Abstract
Objectives
In head and neck cancers, tumour cell repopulation during chemotherapy is one reason for treatment failure. Some of the mechanisms responsible for this repopulation are cell recruitment and abortive division. Due to lack of quantitative data in the literature regarding these mechanisms, the aim of this study was to investigate the interplay between recruitment and abortive division during cisplatin chemotherapy and to quantify the impact of these mechanisms on tumour control.
Materials and methods
An in silico Monte Carlo tumour model was developed to simulate tumour behaviour during chemotherapy. The virtual tumour had the composition and kinetic properties of a biological tumour. Effect of cisplatin on cell cycle and repopulation mechanisms were simulated and interpreted.
Results
Abortive division contributed to cell production within the tumour during chemotherapy. There was a strong relationship between recruitment and tumour growth due to abortive division. This observation was supported by the value of proliferative/stem ratio, which increased from 1.3 to 36, even when using small recruitment parameters.
Conclusions
While abortive division contributed towards tumour repopulation during chemotherapy, this mechanism could be controlled by daily doses of cisplatin. On the other hand, stem cells require an additional cytotoxic agent to overcome repopulation due to cell recruitment. Consequently, repopulation via abortive division during chemotherapy did not entail alterations in treatment schedule, nor dose escalation, to control the tumour.
Introduction
One of the continuing challenges in management of rapidly proliferating tumours, such as squamous cell carcinomas of the head and neck, is tumour cell repopulation during treatment. The main trigger for cell repopulation in tumours with high cell turnover is treatment‐induced cell loss. In normal tissue, this would be a natural reaction to rebuild the initial cell population. However, repopulation in these tumours occurs with rates of proliferation higher than the initial growth rate prior to treatment 1.
It has been shown that this accelerated repopulation response of squamous epithelia is equivalent to the acute response of normal epithelia after injury 2. Thus, if normal and malignant squamous epithelia share the same damage–response system, it follows that mechanisms responsible for normal tissue repopulation also occur in the tumours 2. For unresectable head and neck tumours, radio‐chemotherapy remains the standard treatment regimen, with cisplatin being the most commonly employed chemotherapeutic agent.
Whether due to radiotherapy or chemotherapy, repopulation of cancer cells during treatment is a valid concern and has to be dealt with. During radiotherapy, the biological process of tumour repopulation has been widely studied and methods to overcome repopulation have been clinically implemented. One such example is accelerated radiotherapy protocols, which involve shorter overall treatment times and high number of fractions per day, to counteract effects of tumour regrowth and to overcome loco‐regional treatment failure.
Some mechanisms found to be responsible for tumour cell repopulation during treatment are cell recruitment, abortive division, accelerated repopulation of stem cells and loss of asymmetrical division of stem cells 1, 2 (see Table 1 for definitions). Accelerated stem cell division and abortive division are controlled by tissue hypoplasia, whereas the mechanism of asymmetry loss is dictated by stem cell depletion 3.
Table 1.
Repopulation mechanisms and their effect on different cell types
| Repopulation mechanism | Effect/definition | Type of cells affected |
|---|---|---|
| Cell recruitment | Recycling the quiescent cells into the cell cycle | Quiescent cells |
| Accelerated repopulation | Shortening of cell cycle time | Proliferating stem cells |
| Loss of asymmetrical division | Symmetrical division of stem cells in mitosis (i.e. two daughter stem cells) | Proliferating stem cells |
| Abortive division | Limited number of proliferations after which the cell becomes sterile | Proliferating differentiated cells (finitely proliferating cells or doomed cells) |
As also shown in Table 1, cell recruitment affects quiescent cells which, under certain triggers, can re‐enter the cell cycle and proliferate. Stem cells can contribute towards repopulation by reducing their cell‐cycle time (this process is called accelerated stem cell division) and/or by symmetrical division, where the property of stem cells to divide asymmetrically is lost, so that in the new context, a stem cell divides into two daughter stem cells (instead of a stem and a ‘mortal’ cell). Abortive division is characteristic of proliferative cells, which can undergo a finite number of divisions before ceasing. Despite their finite lifespan, these cells are possible candidates for tumour repopulation during treatment 2.
Cell recruitment is, perhaps, the most natural repopulation mechanism due to an available pool of quiescent cells in G0 phase, which, under certain triggers such as radio/chemotherapy, can re‐enter the cell cycle and restart proliferating. Despite this characteristic mechanism, there are very few quantitative data in the literature regarding percentage and types of cells recruited during treatment and their effect on tumour control. Tubiana 4 has shown that in an untreated tumour population of haemopoietic cells, the proportion of recycling stem cells is below 2% of the total number of cells. Given that the proliferative rate of haematological cancers is greater than that of solid malignancies, the limit of 2% of stem cells recruited could represent the upper limit in the case of solid cancers. Some in silico modelling work analysing the impact of cell recruitment has been performed in the past on solid tumours 5. It was also shown that in head and neck carcinoma, this mechanism is less powerful than accelerated repopulation of stem cells or loss of their asymmetrical division, irrespective of number of cells involved in the re‐cycling process.
Cisplatin (cis‐diamine‐dichloro‐platinum) is the most commonly employed chemotherapeutic agent for management of head and neck cancers. Its effects on tumours have been studied both with and without radiotherapy and numbers of clinical trials have investigated various combined schedules for optimal outcome. The challenge with cisplatin stays in the adverse normal tissue reaction, which can be diminished by using lower, less aggressive doses. One successful trial on head and neck cancer has demonstrated that small, daily doses of cisplatin can lead to better tumour control as well as to better normal tissue tolerance, than weekly, large doses 6.
While chemotherapy is an important component of the treatment protocol, studies regarding repopulation during chemotherapy are scarce 7, 8 and the mechanisms behind the process are still not clearly understood. One important effect of cisplatin on tumour cell population is cell recruitment. This affects quiescent (non‐proliferating) cells, in G0 phase, outside the cell cycle. Cell killing due to cisplatin can trigger some of these cells to re‐enter the cycle and start (restart) proliferating.
Various approaches have been reported to investigate tumour regrowth during treatment. While in vitro studies offer a more direct way to assess immediate effects of therapeutic agents, mathematical models serve as valuable tools to predict effects on a longer timescale 9. Radiobiological models of BED (biologically effective dose) 10 or TCP (tumour control probability) 11 allow comparisons between trials regarding dose compensations due to repopulation, an effective way to evaluate altered fractionation schedules for various tumour sites. Models of cell kinetics of cell death and repopulation built on clinical data can also predict clinical outcome 12. Monte Carlo techniques are often implemented into the modelling process due to the stochastic nature of tumour growth, cell death, and response to treatment 13. For the same reason, the study described here has employed a Monte Carlo modelling approach to investigate two of the mechanisms suggested to be responsible for tumour repopulation.
Previous reports have discussed effects of accelerated repopulation of stem cells and loss of asymmetrical division on tumour control during radio‐ and chemotherapy 5, 8, 14. However, there are no studies in the literature describing the role of abortive division as a repopulation mechanism during chemotherapy, despite experimental evidence supporting its existence 15. Abortive division can interact with the clinical outcome by counterbalancing continuous cell loss due to treatment. Thus, a quantitative analysis in this direction is useful to assess the impact of the mechanism on treatment‐induced overall tumour regrowth.
The goal of this study was to examine the mechanism of abortive cell division and the interplay between it and cell recruitment in the context of head and neck chemotherapy. An in silico model of a squamous cell carcinoma has been built previously, which here serves as background for simulation of tumour repopulation during cisplatin‐based chemotherapy.
Materials and methods
Tumour growth module
A virtual squamous cell carcinoma of the head and neck has been previously developed using a Monte Carlo modelling technique with biologically valid cell kinetic parameters 16. The model follows the growth of a tumour from a temporal perspective. Different modules were created to deal with tumour growth, treatment (chemotherapy), and statistical data harvesting.
The growth module assessed the state of every cell on an hourly basis and appropriate action was taken for each cell. Three cell categories were considered in the model: stem cells (S), finitely proliferating cells (P) (or doomed cells) and non‐proliferating cells (N). While stem and finitely proliferating cells are cycling and able to divide by mitosis, non‐proliferating cells are in G0, outside the cell cycle; they could be triggered back into the cycle as a response to cell loss due to treatment.
Finitely proliferating cells underwent abortive division, meaning that they could still produce daughter cells for a limited number of generations. The number of generations that could be produced to keep tumour growth within realistic parameters was also investigated. P cells divide at mitosis into one further P, or N cells with a probability of 30:70. Stem cells are considered to be immortal, which, from a modelling perspective, translates into self‐preservation, which, in mitosis, gives birth to another cell that can be either S, P or N (with various probabilities). When an N cell is created in mitosis, it immediately enters the quiescent phase. As part of tumour growth, the model incorporated cell loss, which for head and neck cancer, according to the literature, reaches a value of 85%.
In this model, the mean cell‐cycle time of head and neck squamous carcinoma cells was 33 h with a standard deviation of 13.7 h, in agreement with literature data 17. Stem cells contributed 2% of the whole tumour population, P cells 13% and the remaining 85% representing the population of N cells, which were quiescent. The model provides average tumour volume doubling time of 45 days, which is, again, in accordance with the literature data 18.
Given that P cells and S cells are the main contributors to tumour growth and development, the relationship between these two cell categories was investigated during chemotherapy. From a repopulation perspective, the interplay between abortive division of P cells and cell recruitment during chemotherapy was analysed and discussed.
Chemotherapy module
The chemotherapy module simulated cisplatin‐based treatment as per clinical protocols. It employed a daily, low‐dose cisplatin treatment, given over 2 weeks. After 2 weeks, the treatment was stopped and the tumour was left to grow unperturbed, to observe regrowth potential of various cell populations that survived chemotherapy.
The effect of cisplatin on the cell cycle was implemented in the model based on experimental data of Sorenson et al. 19 on L1210/0 cell lines. In their experiment, after administration of similar concentrations of cisplatin as those clinically employed, more than 80% cells underwent cell arrest in G2 phase by day 2. Cell release of the surviving population occurred 4–6 days later, when, at the same time, around half the cells were observed as debris. These observations were translated into the model as follows: Given that cisplatin's cytotoxic activity is manifested by DNA adduct formation with proliferative cells 20, 21, in the model, adduct formation was simulated by randomly ‘marking’ 80% of cycling cells. These ‘marked’ cells were arrested in G2 once they reached that position in the cell cycle. After 48 h, half of the arrested cells were released, while the other half underwent apoptosis over a further 72 h. After each dose of cisplatin, this process of marking, arresting, releasing/killing cells was followed.
As there are no quantitative data in the literature regarding cell recruitment due to chemotherapy, the current model considered various values representing percentage of cells recruited after each cell killing due to cisplatin, to account for both low and high levels of cell repopulation, 5%, 15% and 50%. Within these percentages, 4% cells were considered to have stem cell properties, while the remaining ones were finitely proliferating cells. The interplay between abortive division and cell recruitment was analysed and the impact of P/S ratio (proliferative/stem) on tumour development was illustrated.
Statistical data harvesting module
Statistical data harvesting module collected the following information: total number of tumour cells, number of S, P, N cells, number of cells in each of the four phases of the cell cycle, number of quiescent cells. This information was collected every 100 h during tumour growth phase. During treatment phase, statistical data were collected before and after each treatment, allowing thorough analysis of tumour behaviour at every step of chemotherapy.
Results and discussion
Tumour cell proliferation
Finitely proliferating cells underwent a number of divisions until they were no longer able to divide. In silico sensitivity study of this parameter (that is, number of divisions of finitely proliferating cells) showed that increase in tumour cell population was biologically unrealistic if the number of generations exceeded 3 (Fig. 1) as volume doubling time would be much shorter than the average 45 days, which is characteristic of head and neck tumours. Also, if the number of generations of P cells exceeded 3 (when all the other parameters are kept constant), ratio of S:P:N cells would not be in agreement with biologically realistic tumour composition, as presented in the Materials and methods section. Thus, finitely proliferating cells in the current model underwent division for 3 cell generations after which they stopped dividing.
Figure 1.
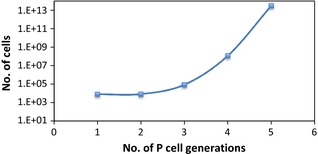
Number of tumour cells as a function of the number of P‐cell generations.
Effect of cisplatin on cell cycle and proliferation
The effect of cisplatin and mechanism of cell recruitment were modelled as described above. Figure 2 presents the sequence of events and their effect on cell distribution along the four phases of the cell cycle. Results of the simulation show that cisplatin had strong impact on cells by arresting a large proportion (80%) of cycling cells in G2 phase. These arrested cells were randomly selected from S and P populations, as dictated by the Monte Carlo model. It is important to note that 85% of cells were initially resting in the quiescent phase, thus only 15% of tumour cells were cycling. The large number of non‐proliferating cells could be an important source of tumour repopulation during treatment.
Figure 2.
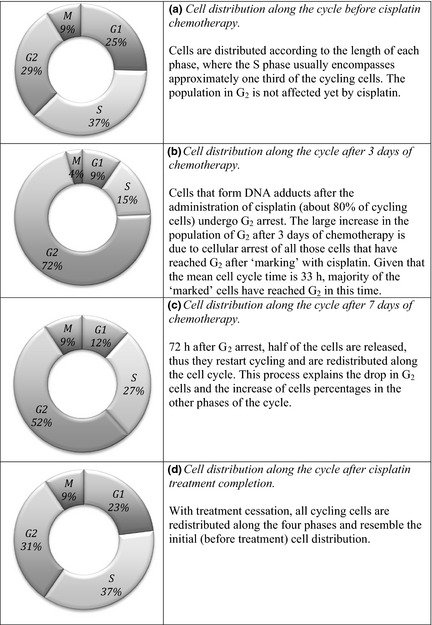
Cell distribution along the cell cycle under cisplatin treatment.
P/S ratio
As mentioned above, cells were recruited from the pool of quiescent cells, representing 85% of the total tumour cells. Recruitment was simulated for various cell percentages, which included both stem and finitely proliferating cells (for example, a recruitment parameter of 5/4 means that 5% cells were recruited from G0, of which 4% had stem cell properties; the rest were finitely proliferating cells that underwent abortive division). Irrespective of the number of recruited P cells, the percentage of stem cells was kept constant (4% total recruited cells), meaning that P/S ratio of recruited cells was also constant and, in our case, equal to 24 (see also Table 2). This meant that for each stem cell recruited, there were 24 finitely proliferating cells re‐cycled from the quiescent phase.
Table 2.
Percentages of re‐cycled stem and proliferating cells as a function of the recruitment parameter
| Cell type | 5/4 recruitment | 15/4 recruitment | 50/4 recruitment |
|---|---|---|---|
| Stem (S) | 0.17 | 0.51 | 1.7 |
| Finitely proliferating (P) | 4.08 | 12.24 | 40.8 |
However, P/S ratio changed drastically once cells were in the cell cycle, due to effects of cisplatin on cycling cells. The random nature of cisplatin's interaction with tumour cells, which can lead to any (or all) of the following outcomes – ‘marking’, ‘arresting’ and ‘killing’ interplayed with cell progression through the cycle, thus affecting P/S ratio. Figure 3 illustrates P/S ratio during chemotherapy for various recruitment parameters compared to the situation when no cell recruitment was simulated.
Figure 3.
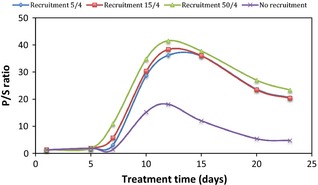
P/S ratio for various cell recruitment parameters.
It can be seen that cell recruitment triggered a large number of cells into the cycle, many of which were destined to undergo abortive division. This process could increase P‐cell population during chemotherapy by as much as a factor of 13 for a low recruitment parameter, and by a factor of 57 for a large recruitment parameter (Fig. 4). To quantify the increase in P cells undergoing abortive division during chemotherapy, a repopulation factor due to P‐cell recruitment (RFP) was defined as the ratio between P‐cell population with and without cell recruitment:
As cell recruitment was triggered by cells loss, the present model simulated cell recruitment after each cell killing process due to cisplatin. As mentioned in the Materials and methods section, cell killing occured to 50% arrested cells about 5 days after administration of cisplatin. Therefore, as shown in Figs 3 and 4, drastic quantitative changes within the P‐cell population were observed towards the end of the first treatment week. Figure 4 indicates exponential increase in RFP during treatment. The steepness of the exponential curve increased with escalation of the recruitment parameter. This exponential behaviour of P cells during recruitment was due to the interplay between abortive cell division and constant population growth.
Figure 4.
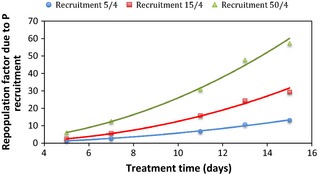
Exponential increase of the repopulation factor due to P‐cell recruitment as a function of treatment time.
The mechanism of cell recruitment during chemotherapy had a double effect on the cell population, which lead to opposite results: first, through recruitment, the number of cycling cells considerably increased (as a function of the recruitment parameter), thus more cells underwent division and contributed to tumour growth. Newly recycled S cells divided indefinitely, unless killed by subsequent dose of cisplatin, while P cells underwent abortive division and contributed to increase in tumour cell number. Figure 5 illustrates that tumour repopulation via abortive division was directly proportional to the recruitment parameter.
Figure 5.
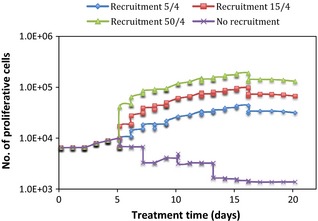
Effect of proliferative cells on tumour response to cisplatin as a function of the recruitment parameter.
Secondly, by increasing the cycling cell population, a larger number of cells was affected and eventually killed by cisplatin, given that cisplatin only marks cycling cells. This situation can be seen from Fig. 6, where the number of S cells was heavily diminished by cisplatin at the end of the first treatment week. Due to the random nature of DNA adduct formation, both S and P cells were evenly affected. However, as the absolute number of S cells was much smaller than the number of P cells, same cell loss from the S population was more noticeable than that from the P‐cell pool. Therefore, cell kill effects were not as drastic in Fig. 5 (P cells) as they were in Fig. 6 (S cells). A further interesting aspect when comparing abortive division of P cells and repopulation of S cells is the situation when no recruitment is considered. In this case, tumour regrowth is merely based on the ability of each cell type to regrow the tumour during or after treatment. The ‘no recruitment’ graph in Fig. 5 shows a very small increase in P‐cell population at the beginning of treatment, which was overcome by cell killing due to cisplatin, shortly afterwards. In comparison, the same graph in Fig. 6 illustrates the potential of S cells to repopulate the tumour after treatment, from surviving cells. The same trend was seen after treatment cessation (from day 16 onwards) for cell recruitment plots: graphs in Fig. 5 have a decreasing final slope, indicating that when the tumour was left to grow unperturbed after the end of treatment, abortive division was not a powerful enough mechanism to re‐grow it. However, Fig. 6 indicates the opposite: irrespective of the recruitment parameter, stem cells were able to repopulate the tumour from the existing S cells, despite their small initial number.
Figure 6.
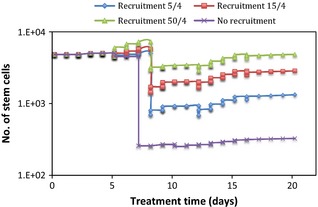
Effect of stem cells on tumour response to cisplatin as a function of the recruitment parameter.
To look more closely at the impact of the recruitment parameter on the two cell types (P and S), after 2 weeks of chemotherapy, the plot in Fig. 7 shows, on a semi logarithmic scale, a supra‐linear relationship between percentage of cells recruited from G0 and total number of P and S cells. The initial shoulder of the curves is given by sudden increase in cell number when the mechanism of cell recruitment was involved, compared to the situation when no recruitment was modelled. Supra‐linear behaviour pattern of both cell types during chemotherapy (when recruitment was activated) shows their potential to repopulate the tumour. This is possibly true during treatment, when other repopulation mechanisms were also triggered by cell loss, but is not valid after treatment when surviving cells had to possess self‐renewal ability to regrow the tumour. Graphs in Figs 5 and 6 confirm the above statement showing that P cells, due to their limited ability to proliferate through abortive division, were not able to rebuild the tumour, while S cells were.
Figure 7.
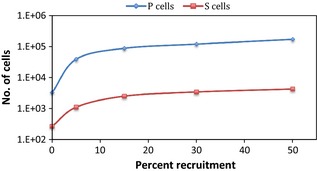
Relationship between the recruitment parameter (percent recruitment) and the number of P and S cells, respectively, at the end of the second treatment week.
Impact of the model results on head and neck cancer chemotherapy
Unresectable locally advanced head and neck cancers are one of the most aggressive tumours, owing to their regrowth potential during treatment. Standard of care in management of head and neck cancers consists of cisplatin‐based chemotherapy combined with radiation 22. One of the main reasons for treatment failure and recurrence in these cancers is tumour repopulation, which is manifested by several mechanisms. Cell recruitment from G0 phase is a common occurrence during treatment and it has a significant impact on tumour population as both stem and finitely proliferating cells are affected by this mechanism.
Stem cells have the ability to proliferate indefinitely; consequently, even a small number of surviving stem cells can contribute to cancer regrowth during treatment. Most importantly, this process controlled by S cells continues after treatment completion. Finitely proliferating cells undergo abortive division, which is shown to be a powerful repopulation mechanism during chemotherapy due to interaction with other repopulation mechanisms such as cell recruitment. Nevertheless, with treatment cessation, these P cells cannot repopulate the tumour via abortive division as cell supply is too weak without any additional sustenance from other repopulation processes.
Thus, we were confronted with two stages of tumour control: (i) during chemotherapy and (ii) after completion of chemotherapy. Tumour control can only be achieved by overcoming repopulation. Cisplatin was shown to be effective when administered on a daily basis 15, a result that might be due to experimental observation where DNA adducts formed by cisplatin disappear after about 24 h 23; this would render weekly administration inefficient. Furthermore, taking into account average cell‐cycle time of head and neck cancers (33 h), daily administration of cisplatin offers a continuous supply of cytotoxic compounds, which proficiently link to DNA of cycling cells. This process eventually leads to apoptosis, or at least sensitizes the cell to a subsequent damage.
In summary:
The interplay between cell recruitment and abortive division created increase in tumour cell population during chemotherapy, which can imply the need for dose escalation. However, abortive division weakens with treatment cessation, when the only challenge remains stem population. Moreover, surviving S cells were shown to be able to regrow the tumour (Fig. 6), meaning that cisplatin as a single agent could not control the tumour. Owing to cisplatin's radio‐sensitizing properties, concurrent radio‐chemotherapy schedules are successful in controlling local disease. This justifies the success of hyperfractionated radiotherapy (radiation doses twice a day) combined with daily cisplatin for locally advanced head and neck cancers 6.
Abortive division of finitely proliferating cells (or doomed cells) contributed to cell production within the tumour, during chemotherapy with cisplatin. There was a strong relationship between cell recruitment during treatment and tumour growth due to abortive division. This was illustrated by behaviour of P/S ratio (proliferative /stem), which increased from a value of 1.3 to P/S = 36 even for the smallest recruitment parameter.
Cell recruitment triggered a large number of cells, many of which were destined to undergo abortive division. This process increased the P‐cell population during chemotherapy by a factor of 13 for a low recruitment parameter (5/4) and by a factor of 57 for a large recruitment parameter (50/4).
With treatment cessation, stem cells remained the main factor in tumour repopulation, while the number of finitely proliferating cells slowly decreased. This means, that while the main target of any treatment should be the pool of stem cells, P cells, which undergo abortive division, can contribute to the whole tumour growth, a process that could misleadingly suggest the need for a larger treatment dose to eradicate the tumour.
Through this work, the role of the computational models as predictive tool in cancer research was proven once again. This virtual tumour incorporated the main kinetic properties of a biological tumour, which allowed simulation of repopulation mechanisms during cisplatin chemotherapy.
While built for simulation of head and neck cancer growth and development, the current in silico model allows input parameter adjustments to accommodate investigation of other tumour types. This represents a direction for future research.
In conclusion, while abortive division contributed to tumour repopulation during chemotherapy, this repopulation mechanism could be controlled by daily doses of cisplatin. On the other hand, due to their self‐renewal ability, stem cells require an additional cytotoxic agent (such as radiotherapy) to overcome repopulation due to cell recruitment. Consequently, repopulation via abortive division during chemotherapy did not require alteration of the treatment schedule, nor dose escalation to control the tumour.
Acknowledgements
This work was supported by a grant of the Ministry of National Education, CNCS‐UEFISCDI, Project no. PN‐II‐ID‐PCE‐2012‐4‐0067.
References
- 1. Withers HR (1993) Treatment‐induced accelerated human tumor growth. Semin. Radiat. Oncol. 3, 135–143. [DOI] [PubMed] [Google Scholar]
- 2. Dörr W (2009) Time factors in normal‐tissue responses to irradiation In: Van der Koegel A, Joiner M, eds. Basic Clinical Radiobiology, 4th edn, pp. 149–157. London, UK: Hodder Arnold. [Google Scholar]
- 3. Dörr W (2003) Modulation of repopulation processes in oral mucosa: experimental results. Int. J. Radiat. Biol. 79, 531–537. [DOI] [PubMed] [Google Scholar]
- 4. Tubiana M (1988) Repopulation in human tumours. Acta Oncol. 27, 83–88. [DOI] [PubMed] [Google Scholar]
- 5. Marcu L, van Doorn T, Olver I (2004) Modelling of post‐irradiation accelerated repopulation in squamous cell carcinomas. Phys. Med. Biol. 49, 3767–3779. [DOI] [PubMed] [Google Scholar]
- 6. Jeremic B, Shibamoto Y, Milicic B, Nikolic N, Dagovic A, Aleksandrovic J et al (2000) Hyperfractionated radiation therapy with or without concurrent low‐dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J. Clin. Oncol. 18, 1458–1464. [DOI] [PubMed] [Google Scholar]
- 7. Davis A, Tannock I (2000) Repopulation of tumour cells between cycles of chemotherapy: a neglected factor. Lancet Oncol. 1, 86–93. [DOI] [PubMed] [Google Scholar]
- 8. Marcu L, Bezak E (2010) Modelling of tumour repopulation after chemotherapy. Australas. Phys. Eng. Sci. Med. 33, 265–270. [DOI] [PubMed] [Google Scholar]
- 9. Zhang M, Das C, Vasquez H, Aguilera D, Zage PE, Gopalakrishnan V et al (2006) Predicting tumor cell repopulation after response: mathematical modeling of cancer cell growth. Anticancer Res. 26, 2933–2936. [PubMed] [Google Scholar]
- 10. Meade S, Sanghera P, McConkey C, Fowler J, Fountzilas G, Glaholm J et al (2013) Revising the radiobiological model of synchronous chemotherapy in head‐and‐neck cancer: a new analysis examining reduced weighting of accelerated repopulation. Int. J. Radiat. Oncol. Biol. Phys. 86, 157–163. [DOI] [PubMed] [Google Scholar]
- 11. Fakir H, Hlatky L, Li H, Sachs R (2013) Repopulation of interacting tumor cells during fractionated radiotherapy: stochastic modeling of the tumor control probability. Med. Phys. 40, 121716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirkpatrick JP, Marks LB (2004) Modeling killing and repopulation kinetics of subclinical cancer: direct calculations from clinical data. Int. J. Radiat. Oncol. Biol. Phys. 58, 641–654. [DOI] [PubMed] [Google Scholar]
- 13. Harriss‐Phillips WM, Bezak E, Yeoh E (2012) The HYP‐RT hypoxic tumour radiotherapy algorithm and accelerated repopulation dose per fraction study. Comput. Math. Methods Med. 2012, 363564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harriss‐Phillips WM, Bezak E, Yeoh EK (2011) Monte Carlo radiotherapy simulations of accelerated repopulation and reoxygenation for hypoxic head and neck cancer. Br. J. Radiol. 84, 903–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crowther PJ, Cooper IA, Woodcock DM (1985) Biology of cell killing by 1‐beta‐D‐arabinofuranosylcytosine and its relevance to molecular mechanisms of cytotoxicity. Cancer Res. 45, 4291–4300. [PubMed] [Google Scholar]
- 16. Marcu L, van Doorn T, Olver I, Zavgorodni S (2002) Growth of a virtual tumour using probabilistic methods of cell generation. Australas. Phys. Eng. Sci. Med. 25, 155–161. [DOI] [PubMed] [Google Scholar]
- 17. Hall EJ (2000) Radiobiology for the Radiologist, 5th edn, pp. 382–386. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- 18. Tannock IF, Hill RP (1992) The Basic Science of Oncology, 2nd edn, pp. 155–176. New York: McGraw‐Hill. [Google Scholar]
- 19. Sorenson C, Barry M, Eastman A (1990) Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J. Natl. Cancer Inst. 82, 749–754. [DOI] [PubMed] [Google Scholar]
- 20. Téletchéa S, Skauge T, Sletten E, Kozelka J (2009) Cisplatin adducts on a GGG sequence within a DNA duplex studied by NMR spectroscopy and molecular dynamics simulations. Chemistry 15, 12320–12337. [DOI] [PubMed] [Google Scholar]
- 21. Cerón‐Carrasco JP, Jacquemin D, Cauët E (2012) Cisplatin cytotoxicity: a theoretical study of induced mutations. Phys. Chem. Chem. Phys. 14, 12457–12464. [DOI] [PubMed] [Google Scholar]
- 22. Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C, Bourhis J et al (2011) Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): a comprehensive analysis by tumour site. Radiother. Oncol. 100, 33–40. [DOI] [PubMed] [Google Scholar]
- 23. Welters MJ, Fichtinger‐Schepman AM, Baan RA, Jacobs‐Bergmans AJ, Kegel A, van der Vijgh WJ et al (1999) Pharmacodynamics of cisplatin in human head and neck cancer: correlation between platinum content, DNA adduct levels and drug sensitivity in vitro and in vivo. Br. J. Cancer 79, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


