Acyclovir (ACV) resistance-associated mutations in two recombinant herpes simplex virus 1 (HSV-1) clones were compared. Recombinant HSV-1 lacking its thymidine kinase (TK) and expressing varicella-zoster virus (VZV) TK ectopically had no mutations in the VZV TK gene.
KEYWORDS: DNA polymerase, acyclovir, acyclovir resistant, herpes simplex virus 1, thymidine kinase, varicella-zoster virus
ABSTRACT
Acyclovir (ACV) resistance-associated mutations in two recombinant herpes simplex virus 1 (HSV-1) clones were compared. Recombinant HSV-1 lacking its thymidine kinase (TK) and expressing varicella-zoster virus (VZV) TK ectopically had no mutations in the VZV TK gene. In contrast, recombinant HSV-1 expressing HSV-1 TK ectopically harbored mutations in the HSV-1 TK gene. These results suggest that the relatively low frequency of ACV-resistant VZV is a consequence of the characteristics of the TK gene.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) and varicella-zoster virus (VZV) establish latency in ganglion cells and reactivate under certain conditions to cause recurrent vesicular lesions (1). Typically, reactivation of HSV-1 results in herpes labialis, periorbital herpes, herpes keratitis, or recurrent genital herpes, whereas reactivation of VZV causes zoster (1). These recurrent diseases occur more frequently, and are more severe, in immunocompromised patients than in immunocompetent patients (2). Acyclovir (ACV), a guanosine analog, is a drug used to treat patients with HSV-1 and VZV diseases. The mechanism of action of ACV is as follows: ACV is phosphorylated by the viral thymidine kinase (TK) to yield ACV monophosphate and further phosphorylated by host cellular kinases to yield ACV triphosphate, which then competes with dGTP for the viral DNA polymerase (DNApol) (3–5). Occasionally, ACV-resistant (ACVr) HSV-1 and VZV are induced in immunocompromised patients (2, 6, 7). It is suggested that the occurrence of viral TK-associating ACVr mutations in VZV is much less frequent than in HSV-1. ACVr VZV is reported rarely in immunocompromised patients (8–23). In contrast, ACVr HSV-1 occurs in 3.5% to 10% of immunocompromised patients (2, 24–28). It is thought that the emergence of ACVr VZV is less likely than that of HSV-1, because VZV is approximately 100 times less sensitive to ACV than HSV-1 (29). The ACV sensitivity of a chimeric HSV-1 lacking the original HSV-1 TK gene, but harboring the VZV TK gene, is the same as that of VZV (29). However, the precise mechanism underlying the difference in the likelihood of emergence of ACVr viruses is unclear.
One possible mechanism may be based on the particular characteristics of VZV TK and HSV-1 TK; i.e., viral TK determines the sensitivity to ACV (29). To clarify the hypothesis, we generated two recombinant HSV-1 viruses: HSV-1_VZV-TK expressing the TK gene of VZV strain vOka (GenBank accession AB097932.1) under the control of the cytomegalovirus (CMV) promoter and from which the HSV-1 TK gene was deleted, and HSV-1_HSV1-TK, in which the VZV TK gene of HSV-1_VZV-TK was replaced with that for HSV-1 TK. We then compared the ACV resistance-associated mutations in recombinant HSV-1 clones.
HSV-1_VZV-TK was generated using a two-step Red recombination system, as described previously (30–32). A DNA fragment containing nucleotides (nt) 1 to 44 of the VZV TK gene, the I-SceI restriction site and the kanamycin resistance gene from pEP-KanS (32), the CMV promoter, and the VZV TK gene and its poly(A) region were amplified using primers 5′-GCGGTACCATGTCAACGGATAAAACCGATGTAAAAATGGGCGTTTTGCGTATGATGACGACGATAAGTAGGG-3′ and 5′-GCGGTACCGCCAGTGTTACAACCAATTAACC-3′. The DNA fragment was then inserted between the UL50 and UL51 genes using primers 5′-ATCTCATCTTTCCTGTGTGTAGTTGTTTCTGTTGGAGGCCTGTGGGTCTAACATTGATTATTGACTAGTTATTAA-3′ and 5′-TTCATCCAACCCGTGTGTTCTGTGTTTGTGGGATGGAGGGGCGGGTGTGAATCTTTTTACTGGTACATACGTAAA-3′, as described previously (30–32). Next, the HSV-1 TK gene was replaced with the kanamycin resistance gene using primers 5′-TTATTGCCGTCATAGCGCGGGTTCCTTCCGGTATTGTCTCCTTCCGTGTTAGGATGACGACGATAAGTAGGG-3′ and 5′-TCCGCCTGGAGCAGAAAATGCCCACGCTACTGCGGGTTTATATAGACGGTAACACGGAAGGAGACAATACCAACCAATTAACCAATTCTGATTAG-3′ (30–32). HSV-1_HSV1-TK was constructed by replacing the VZV TK gene of HSV-1_VZV-TK with the TK gene of HSV-1 strain F (GenBank accession GU734771.1). The DNA fragment containing nt 1 to 44 of the HSV-1 TK gene, the I-SceI restriction site and the Zeocin resistance gene from pUC-Zeo (33), the CMV promoter, and the HSV-1 TK gene and its poly(A) region were amplified using primers 5′-GCGGTACCATGGCTTCGTACCCCTGCCATCAACACGCGTCTGCGTTCGACCACGGGGATCTAGGGATAACAG-3′ and 5′-GCGGTACCATTACGCCAAGCTTGCATGC-3′ and then inserted between the UL50 and UL51 genes using primers 5′-ATCTCATCTTTCCTGTGTGTAGTTGTTTCTGTTGGAGGCCTGTGGGTCTAACATTGATTATTGACTAGTTATTAA-3′ and 5′-TTCATCCAACCCGTGTGTTCTGTGTTTGTGGGATGGAGGGGCGGGTGTGATTTATTCTGTCTTTTTATTGCCGTC-3′. The half maximal inhibitory concentration (IC50) of ACV to HSV-1_VZV-TK was higher than those to HSV-1 F and HSV-1_HSV1-TK in both Vero cells and MRC-5 cells; also, the sensitivity of HSV-1_VZV-TK in MRC-5 cells to ACV was similar to that of VZV vOka (Table 1). In contrast, the sensitivities of HSV-1_HSV1-TK to ACV were similar to those of HSV-1 F in both cells (Table 1). These results suggest that the ACV sensitivity of HSV-1 and VZV depends on the characteristics of viral TK.
TABLE 1.
Inhibitory effect of ACV on parental and recombinant viruses, as determined in a plaque reduction assay in Vero and MRC-5 cells
| Virus | IC50 (μg/ml) ina: |
|
|---|---|---|
| Vero cells | MRC-5 cells | |
| HSV-1 F | 0.3 ± 0.5 | 0.1 ± 0.04 |
| HSV-1_VZV-TK | 2.1 ± 0.5 | 3.4 ± 1.4 |
| HSV-1_HSV1-TK | 0.3 ± 0.03 | 0.1 ± 0.03 |
| VZV vOka | NDb | 1.0 ± 0.5 |
Values include standard deviations from three independent tests.
ND, not determined.
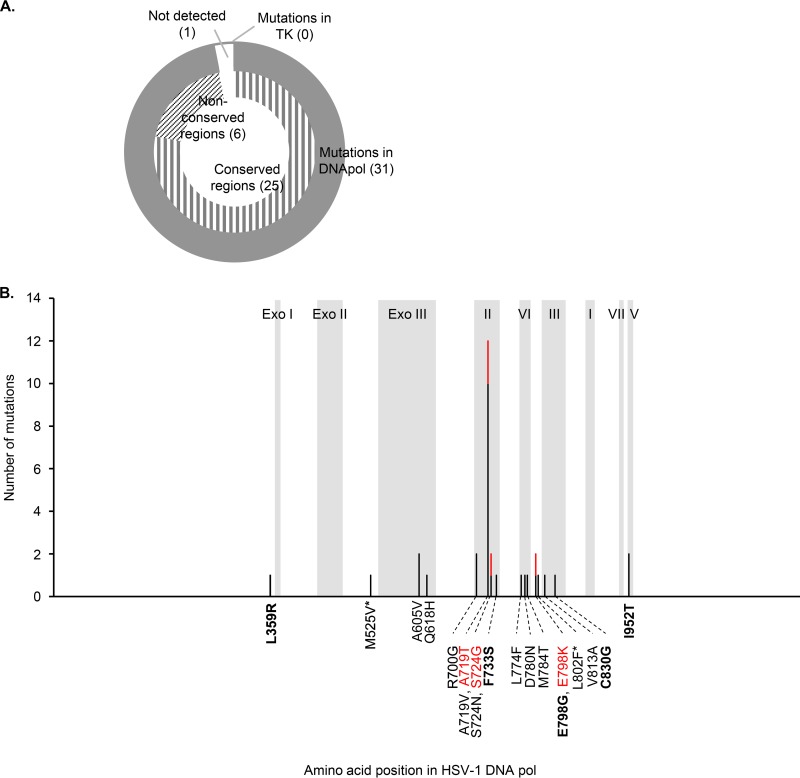
ACVr HSV-1_VZV-TK and ACVr HSV-1_HSV1-TK clones were generated by serial passage of HSV-1_VZV-TK and HSV-1_HSV1-TK, respectively, in the presence of increasing concentrations of ACV, as described previously (34, 35). Thirty-two ACVr HSV-1_VZV-TK clones were obtained, and the sequences of the viral TK and DNApol genes were determined. Compared with the original HSV-1_VZV-TK clones, none of the ACVr HSV-1_VZV-TK clones harbored mutations in the TK gene. However, 31 ACVr clones harbored mutations in the DNApol gene; all were single or double nucleotide mutations that resulted in the substitution of one or two amino acids, respectively (Fig. 1A). Twenty-five ACVr clones harbored amino acid substitutions in the conserved regions of DNApol, whereas the other six harbored amino acid substitutions in nonconserved regions (Fig. 1). Twelve ACVr clones harbored substitutions at amino acid 719 of DNApol (Fig. 1B). L356R, F733S, E798G, C830G, and I952T in DNApol were identified as novel amino acid substitutions that conferred ACV resistance (Fig. 1B). Of 31 nucleotide substitutions, 12 (approximately 40%) were an A/T to G/C switch in the HSV-1 DNApol gene. Although VZV TK has affinity not only for thymidine but also for deoxycytidine (36), there was no significant preference with respect to nucleotide substitutions in the HSV-1 DNApol gene. One ACVr clone harbored no mutations in either the TK or DNApol genes compared to those of the original HSV-1_VZV-TK (Fig. 1A), suggesting that not only viral TK and DNApol but also other factors affect the functional mechanism of action of ACV in this recombinant virus. Further studies are needed to clarify the mechanism underlying the generation of clones that are resistant to ACV.
FIG 1.
ACV resistance-associated mutations detected in ACVr HSV-1_VZV-TK clones. (A) The pie chart shows the regions in which ACV resistance-associated mutations were detected. The numbers in parentheses denote the number of ACVr HSV-1_VZV-TK mutations. (B) Mutations detected, and their frequencies, in HSV-1 DNApol. The y axis indicates the number of clones harboring the mutations detected at a given amino acid position. Amino acid changes are shown on the x axis, and the color of the letters corresponds to the color of the bars. The gray boxes indicate the conserved regions in DNApol of HSV-1 strain F (GenBank accession GU734771.1): Exo I to III (amino acids 363 to 373, 437 to 469, and 531 to 627), II (amino acids 694 to 736), VI (amino acids 772 to 791), III (amino acids 805 to 845), I (amino acids 881 to 896), VII (amino acids 938 to 946), and V (amino acids 953 to 963). Novel mutations are shown in boldface font. Two amino acid substitutions were detected in one ACVr HSV-1_VZV-TK clone; these are indicated by asterisks.
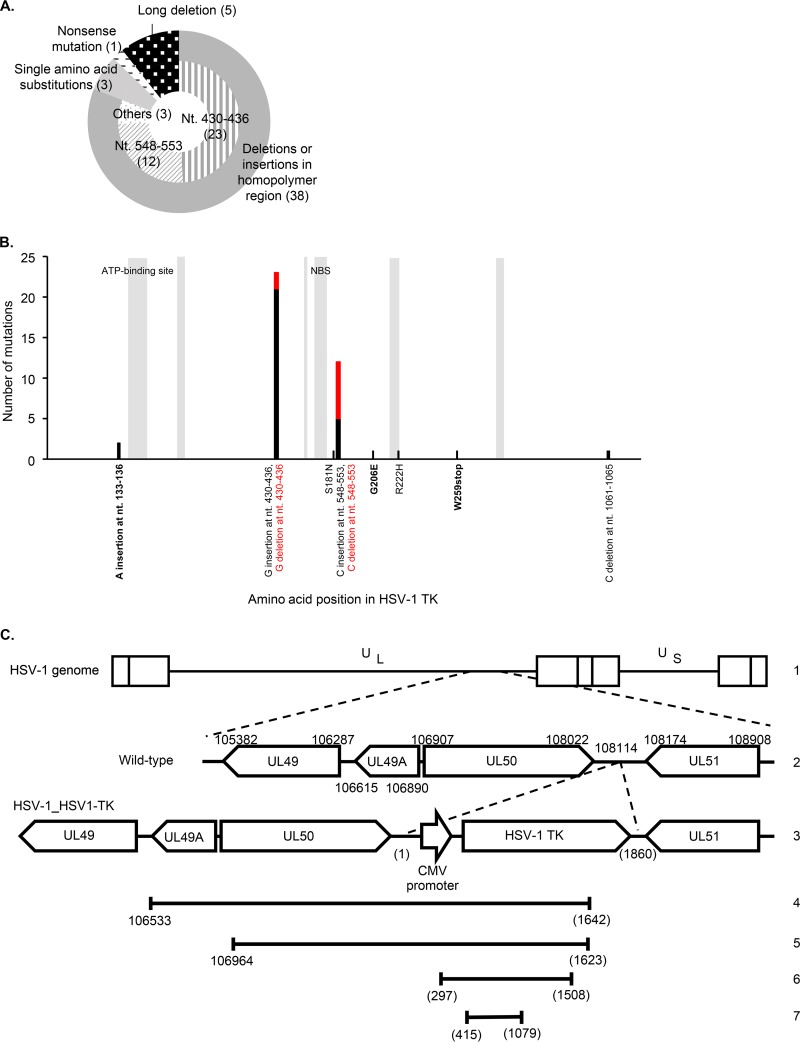
Forty-seven ACVr HSV-1_HSV1-TK clones were obtained as described above, and all harbored mutations in the HSV-1 TK gene but not in the DNApol gene. These results suggest that ACV resistance-associated mutations are less likely to occur in VZV TK than in HSV-1 TK. This is supported, at least partially, by the fact that the HSV-1 TK gene contains homopolymer regions in which nucleotide insertions or deletions are common (37–40). Thirty-eight clones harbored insertion or deletion mutations in the homopolymer stretch regions, and 23 of the 38 clones harbored a single insertion of guanine at nt 430 to 436 in the 7-G homopolymer stretch within the HSV-1 TK gene (Fig. 2A). Four clones harbored single point nucleotide substitutions at different locations, resulting in a single amino acid substitution or a nonsense mutation (Fig. 2A). A single insertion of adenine at nt 133 to 136 and W259stop and G206E substitutions in HSV-1 TK were novel mutations (Fig. 2B). Five clones lost a long sequence of nucleotide residues, including part of the inserted HSV-1 TK gene (Fig. 2C). One clone lost a long nucleotide sequence stretching from the UL49 gene to the HSV-1 TK gene (Fig. 2C, line 4). Another clone lost a long sequence stretching from the UL50 gene to the HSV-1 TK gene (Fig. 2C, line 5). The other three clones lost a long sequence within the transfer region (Fig. 2C, lines 6 and 7); two of these deletions showed the same pattern (Fig. 2C, line 6). Such long deletions may be due to homologous recombination, although no homologous regions were found in and around the deleted regions. It is possible that the phenomenon was specific to recombinant HSV-1_HSV1-TK, as it has not been reported in any other ACVr HSV-1 isolates examined in in vitro or clinical studies.
FIG 2.
ACV resistance-associated mutations detected in ACVr HSV-1_HSV1-TK clones. (A) The pie chart shows the patterns of mutations in the HSV-1 TK gene. Nt, nucleotide. The numbers in parentheses denote the number of ACVr HSV-1_HSV1-TK clones. (B) Frequency of each mutation detected in HSV-1 TK. The y axis indicates the number of mutations detected at a given amino acid position. The mutations detected are shown on the x axis, and the color of the letters corresponds to the color of the bars. The gray boxes indicate the conserved regions within HSV-1 TK. NBS, nucleoside binding site. Novel mutations are shown in boldface font. (C) Schematic representation of the long sequence deletions detected in five ACVr HSV-1_HSV1-TK clones. Line 1, the whole-genome structure of wild-type HSV-1 F; line 2, the related domains of HSV-1 F; line 3, the related domains of HSV-1_HSV1-TK; lines 4 to 7, nucleic acid deletions detected in ACVr HSV-1_HSV1-TK clones. The same deletion (line 6) was detected in two clones. Other deletions (lines 4, 5, and 7) were each detected in a single clone. Numbers indicate the location of nucleic acids in the HSV-1 F genome (GenBank GU734771.1). The numbers in parentheses indicate the location of nucleic acids within the region inserted.
In conclusion, the data presented herein suggest that the mechanism underlying differences in the likelihood of emergence of ACVr HSV-1 and ACVr VZV is due to the characteristics of HSV-1 TK and VZV TK. In addition, as HSV-1_VZV-TK induced ACV resistance-associated mutations only in the HSV-1 DNApol gene, this system could be used to enrich the database of HSV-1 DNApol gene-associated ACVr mutations.
ACKNOWLEDGMENTS
HSV-1 F was provided by Y. Kawaguchi (The University of Tokyo, Japan) with permission from B. Roizman (University of Chicago, IL). Escherichia coli GS1783 containing pYEbac102 was provided by Y. Kawaguchi, with permission from G. A. Smith (Northwestern University, IL) and N. Osterrieder (Freie Universität, Berlin, Germany) (31). pEP-KanS was provided by Y. Kawaguchi, with permission from N. Osterrieder. VZV strain vOka was provided by BIKEN Group, Osaka, Japan.
We thank Y. Fukui and M. Tsuda for technical and clerical assistance.
This work was supported by grants-in-aid from the Ministry of Health, Labor and Welfare science research grant(s) for research on measures for emerging and reemerging infections (intractable infectious diseases in organ transplant recipients [H21-Shinko-Ippan-009]), and from the Ministry of Education, Culture, Sports, Science and Technology of Japan (numbers 21591402, 24591591, 15K09675, and 15K19594).
REFERENCES
- 1.Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B. (ed) 2013. Fields virology, 6th ed Wolters Kluwer Health, Philadelphia, PA. [Google Scholar]
- 2.Piret J, Boivin G. 2011. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother 55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elion GB. 1983. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother 12:9–17. doi: 10.1093/jac/12.suppl_B.9. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert C, Bestman-Smith J, Boivin G. 2002. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist Updat 5:88–114. doi: 10.1016/S1368-7646(02)00021-3. [DOI] [PubMed] [Google Scholar]
- 5.Morfin F, Thouvenot D. 2003. Herpes simplex virus resistance to antiviral drugs. J Clin Virol 26:29–37. doi: 10.1016/S1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura K, Hayakawa J, Akahoshi Y, Harada N, Nakano H, Kameda K, Ugai T, Wada H, Yamasaki R, Ishihara Y, Sakamoto K, Ashizawa M, Sato M, Terasako-Saito K, Kimura S, Kikuchi M, Nakasone H, Yamazaki R, Kanda J, Kako S, Tanihara A, Nishida J, Kanda Y. 2015. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus and varicella zoster virus diseases after autologous hematopoietic stem cell transplantation. Int J Hematol 102:230–237. doi: 10.1007/s12185-015-1810-4. [DOI] [PubMed] [Google Scholar]
- 7.Sandherr M, Hentrich M, von Lilienfeld-Toal M, Massenkeil G, Neumann S, Penack O, Biehl L, Cornely OA. 2015. Antiviral prophylaxis in patients with solid tumours and haematological malignancies–update of the Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO). Ann Hematol 94:1441–1450. doi: 10.1007/s00277-015-2447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boivin G, Edelman CK, Pedneault L, Talarico CL, Biron KK, Balfour HH. Jr, 1994. Phenotypic and genotypic characterization of acyclovir-resistant varicella-zoster viruses isolated from persons with AIDS. J Infect Dis 170:68–75. doi: 10.1093/infdis/170.1.68. [DOI] [PubMed] [Google Scholar]
- 9.Bryan CJ, Prichard MN, Daily S, Jefferson G, Hartline C, Cassady KA, Hilliard L, Shimamura M. 2008. Acyclovir-resistant chronic verrucous vaccine strain varicella in a patient with neuroblastoma. Pediatr Infect Dis J 27:946–948. doi: 10.1097/INF.0b013e318175d85c. [DOI] [PubMed] [Google Scholar]
- 10.Crassard N, Souillet AL, Morfin F, Thouvenot D, Claudy A, Bertrand Y. 2007. Acyclovir-resistant varicella infection with atypical lesions in a non-HIV leukemic infant. Acta Paediatr 89:1497–1499. doi: 10.1111/j.1651-2227.2000.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 11.Fillet AM, Dumont B, Caumes E, Visse B, Agut H, Bricaire F, Huraux JM. 1998. Acyclovir-resistant varicella-zoster virus: phenotypic and genetic characterization. J Med Virol 55:250–254. doi:. [DOI] [PubMed] [Google Scholar]
- 12.Hatchette T, Tipples GA, Peters G, Alsuwaidi A, Zhou J, Mailman TL. 2008. Foscarnet salvage therapy for acyclovir-resistant varicella zoster: report of a novel thymidine kinase mutation and review of the literature. Pediatr Infect Dis J 27:75–77. doi: 10.1097/INF.0b013e3181598315. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson MA, Berger TG, Fikrig S, Becherer P, Moohr JW, Stanat SC, Biron KK. 1990. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 112:187–191. doi: 10.7326/0003-4819-112-3-187. [DOI] [PubMed] [Google Scholar]
- 14.Linnemann CC Jr, Biron KK, Hoppenjans WG, Solinger AM. 1990. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS 4:577–579. doi: 10.1097/00002030-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Lyall EG, Ogilvie MM, Smith NM, Burns S. 1994. Acyclovir resistant varicella zoster and HIV infection. Arch Dis Child 70:133–135. doi: 10.1136/adc.70.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morfin F, Thouvenot D, De Turenne-Tessier M, Lina B, Aymard M, Ooka T. 1999. Phenotypic and genetic characterization of thymidine kinase from clinical strains of varicella-zoster virus resistant to acyclovir. Antimicrob Agents Chemother 43:2412–2416. doi: 10.1128/AAC.43.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahwa S, Biron K, Lim W, Swenson P, Kaplan MH, Sadick N, Pahwa R. 1988. Continuous varicella-zoster infection associated with acyclovir resistance in a child with AIDS. JAMA 260:2879–2882. doi: 10.1001/jama.1988.03410190127035. [DOI] [PubMed] [Google Scholar]
- 18.Safrin S, Berger TG, Gilson I, Wolfe PR, Wofsy CB, Mills J, Biron KK. 1991. Foscarnet therapy in five patients with AIDS and acyclovir-resistant varicella-zoster virus infection. Ann Intern Med 115:19–21. doi: 10.7326/0003-4819-115-1-19. [DOI] [PubMed] [Google Scholar]
- 19.Saint-Leger E, Caumes E, Breton G, Douard D, Saiag P, Huraux JM, Bricaire F, Agut H, Fillet AM. 2001. Clinical and virologic characterization of acyclovir-resistant varicella-zoster viruses isolated from 11 patients with acquired immunodeficiency syndrome. Clin Infect Dis 33:2061–2067. doi: 10.1086/324503. [DOI] [PubMed] [Google Scholar]
- 20.Sauerbrei A, Taut J, Zell R, Wutzler P. 2011. Resistance testing of clinical varicella-zoster virus strains. Antiviral Res 90:242–247. doi: 10.1016/j.antiviral.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Snoeck R, Gérard M, Sadzot-Delvaux C, Andrei G, Balzarini J, Reymen D, Ahadi N, De Bruyn JM, Piette J, Rentier B, Clumeck N, De Clercq E. 1994. Meningoradiculoneuritis due to acyclovir-resistant varicella zoster virus in an acquired immune deficiency syndrome patient. J Med Virol 42:338–347. doi: 10.1002/jmv.1890420404. [DOI] [PubMed] [Google Scholar]
- 22.Talarico CL, Phelps WC, Biron KK. 1993. Analysis of the thymidine kinase genes from acyclovir-resistant mutants of varicella-zoster virus isolated from patients with AIDS. J Virol 67:1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visse B, Dumont B, Huraux JM, Fillet AM. 1998. Single amino acid change in DNA polymerase is associated with foscarnet resistance in a varicella-zoster virus strain recovered from a patient with AIDS. J Infect Dis 178:S55–S57. doi: 10.1086/514257. [DOI] [PubMed] [Google Scholar]
- 24.Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. 2003. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev 16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christophers J, Clayton J, Craske J, Ward R, Collins P, Trowbridge M, Darby G. 1998. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob Agents Chemother 42:868–872. doi: 10.1128/AAC.42.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Englund JA, Zimmerman ME, Swierkosz EM, Goodman JL, Scholl DR, Balfour HH Jr. 1990. Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med 112:416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- 27.Nugier F, Colin JN, Aymard M, Langlois M. 1992. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J Med Virol 36:1–12. doi: 10.1002/jmv.1890360102. [DOI] [PubMed] [Google Scholar]
- 28.Stranska R, Schuurman R, Nienhuis E, Goedegebuure IW, Polman M, Weel JF, Wertheim-Van Dillen PM, Berkhout RJ, van Loon AM. 2005. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J Clin Virol 32:7–18. doi: 10.1016/j.jcv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Bevilacqua F, Davis-Poynter N, Worrallo J, Gower D, Collins P, Darby G. 1995. Construction of a herpes simplex virus/varicella-zoster virus (HSV/VZV) thymidine kinase recombinant with the pathogenic potential of HSV and a drug sensitivity profile resembling that of VZV. J Gen Virol 76:1927–1935. doi: 10.1099/0022-1317-76-8-1927. [DOI] [PubMed] [Google Scholar]
- 30.Kato A, Tanaka M, Yamamoto M, Asai R, Sata T, Nishiyama Y, Kawaguchi Y. 2008. Identification of a physiological phosphorylation site of the herpes simplex virus 1-encoded protein kinase Us3 which regulates its optimal catalytic activity in vitro and influences its function in infected cells. J Virol 82:6172–6189. doi: 10.1128/JVI.00044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka M, Kagawa H, Yamanashi Y, Sata T, Kawaguchi Y. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J Virol 77:1382–1391. doi: 10.1128/JVI.77.2.1382-1391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tischer BK, von Einem J, Kaufer B, Osterrieder N. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- 33.Yoshikawa T, Fujii H, Okutani A, Shibamura M, Omura N, Egawa K, Kato H, Inagaki T, Harada S, Yamada S, Morikawa S, Saijo M. 2018. Construction and characterization of bacterial artificial chromosomes harboring the full-length genome of a highly attenuated vaccinia virus LC16m8. PLoS One 13:e0192725. doi: 10.1371/journal.pone.0192725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saijo M, Suzutani T, De Clercq E, Niikura M, Maeda A, Morikawa S, Kurane I. 2002. Genotypic and phenotypic characterization of the thymidine kinase of ACV-resistant HSV-1 derived from an acyclovir-sensitive herpes simplex virus type 1 strain. Antiviral Res 56:253–262. doi: 10.1016/S0166-3542(02)00131-6. [DOI] [PubMed] [Google Scholar]
- 35.Wang LX, Takayama-Ito M, Kinoshita-Yamaguchi H, Kakiuchi S, Suzutani T, Nakamichi K, Lim CK, Kurane I, Saijo M. 2013. Characterization of DNA polymerase-associated acyclovir-resistant herpes simplex virus type 1: mutations, sensitivity to antiviral compounds, neurovirulence, and in-vivo sensitivity to treatment. Jpn J Infect Dis 66:404–410. doi: 10.7883/yoken.66.404. [DOI] [PubMed] [Google Scholar]
- 36.Harrison PT, Thompson R, Davison AJ. 1991. Evolution of herpesvirus thymidine kinases from cellular deoxycytidine kinase. J Gen Virol 72:2583–2586. doi: 10.1099/0022-1317-72-10-2583. [DOI] [PubMed] [Google Scholar]
- 37.Andrei G, Topalis D, Fiten P, McGuigan C, Balzarini J, Opdenakker G, Snoeck R. 2012. In vitro-selected drug-resistant varicella-zoster virus mutants in the thymidine kinase and DNA polymerase genes yield novel phenotype-genotype associations and highlight differences between antiherpesvirus drugs. J Virol 86:2641–2652. doi: 10.1128/JVI.06620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burrel S, Deback C, Agut H, Boutolleau D. 2010. Genotypic characterization of UL23 thymidine kinase and UL30 DNA polymerase of clinical isolates of herpes simplex virus: natural polymorphism and mutations associated with resistance to antivirals. Antimicrob Agents Chemother 54:4833–4842. doi: 10.1128/AAC.00669-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill EL, Hunter GA, Ellis MN. 1991. In vitro and in vivo characterization of herpes simplex virus clinical isolates recovered from patients infected with human immunodeficiency virus. Antimicrob Agents Chemother 35:2322–2328. doi: 10.1128/AAC.35.11.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt S, Bohn-Wippert K, Schlattmann P, Zell R, Sauerbrei A. 2015. Sequence analysis of herpes simplex virus 1 thymidine kinase and DNA polymerase genes from over 300 clinical isolates from 1973 to 2014 finds novel mutations that may be relevant for development of antiviral resistance. Antimicrob Agents Chemother 59:4938–4945. doi: 10.1128/AAC.00977-15. [DOI] [PMC free article] [PubMed] [Google Scholar]