
- 14. Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, et al. MicroRNA‐143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2015;117:870–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, et al. The microRNA‐328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L‐type calcium channel‐alpha1C. Hypertension. 2012;59:1006–1013. [DOI] [PubMed] [Google Scholar]
- 16. Li S, Ran Y, Zhang D, Chen J, Li S, Zhu D. MicroRNA‐138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1. Biochem J. 2013;452:281–291. [DOI] [PubMed] [Google Scholar]
- 17. Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, et al. Interleukin‐6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3‐microRNA cluster 17/92 pathway. Circ Res. 2009;104:1184–1191. [DOI] [PubMed] [Google Scholar]
- 18. Kang K, Peng X, Zhang X, Wang Y, Zhang L, Gao L, et al. MicroRNA‐124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J Biol Chem. 2013;288:25414–25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest. 1998;114:225S–230S. [DOI] [PubMed] [Google Scholar]
- 20. Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams BD, Wali VB, Cheng CJ, Inukai S, Booth CJ, Agarwal S, et al. miR‐34a silences c‐SRC to attenuate tumor growth in triple negative breast cancer. Cancer Res. 2016;76:927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu YW, Sun M, Xia R, Zhang EB, Liu XH, Zhang ZH, et al. LincHOTAIR epigenetically silences miR34a by binding to PRC2 to promote the epithelial‐to‐mesenchymal transition in human gastric cancer. Cell Death Dis. 2015;6:e1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang Y, et al. miR‐497 and miR‐34a retard lung cancer growth by co‐inhibiting cyclin E1 (CCNE1). Oncotarget. 2015;6:13149–13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu K, Huang J, Xie M, Yu Y, Zhu S, Kang R, et al. MIR34A regulates autophagy and apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy. 2014;10:442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu G, Yao W, Xiao W, Li H, Xu H, Lang B. MicroRNA‐34a functions as an anti‐metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J Exp Clin Cancer Res. 2014;33:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez‐Arroyo J, Voelkel NF, et al. p53 Gene deficiency promotes hypoxia‐induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol. 2011;300:L753–L761. [DOI] [PubMed] [Google Scholar]
- 27. Ma J, Liang S, Wang Z, Zhang L, Jiang J, Zheng J, et al. ROCK pathway participates in the processes that 15‐hydroxyeicosatetraenoic acid (15‐HETE) mediated the pulmonary vascular remodeling induced by hypoxia in rat. J Cell Physiol. 2010;222:82–94. [DOI] [PubMed] [Google Scholar]
- 28. Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, et al. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L354–L363. [DOI] [PubMed] [Google Scholar]
- 29. Girerd B, Perros F, Antigny F, Humbert M, Montani D. KCNK3: new gene target for pulmonary hypertension? Expert Rev Respir Med. 2014;8:385–387. [DOI] [PubMed] [Google Scholar]
- 30. Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA‐21 plays a role in hypoxia‐mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol. 2010;299:L861–L871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, et al. Transactivation of miR‐34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ji X, Wang Z, Geamanu A, Goja A, Sarkar FH, Gupta SV. Delta‐tocotrienol suppresses Notch‐1 pathway by upregulating miR‐34a in nonsmall cell lung cancer cells. Int J Cancer. 2012;131:2668–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, et al. PDGF signalling controls age‐dependent proliferation in pancreatic beta‐cells. Nature. 2011;478:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sekkach Y, Mekouar F, Jira M, Elqatni M, Elomri N, Fatihi J, et al. Durable efficacity and remission after treatment with imatinib mesylate for FIP1L1‐PDGFRA transcript negative associated eosinophilic cardiomyopathy. Ann Pharm Fr. 2011;69:277–281. [DOI] [PubMed] [Google Scholar]
- 35. Legrand F, Renneville A, Macintyre E, Mastrilli S, Ackermann F, Cayuela JM, et al. The spectrum of FIP1L1‐PDGFRA‐associated chronic eosinophilic leukemia: new insights based on a survey of 44 cases. Medicine. 2013;5:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perros F, Montani D, Dorfmuller P, Durand‐Gasselin I, Tcherakian C, Le Pavec J, et al. Platelet‐derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:81–88. [DOI] [PubMed] [Google Scholar]
- 37. Wei T, Zhang LN, Lv Y, Ma XY, Zhi L, Liu C, et al. Overexpression of platelet‐derived growth factor receptor alpha promotes tumor progression and indicates poor prognosis in hepatocellular carcinoma. Oncotarget. 2014;5:10307–10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992;86:723–729. [DOI] [PubMed] [Google Scholar]
- 39. Negash S, Narasimhan SR, Zhou W, Liu J, Wei FL, Tian J, et al. Role of cGMP‐dependent protein kinase in regulation of pulmonary vascular smooth muscle cell adhesion and migration: effect of hypoxia. Am J Physiol Heart Circ Physiol. 2009;297:H304–H312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA‐mediated microRNA maturation. Nature. 2008;454:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129:786–797. [DOI] [PubMed] [Google Scholar]
- 42. Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, et al. Role for miR‐204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA. 2007;104:11418–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, et al. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3‐{beta}/NFAT axis. Circulation. 2009;120:1231–1240. [DOI] [PubMed] [Google Scholar]
- 45. Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, et al. Signal transducers and activators of transcription‐3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


