The most frequent ailment for which antibiotics are prescribed is otitis media (ear infections), which is most commonly caused by Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. Treatment of otitis media is complicated by the fact that the bacteria in the middle ear typically form biofilms, which can be recalcitrant to antibiotic treatment.
KEYWORDS: Haemophilus influenzae, Streptococcus pneumoniae, otitis media
ABSTRACT
The most frequent ailment for which antibiotics are prescribed is otitis media (ear infections), which is most commonly caused by Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. Treatment of otitis media is complicated by the fact that the bacteria in the middle ear typically form biofilms, which can be recalcitrant to antibiotic treatment. Furthermore, bacterial respiratory infections can be greatly exacerbated by viral coinfection, which is particularly evidenced by the synergy between influenza and S. pneumoniae. In this study, we sought to ascertain the in vivo efficacy of aminomethyl spectinomycin lead 1950, an effective antibacterial agent both in vitro and in vivo against Streptococcus pneumoniae in the context of complex respiratory infections and acute otitis media. A single dose of 1950 significantly reduced bacterial burden in the respiratory tract for all three pathogens, even when species were present in a coinfection model. Additionally, a single dose of 1950 effectively reduced pneumococcal acute otitis media from the middle ear. The agent 1950 also proved efficacious in the context of influenza-pneumococcal super infection. These data further support the development of this family of compounds as potential therapeutic agents against the common causes of complex upper respiratory tract infections and acute otitis media.
INTRODUCTION
The increasing prevalence of antibiotic-resistant bacteria among bacterial pathogens necessitates the development of novel compounds to expand the therapeutic pipeline. Chemical modification of antibiotics to increase potency as well as to bypass resistance mechanisms has led to many successful new treatment options. Examples of this successful strategy include multiple generations of β-lactams, macrolide antibiotics, and aminoglycosides (1, 2). However, to date there are no approved modified spectinomycin analogs in clinical use.
Our efforts have focused on identifying expanded-spectrum spectinomycin analogs by using structure-guided design methods, which recently have become assessible through ribosome crystallography (3). These efforts have yielded two new classes of spectinomycin derivatives, namely, spectinamides (4, 5) and aminomethyl spectinomycins (amSPCs), that resulted in new therapeutic possibilities for the treatment of multidrug-resistant bacterial infections (6). The success of evading efflux mechanisms while retaining effective inhibition of the bacterial target through chemical variation underscores the importance of target-based activity and mechanisms of cellular uptake and efflux for optimal development (7). These results highlight the importance of reevaluating existing antibiotic scaffolds to identify structural analogs that may possess distinctive in vitro and in vivo characteristics to identify new therapeutic regimens for difficult-to-treat pathogens. It is our position that spectinomycin provides a unique platform to achieve this goal.
Spectinomycin inhibits protein synthesis via a rather unique interaction at the head domain of the 30S ribosomal subunit (8). Previously published chemical modification of the N-benzyl aminomethyl spectinomycins indicated that these compounds demonstrate potent antibacterial activity and superiority over the parent spectinomycin for the treatment of invasive pneumococcal infection (6). This series of N-benzyl-substituted 3′-(R)-3′-aminomethyl-3′-hydroxy spectinomycins also demonstrated potency in vitro against other pathogens commonly associated with respiratory tract infections, including Haemophilus influenzae and Moraxella catarrhalis (6). These promising in vitro and in vivo efficacy trials led us to hypothesize that this series may be efficacious against other mucosal pathogens and diseases.
Acute otitis media is the most frequently diagnosed infection of children and is the most common reason for prescribing antibiotics to children in the United States (9). Despite prolonged exposure to broad-spectrum antibiotics, a high percentage of children who experience acute otitis media will have frequent recurrences of infection (10, 11). The most common pathogens responsible for otitis media are H. influenzae, Streptococcus pneumoniae, and M. catarrhalis (12). When multiple infectious agents are present, the subsequent risk for developing acute otitis media is higher than that of carrying any individual pathogen (13). Bacterial pathogens, such as pneumococci, have a propensity to form biofilms during episodes of otitis media, which are inherently difficult to clear by antibiotics (14). Another challenge of treatment is achieving sufficient antibiotic concentration and duration of exposure in the middle ear to reduce and ultimately eliminate the bacterial infection (15). Treatment can also be confounded by the presence of multiple bacterial species that may have distinct antibiotic resistance profiles and can potentially confer protection based on their capacity to inactivate antibiotics, as seen in cases such as the β-lactamases (16–18). As such, antibiotics that successfully target these three pathogens with sufficient efficacy in the middle ear would provide an attractive new avenue for treatment options for acute otitis media.
In addition to being the most prominent bacterial causes of acute otitis media, H. influenzae, M. catarrhalis, and S. pneumoniae are also among the most commonly associated pathogens in community-acquired pneumonia (19). Pneumonia and influenza remain the leading infectious causes of death in the United States despite widespread availability of both the pneumococcal conjugate and seasonal influenza vaccines (20). Previous work demonstrated robust in vitro activity of the amSPCs against these common bacterial species (6). In addition, 1950 showed excellent in vivo protection against pneumococcal pneumonia, showing greater efficacy than what might be expected from in vitro susceptibility testing. Based on this result, we hypothesized that 1950 could be leveraged to effectively treat both acute otitis media and pneumonia caused by H. influenzae, M. catarrhalis, and S. pneumoniae. Robust protective efficacy was achieved both during pulmonary and acute otitis models of murine infection. Protection was engendered in the context of more complex infections with either multiple bacterial pathogens or with influenza coinfection. These results underscore the potential therapeutic efficacy of amSPCs for the treatment of respiratory infections.
RESULTS
Aminomethyl spectinomycins are effective against acute otitis media.
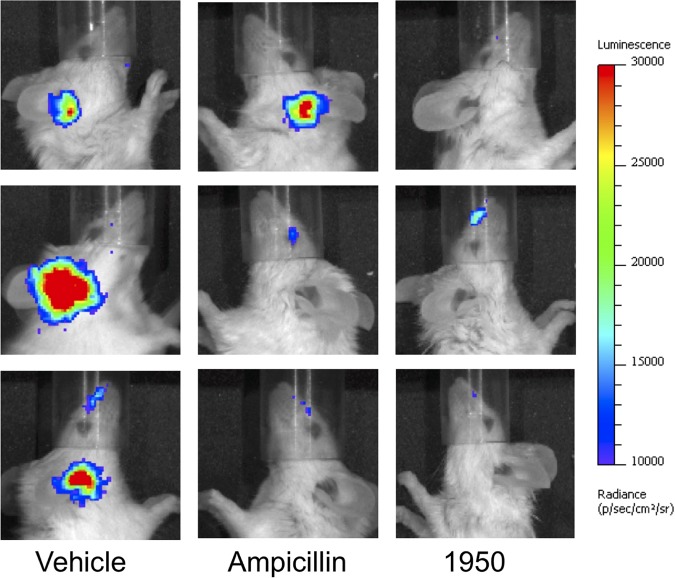
In the S. pneumoniae-infected animals, the 1950 treatment resulted in a significant decrease in bacterial burden that was statistically indistinguishable from that in the ampicillin-treated group (Fig. 1A). For the animals infected with H. influenzae, 1950 resulted in a statistically significant improvement over the vehicle control and ampicillin-treated groups (Fig. 1B). This strain of Haemophilus encodes a β-lactamase and, hence, resistance to ampicillin is expected (21). It should be noted this model requires translocation of the bacteria from the respiratory tract into the middle ear, imparting a significant population bottleneck that results in a high degree of variation in ear titers. Various degrees of immune-mediated clearance mechanisms can also result in the high variability inherent in this model. In our models, no translocation of M. catarrhalis was observed into the middle ear; hence, this pathogen was not included in the treatment efficacy trials for acute otitis media, although 1950 was found to inhibit M. catarrhalis effectively in vitro. This finding indicated that administration of 1950, whose structure is detailed in Fig. 1C, is effective for the treatment of acute otitis media for both S. pneumoniae and H. influenzae in concordance with the in vitro susceptibility testing of the strains used in this study (Fig. 1D). In addition to measuring bacterial titers in mice infected with pneumococcus, we also used Xenogen imaging to monitor bacterial burden by luminescence. As seen in Fig. 2, mice receiving vehicle alone had robust luminescent signals from the ears from the S. pneumoniae infection, whereas these signals were notably reduced or absent completely in both the ampicillin- and 1950-treated groups.
FIG 1.
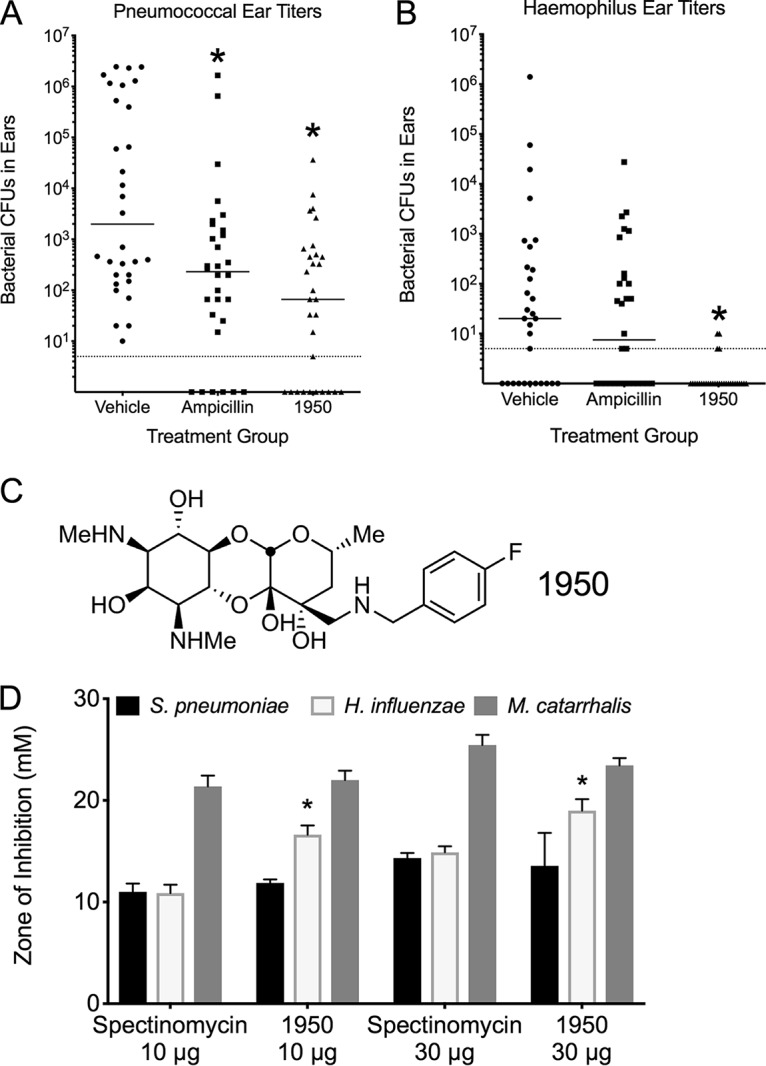
Aminomethyl spectinomycin effective for treatment of acute otitis media. Mice infected with S. pneumoniae (A) or H. influenzae (B) and subsequently treated with a single dose of vehicle, ampicillin, or 1950. Bacterial burden in the ears was measured by CFU enumeration at 24 h postchallenge. Groups were compared by Mann-Whitney U testing. *, indicates P < 0.05 compared with vehicle control group for (A) and (B). Each data point represents an individual ear. Dotted line indicates the limit of detection. (C) Structure of aminomethyl spectinomycin analog 1950. (D) The zone of inhibition was compared for each species between spectinomycin and 1950 by Mann-Whitney U testing, with the asterisk (*) indicating P < 0.05.
FIG 2.
Bioluminescent images of pneumococcal acute otitis media. Representative images from vehicle control, ampicillin-treated, and 1950-treated groups demonstrate a marked reduction in luminescent signal detected from the ears. Images were taken at 24 h postchallenge immediately prior to tissue harvest. Scale bar indicates luminescent intensity.
Protection against Gram-positive and Gram-negative pneumonia.
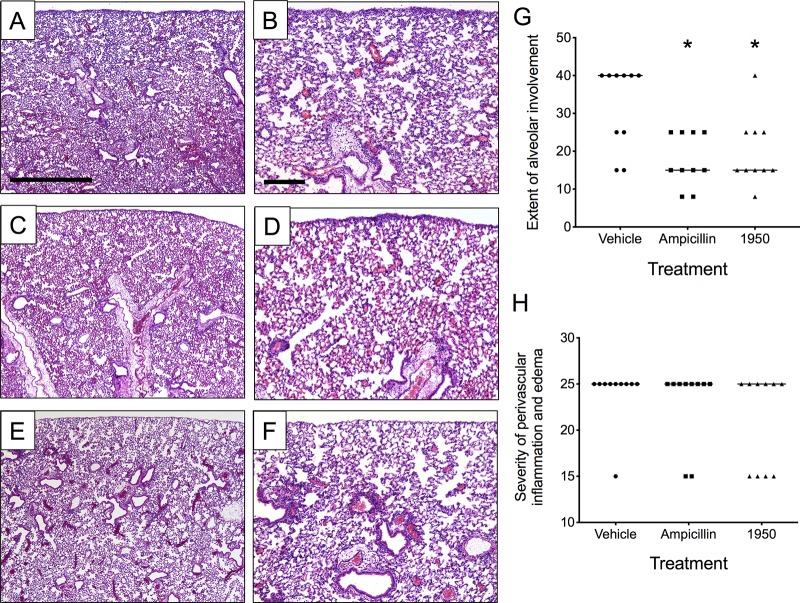
We next sought to determine the efficacy of 1950 in the reduction of bacterial burden in the respiratory tract following infection. For the pneumococcal challenge, a single dose of 1950 reduced the bacterial lung burden to levels indistinguishable from those in the ampicillin-treated group (Fig. 3A). For both the H. influenzae and M. catarrhalis challenge groups, 1950 treatment significantly reduced bacterial burden by approximately two-logs to levels approaching the limit of detection of the assay (Fig. 3B and C). Treatment with 1950 significantly reduced the extent of alveolar involvement in terms of lung inflammation to an extent indistinguishable from ampicillin in the context of pneumococcal pneumonia (Fig. 4). No significant differences in the extent of alveolar involvement or perivascular inflammation were observed by histopathology in either H. influenzae and M. catarrhalis at 24 h following challenge (data not shown). This result supports our hypothesis that treatment with 1950 would be effective against these Gram-positive and Gram-negative pathogens in the context of pulmonary infection.
FIG 3.
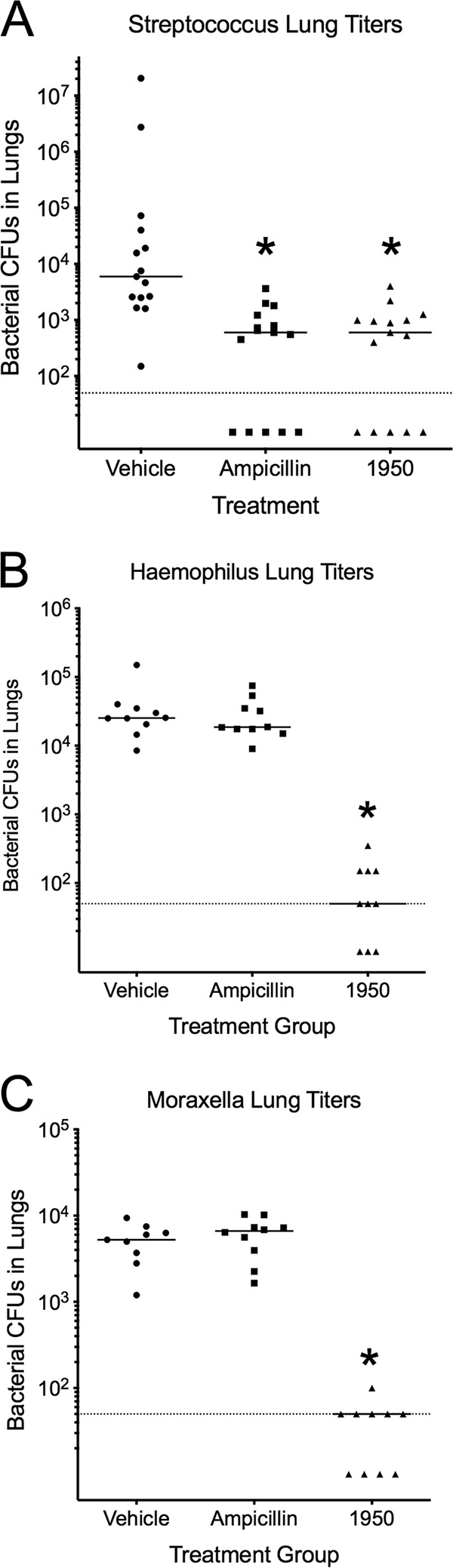
Efficacy of 1950 against etiological agents of community-acquired pneumonia. Bacterial burden in the lungs following treatment with vehicle, ampicillin, or 1950 in mice infected with S. pneumoniae (A), H. influenzae (B), or M. catarrhalis (C). Bacterial burden in the lungs was measured by CFU enumeration. The strains of H. influenzae and M. catarrhalis used in these challenges are resistant to ampicillin. Groups were compared by Mann-Whitney U testing. *, indicates P < 0.05 compared with vehicle control group. Each data point represents titers from one animal. Dotted line indicates the limit of detection.
FIG 4.
The 1950 treatment reduces the extent of alveolar involvement during pneumococcal pneumonia. Representative lung sections subjected hematoxylin and eosin staining of vehicle (A, B), ampicillin (C, D), and 1950 (E, F) at 24 h postchallenge. Scale bar for ×4 magnification in (A, C, and E) is 1 mm and indicated in (A). Scale bar for ×10 magnification in (B, D, and F) is 200 μm and indicated in (A). Pathologist scores for (G) indicated 1950 significantly reduced the extent of alveolar involvement in lung inflammation in a manner indistinguishable from ampicillin treatment. No differences were observed at this time point in terms of severity of perivascular inflammation and edema (H). Groups were compared by Mann-Whitney U testing. *, indicates P < 0.05 compared with vehicle control group. Each data point represents scores from one animal.
Efficacy of 1950 against multispecies bacterial challenges.
After determining the potency against single pathogens, we tested 1950 in the context of pneumococcal pneumonia complicated by coinfection with either H. influenzae or M. catarrhalis. In the pneumococcus and H. influenzae coinfection group, 1950 significantly (P < 0.05, Mann-Whitney U test) reduced the bacterial burden of both pathogens (Fig. 5A and B). In contrast, ampicillin treatment did not significantly reduce the bacterial burden of either pathogen, indicating the protective capacity of H. influenzae in allowing the pneumococcus to evade clearance by ampicillin. This observation was mirrored in the context of Moraxella/pneumococcal coinfection, whereby treatment with 1950 significantly reduced the bacterial burden of both bacterial pathogens, while the ampicillin treatment resulted in no significant reduction in bacterial burden in the lungs (Fig. 5C and D). These observations indicate that the presence of β-lactamase-producing H. influenzae or M. catarrhalis can interfere with the elimination of sensitive pneumococci in the context of complex bacterial pneumonia. Furthermore, the data indicate the efficacy of 1950 in reducing bacterial lung burden of the respective pathogens regardless of whether the infective agents are present alone or in combination. While we only have preliminary data thus far, these results bolster our position that modified chemical matter, such as the amSPCs, can provide protection against invasive pathogens that would otherwise be resistant to other therapeutic treatments, such as ampicillin.
FIG 5.
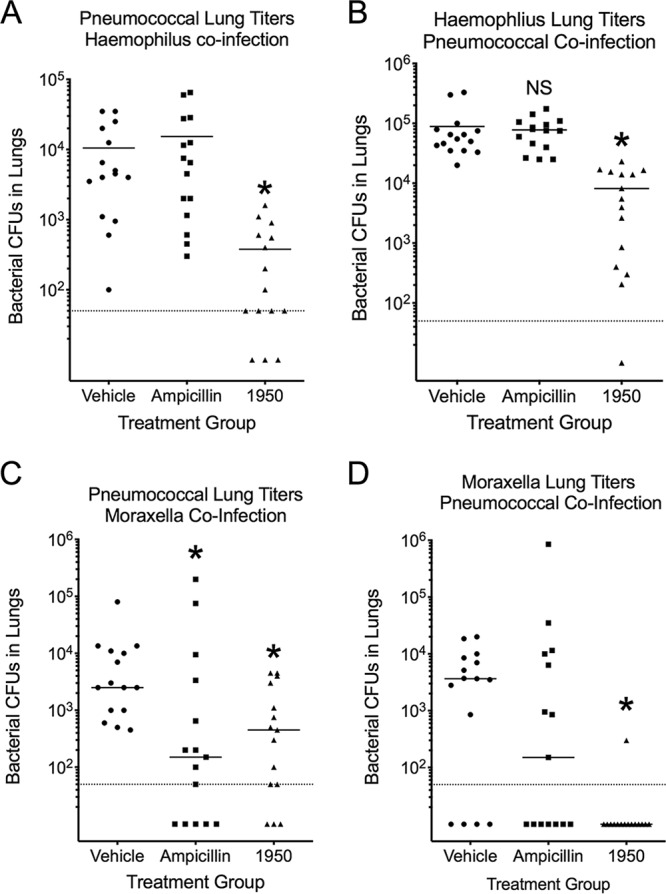
Efficacy of 1950 in mixed bacterial infections. Bacterial burden from S. pneumoniae/H. influenzae coinfected lungs (A, B) and S. pneumoniae/M. catarrhalis coinfected infected lungs (C, D) following treatment with vehicle, ampicillin, or 1950. Bacterial burden in the lungs was measured by CFU enumeration. Groups were compared by Mann-Whitney U testing. *, indicates P < 0.05 compared with vehicle control group. Each data point represents 1 animal. Dotted line indicates the limit of detection.
Efficacy of 1950 in an influenza-pneumococcal superinfection model.
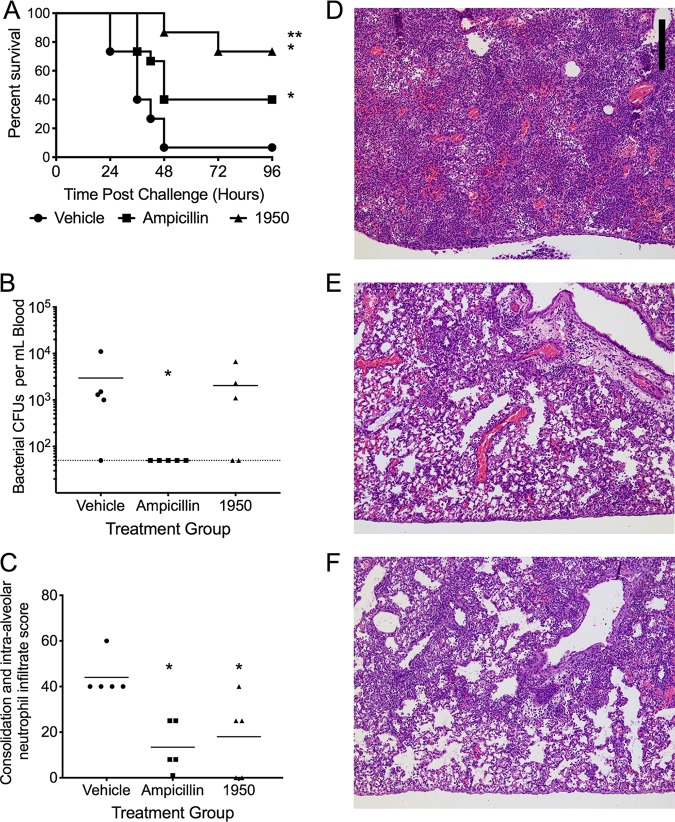
Viral coinfection can greatly exacerbate subsequent bacterial infections, an observation seen clinically and effectively recapitulated in murine models (22). Due to the severity of the secondary bacterial infection and the propensity for bacteriolytic antibiotics to exacerbate pulmonary damage and mortality, we explored if our compound would be able to alleviate damage and, thereby, decrease the mortality levels of such an infection. Indeed, treatment with 1950 conferred significantly greater protection against mortality than did either vehicle control or ampicillin treatment (Fig. 6A). This result was of particular interest as the bacterial burden at 24 h postchallenge was undetectable in the ampicillin-treated animals, whereas bacteria were recovered from the bloodstream of both the vehicle and 1950-treated animals (Fig. 6B). Ampicillin and 1950 significantly reduced the areas of pulmonary consolidation and intra-alveolar neutrophils (Fig. 6C), as observed by histopathological scoring of lung tissues at 24 h following bacterial challenge (Fig. 6D to F). These data indicate that 1950 is effective against influenza-mediated coinfection with S. pneumoniae.
FIG 6.
Efficacy of 1950 in an influenza-pneumococcal superinfection model. Murine survival following previous infection with influenza and subsequent S. pneumoniae challenge (A). Mice were treated starting 6 h post-bacterial challenge and twice daily thereafter, with data representing overall survival time. Bacterial burden in bloodstream 24 h postchallenge (B). Pathology scores of levels of consolidation and alveolar neutrophil infiltrate in lung tissue 24 h postchallenge (C). Representative histopathology images of vehicle (D), ampicillin treated (E), and 1950 treated (F) at 24 h following bacterial challenge. Scale bar represents 200 μm and indicated in (D). *, indicates P < 0.05 by Mantel log-rank test compared with vehicle; **, indicates P < 0.05 by Mantel log-rank test compared with ampicillin.
DISCUSSION
Successful treatment of bacterial infections is a complicated process involving not only on-target potency but also influx/efflux kinetics, tissue bioavailability, and immune response among other considerations. We found that administering 1950 dramatically reduced the bacterial burden of both H. influenzae and S. pneumoniae in a murine model of acute otitis media following a single dose of antibiotic once animals became symptomatic. Although sterilization was not achieved with a single dose, the bacterial burden was significantly reduced for both pathogens, indicating that a sufficient drug concentration was achieved in the middle ear to reduce the bacterial burden. From the data collected, we concluded that 1950 is a potential candidate for the treatment of acute otitis media.
We further tested the ability of 1950 to reduce bacterial burden in the lungs using three of the frequent causes of community-acquired pneumonia, namely, Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. In agreement with previous in vitro susceptibility testing, administration of a single dose of 1950 significantly reduced the bacterial burden of all three pathogens in the lungs. While antibiotic treatment effectively reduced pathogen burden in the lung tissues, it is important to note these infections are undertaken in immunocompetent animals and, as such, innate immune-mediated clearance mechanisms remain operative and reduce bacterial burden significantly during the time frame of these experiments. This finding highlights the effectiveness of amSPCs at eliminating both Gram-positive and Gram-negative bacterial respiratory infections caused by the major etiologic agents of community-acquired pneumonia.
Efficacious treatment of these types of infections can be confounded by the presence of multiple pathogens, which can exacerbate the infection or complicate antibiotic therapy. This was modeled by using pneumococcal coinfection with either H. influenzae, which encodes β-lactamase, or M. catarrhalis. As expected, coinfection with H. influenzae resulted in a corresponding protective effect for the pneumococcus in response to ampicillin treatment, rendering the treatment ineffective. In contrast, the 1950 treatment groups had significantly reduced bacterial burden in the lungs for both H. influenzae and S. pneumoniae. A similar trend was observed with M. catarrhalis, whereby ampicillin treatment was less effective at clearing M. catarrhalis in the context of a mixed infection, and 1950 was effective against both M. catarrhalis and S. pneumoniae. These results indicate that amSPCs may prove efficacious in treating mixed bacterial infections that would otherwise be untreatable with β-lactam antibiotics.
The final aspect investigated was the use of this compound to treat pneumococcal infection in the context of an influenza virus coinfection. Previous work demonstrated that protein synthesis inhibitors are more effective for the treatment of secondary pneumococcal pneumonia following influenza infection (23). The proposed mechanism for this observation is the release of the pore-forming toxin pneumolysin, which causes extensive cellular damage following exposure of the pneumococcus to bacteriolytic antibiotics, such as β-lactams. We observed a similar trend, whereby mice treated with ampicillin did significantly worse than those treated with 1950, which is in agreement with previous findings about protein synthesis-targeting antibiotics. Interestingly, ampicillin was more effective at reducing the level of bacteremia than 1950 at 24 h postchallenge, although mice in this treatment group had poorer survival. However, the level of pulmonary consolidation observed in the lungs was similar between the 1950 and the ampicillin groups and was markedly reduced compared with that of the vehicle controls in the context of viral coinfection. Altogether, the data obtained indicate that amSPC 1950 is potent against common causative agents of community-acquired pneumonia and acute otitis in murine models of the diseases, supporting the application of this drug scaffold as a potential therapeutic treatment for these infections.
MATERIALS AND METHODS
Compound synthesis and quality control.
Compound 1950 was synthesized according to the procedure reported previously (6). The purity and identity of the compound were determined using reverse-phase ultraperformance liquid chromatography (UPLC) analysis with evaporative light scattering detector (ELSD)/mass spectrometry (MS) detection and by 1H nuclear magnetic resonance (NMR). All samples were confirmed to be >95% pure before being submitted for biological testing.
Media and growth conditions.
S. pneumoniae strain BHN97x (24) was grown on tryptic soy agar (TSA; EMD Chemicals, New Jersey) supplemented with 3% sheep blood or in CY medium, a defined semisynthetic casein liquid medium (25) supplemented with 0.5% yeast extract. Cultures of S. pneumoniae were inoculated from either frozen stock or newly streaked TSA blood plates and incubated at 37°C in 5% CO2. Nontypeable H. influenzae 86-028NP originally isolated from a patient with chronic otitis media, which carries a broad-spectrum β-lactamase, was grown as previously described (21). M. catarrhalis O53E (26) was cultured in brain heart infusion (BHI) broth supplemented with 0.5% yeast extract. To differentiate bacterial counts during mixed infections, aliquots were cultured on BHI agar plates with 3 μg/ml vancomycin to isolate M. catarrhalis, on chocolate agar plates with bacitracin (Remel, San Diego, CA) to isolate H. influenzae, and on TSA plates with 3% sheep blood and 400 μg/ml kanamycin to isolate S. pneumoniae BHN97x, which carries a kanamycin-resistance gene associated with insertion of the bioluminescent marker.
Susceptibility testing was undertaken by disc diffusion in accordance with EUCAST guidelines. To a sterile 6-mm filter disc, 10 μg or 30 μg of either spectinomycin or 1950 was applied in a volume of 10 μl. The antibiotic-infused discs were overlaid with freshly plated M. catarrhalis, S. pneumoniae, or H. influenzae onto Muller-Hinton agar supplemented with 20 μg/liter NAD and 5% horse blood (Edge Biologics, Memphis, TN). Plates were incubated at 37°C in 5% CO2 and the diameter of the zone of growth inhibition measured following an 18 h incubation. Experiments were repeated at least four times.
Mouse studies.
All experiments involving animals were performed with prior approval of and in accordance with guidelines of the St. Jude Institutional Animal Care and Use Committee. The St. Jude laboratory animal facilities are fully accredited by the American Association for Accreditation of Laboratory Animal Care. Laboratory animals were maintained in accordance with the applicable portions of the Animal Welfare Act and the guidelines prescribed in the Department of Health and Human Services (DHHS) publication, Guide for the Care and Use of Laboratory Animals. All mice were maintained in biosafety level 2 (BSL2) facilities, and all experiments were done while the mice were under inhaled isoflurane (2.5%) anesthesia. Mice were monitored daily for signs of infection. This work was approved under IACUC protocol number 538-100013-04/12 R1.
For bacterial burden and survival studies, all bacteria were grown in their respective medium and diluted according to a previously determined standard curve. Bacteria were enumerated to ensure that the proper number of bacteria was used in infection for each challenge. Previous work established models of acute otitis media in the murine model in which bacteria are readily detectable in the middle ear (27, 28). For the acute otitis media studies, bacteria were introduced into 7-week-old female BALB/c mice (Jackson Laboratory) via intranasal (IN) administration of 106 CFU S. pneumoniae or 107 CFU of either H. influenza or M. catarrhalis in phosphate-buffered saline (PBS) (100 μl) either alone or with S. pneumoniae. At 6 h following infection, the time point at which bacteria are initially detected in the middle ear (28), mice were treated with a single dose of 1950 at a concentration of 25 mg/kg of body weight in Plasma-Lyte or 25 mg/kg ampicillin, to which the pneumococcal strain is acutely sensitive (positive control); mice treated with an equivalent volume of Plasma-Lyte alone were used as a vehicle control. This dosage was chosen as we wanted to ensure a high dose that was well tolerated and below the maximum tolerated dose of >100 mg/kg. We opted for the subcutaneous injection route for the antibiotics due to the rationale that substances administered subcutaneously often are absorbed at a lower rate than other parenteral routes, providing a more sustained effect (29). Mice were allowed to recover for 18 h following treatment. At 24 h after infection, mice were humanely euthanized via CO2 asphyxiation, and cervical dislocation was performed.
Lungs were harvested into 500 μl ice-cold PBS. For middle ear harvest, the head was detached and then skin and the outer ear were removed. An incision was made along the midline of the skull, and another perpendicular one was made just behind the eyes. The top of the skull and the brain were removed. A vertical incision was made just behind the ear canal. Connective tissue and muscle were removed to expose the middle ear, which was carefully excised and placed in 1 ml of chilled PBS. The lungs and middle ears were then homogenized and subsequently plated to ascertain bacterial burden in the respective tissues.
To model influenza-pneumococcal superinfection, mice were infected IN with 100× 50% tissue culture infective dose (TCID50) influenza pH1N1 A/California/09 and monitored for 7 days. At day 7, influenza-infected mice were challenged with 104 CFU S. pneumoniae D39x. At 6 h postchallenge and every 12 h thereafter (q12H), mice were treated with vehicle (Plasma-Lyte), ampicillin (25 mg/kg), or 1950 (25 mg/kg) via subcutaneous injection. Mice were monitored for mortality and bacterial burden in the bloodstream.
Histological analysis.
At 24 h after infection and 18 hours following treatment, mice were humanely euthanized via CO2 asphyxiation, and cervical dislocation was performed. After euthanasia, mice were perfused with 3% paraformaldehyde, and lungs were excised and instilled with paraformaldehyde under constant pressure to expand the lungs to approximately physiological dimensions and embedded in paraffin for hematoxylin and eosin (H&E) staining. The severity of perivascular inflammation/edema and the extent of alveolar lesions were assessed and graded in a blind manner by a veterinary pathologist. Separate severity grades for lesions in both locations (perivascular/peribronchiolar and alveoli) were assigned as follows: 0, no lesions detected; 1, minimal, rare, barely detectable lesions; 2, mild multifocal, small focal, or widely separated lesions; 3, moderate, multifocal, and prominent lesions; 4, marked, extensive-to-coalescing areas; and 5, severe and extensive lesions with pulmonary consolidation. These severity grades were then converted to weighted semiquantitative scores as follows: 0, 0; 1, 1; 2, 15; 3, 40; 4, 80; and 5, 100.
Statistical analysis.
For bacterial burden comparisons and histopathological scoring, Mann-Whitney U testing was utilized, with a P value less than 0.05 considered to be significant. For survival analysis, log-rank testing of survival time was utilized, with a P value less than 0.05 considered to be significant.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI111449 to R.L. and American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital. J.W.R. is supported by 1U01AI124302 and 1R01AI110618.
REFERENCES
- 1.Fischbach MA, Walsh CT. 2009. Antibiotics for emerging pathogens. Science 325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright GD, Sutherland AD. 2007. New strategies for combating multidrug-resistant bacteria. Trends Mol Med 13:260–267. doi: 10.1016/j.molmed.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Borovinskaya MA, Shoji S, Holton JM, Fredrick K, Cate JH. 2007. A steric block in translation caused by the antibiotic spectinomycin. ACS Chem Biol 2:545–552. doi: 10.1021/cb700100n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RE, Hurdle JG, Liu J, Bruhn DF, Matt T, Scherman MS, Vaddady PK, Zheng Z, Qi J, Akbergenov R, Das S, Madhura DB, Rathi C, Trivedi A, Villellas C, Lee RB, Rakesh, Waidyarachchi SL, Sun D, McNeil MR, Ainsa JA, Boshoff HI, Gonzalez-Juarrero M, Meibohm B, Bottger EC, Lenaerts AJ. 2014. Spectinamides: a new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat Med 20:152–158. doi: 10.1038/nm.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson GT, Scherman MS, Bruhn DF, Liu J, Hastings C, McNeil MR, Butler MM, Bowlin TL, Lee RB, Lee RE, Lenaerts AJ. 2017. Spectinamides are effective partner agents for the treatment of tuberculosis in multiple mouse infection models. J Antimicrob Chemother 72:770–777. doi: 10.1093/jac/dkw467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruhn DF, Waidyarachchi SL, Madhura DB, Shcherbakov D, Zheng Z, Liu J, Abdelrahman YM, Singh AP, Duscha S, Rathi C, Lee RB, Belland RJ, Meibohm B, Rosch JW, Bottger EC, Lee RE. 2015. Aminomethyl spectinomycins as therapeutics for drug-resistant respiratory tract and sexually transmitted bacterial infections. Sci Transl Med 7:288ra75. doi: 10.1126/scitranslmed.3010572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Bruhn DF, Lee RB, Zheng Z, Janusic T, Scherbakov D, Scherman MS, Boshoff HI, Das S, Rakesh, Waidyarachchi SL, Brewer TA, Gracia B, Yang L, Bollinger J, Robertson GT, Meibohm B, Lenaerts AJ, Ainsa J, Bottger EC, Lee RE. 2017. Structure-activity relationships of spectinamide antituberculosis agents: a dissection of ribosomal inhibition and native efflux avoidance contributions. ACS Infect Dis 3:72–88. doi: 10.1021/acsinfecdis.6b00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter AP, Clemons WM, Brodersen DE, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407:340–348. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- 9.Grijalva CG, Nuorti JP, Griffin MR. 2009. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 302:758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faden H. 2001. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr 160:407–413. doi: 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 11.Pelton SI. 2002. Acute otitis media in an era of increasing antimicrobial resistance and universal administration of pneumococcal conjugate vaccine. Pediatr Infect Dis J 21:599–604; discussion 613–614. doi: 10.1097/00006454-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 12.Casey JR, Adlowitz DG, Pichichero ME. 2010. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 29:304–309. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Revai K, Mamidi D, Chonmaitree T. 2008. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis 46:e34–e37. doi: 10.1086/525856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid SD, Hong W, Dew KE, Winn DR, Pang B, Watt J, Glover DT, Hollingshead SK, Swords WE. 2009. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis 199:786–794. doi: 10.1086/597042. [DOI] [PubMed] [Google Scholar]
- 15.Craig WA, Andes D. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J 15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton RB, Rigby PJ, Wiertsema SP, Filion P, Langlands J, Coates HL, Vijayasekaran S, Keil AD, Richmond PC. 2011. Multi-species bacterial biofilm and intracellular infection in otitis media. BMC Pediatr 11:94. doi: 10.1186/1471-2431-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holder RC, Kirse DJ, Evans AK, Peters TR, Poehling KA, Swords WE, Reid SD. 2012. One third of middle ear effusions from children undergoing tympanostomy tube placement had multiple bacterial pathogens. BMC Pediatr 12:87. doi: 10.1186/1471-2431-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez JA, Anzueto AR. 2011. Changing needs of community-acquired pneumonia. J Antimicrob Chemother 66:iii3–iii9. doi: 10.1093/jac/dkr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. 2016. Deaths: final data for 2014. Natl Vital Stat Rep 65:1–122. [PubMed] [Google Scholar]
- 21.Harrison A, Dyer DW, Gillaspy A, Ray WC, Mungur R, Carson MB, Zhong H, Gipson J, Gipson M, Johnson LS, Lewis L, Bakaletz LO, Munson RS Jr, 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol 187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCullers JA. 2014. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 12:252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 23.Karlstrom A, Boyd KL, English BK, McCullers JA. 2009. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J Infect Dis 199:311–319. doi: 10.1086/596051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. 2010. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis 202:1287–1295. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacks S, Hotchkiss RD. 1960. A study of the genetic material determining enzyme in the pneumococcus. Biochim Biophys Acta 39:508–517. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 26.Helminen ME, Maciver I, Latimer JL, Klesney-Tait J, Cope LD, Paris M, McCracken GH Jr, Hansen EJ. 1994. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis 170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 27.Mina MJ, Klugman KP, Rosch JW, McCullers JA. 2015. Live attenuated influenza virus increases pneumococcal translocation and persistence within the middle ear. J Infect Dis 212:195–201. doi: 10.1093/infdis/jiu804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosch JW, Iverson AR, Humann J, Mann B, Gao G, Vogel P, Mina M, Murrah KA, Perez AC, Edward Swords W, Tuomanen EI, McCullers JA. 2014. A live-attenuated pneumococcal vaccine elicits CD4+ T-cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol Med 6:141–154. doi: 10.1002/emmm.201202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613. [PMC free article] [PubMed] [Google Scholar]